Abstract
Aim & methods: This trial investigated long-term (56-week treatment/24-week follow-up) use of subcutaneous tanezumab (5 or 10 mg every 8 weeks) or oral celecoxib (200 mg/day) in Japanese patients with chronic low back pain. Results & conclusion: Tanezumab safety was consistent with previous studies, except overall adverse events (tanezumab 5 mg = 63.0%, tanezumab 10 mg = 54.8%, celecoxib = 67.4%) and events of abnormal peripheral sensation (tanezumab 5 mg = 9.8%, tanezumab 10 mg = 4.3%, celecoxib = 4.3%) were more frequent with 5 mg than 10 mg tanezumab. Joint safety event rates were 1.1% for tanezumab 5 mg, 2.2% for tanezumab 10 mg and 0% for celecoxib. All treatments improved pain and function throughout the treatment period.
Clinical trial registration number: NCT02725411
Lay abstract
In this study, researchers looked at the safety of tanezumab (a medication that blocks nerve growth factor) in Japanese people with chronic low back pain (CLBP). Researchers also looked at how well tanezumab improves the symptoms (pain and difficulty doing activities) of CLBP. People in the study were given oral (taken by mouth) celecoxib (a medication commonly used to treat CLBP) or injections of tanezumab (5 or 10 mg doses) under the skin of the belly or upper leg every 8 weeks for a total of 56 weeks. Side effects (something expected or unexpected that people experienced during the study that may or may not be due to the medication they received) occurred in 63.0% of people receiving tanezumab 5 mg, 54.8% of people receiving tanezumab 10 mg and 67.4% of patients receiving celecoxib. More people receiving tanezumab 5 mg (9.8% of people) had a side effect related to abnormal peripheral sensation (tingling, burning, numbness or sensitivity to heat or cold hands or feet) than people receiving tanezumab 10 mg (4.3% of people) or celecoxib (4.3% of people). More people receiving tanezumab (5 mg = 1.1% of people, 10 mg = 2.2% of people) had a problem with one of their joints (knees or hips) during the study than people receiving celecoxib (0% of people). All treatments improved pain and the ability to do activities. Overall, the researchers concluded that tanezumab was well tolerated in most people and may improve the symptoms of CLBP.
Low back pain is a major contributor to disability in Japan where the estimated prevalence of chronic low back pain (CLBP) among middle-aged and elderly adults is 14–25% [Citation1–5]. CLBP negatively affects quality of life and has significant economic impact on patients and Japanese society as a whole [Citation6].
A comprehensive approach including pharmacological and non-pharmacological therapies is recommended for CLBP [Citation7]. However, a need remains for safe and effective pharmacological agents that improve patient quality of life [Citation8]. NSAIDs (nonsteroidal anti-inflammatory drugs) are the most commonly prescribed agents for CLBP in Japan but carry potentially serious gastrointestinal, renal and cardiovascular risks [Citation9–11]. Further, NSAIDs and opioids demonstrate only mild-to-moderate effects on CLBP, no-to-mild effects on function, and clinical trial data regarding their long-term use is limited [Citation7,Citation9,Citation12].
NGF promotes neuronal sensitization that appears to modulate pain intensity in CLBP syndromes independent of degenerative changes disclosed by axial imaging or segmental instability [Citation13–15]. In rodents, a majority of fibers innervating intervertebral discs express NGF receptors and NGF injection into the multifidus muscle of the lower back sensitizes neurons projecting to this muscle in response to mechanical stimulation [Citation16,Citation17]. In humans, increased NGF levels and enhanced nerve density are observed in resected intervertebral discs associated with painful symptoms compared with discs from asymptomatic segments [Citation18,Citation19].
Tanezumab, a monoclonal antibody against NGF, has been evaluated for the treatment of osteoarthritis and CLBP [Citation13,Citation14,Citation20–24]. Here, we report findings from a randomized, double-blind, celecoxib-controlled, Phase III study evaluating long-term safety (primary objective) and efficacy (secondary objective) of subcutaneous (SC) tanezumab in Japanese patients with moderate-to-severe CLBP.
Patients & methods
Patients
Patients aged ≥18 years with CLBP (primary location between the 12th thoracic vertebra and lower gluteal folds, with or without radiation into the posterior thigh [category 1 or 2 per Quebec Task Force in Spinal Disorders]) of ≥3 months’ duration were eligible. Key inclusion criteria included a Low Back Pain Intensity (LBPI) score ≥5 (0 = no pain to 10 = worst possible pain) at screening and baseline, and a Patient’s Global Assessment of Low Back Pain score of fair, poor, or very poor at baseline. The Patient Global Assessment of Low Back Pain asks patients the question “Considering all the ways your low back pain affects you, how are you doing today?” with the following answer choices: 1 = very good (asymptomatic and no limitation of normal activities), 2 = good (mild symptoms and no limitation of normal activities), 3 = fair (moderate symptoms and limitation of some normal activities), 4 = poor (severe symptoms and inability to carry out most normal activities), 5 = very poor (very severe symptoms which are intolerable and inability to carry out all normal activities). Patients were required to be experiencing some benefit from, and ability to tolerate, a stable (≥5 days/week in the 30 days prior to baseline) regimen of oral NSAID therapy (celecoxib 100 mg twice daily, loxoprofen 120–180 mg/day, or meloxicam 5–15 mg/day) but still require additional pain relief at screening. Key exclusion criteria included a history of lumbosacral radiculopathy, diagnosis of osteoarthritis of the knee or hip based on American College of Rheumatology combined clinical and radiographic criteria, Kellgren-Lawrence (KL)-based radiographic evidence of hip (grade ≥2) or knee (grade ≥3) osteoarthritis, or radiographic evidence and symptoms of osteoarthritis of the shoulders. Patients with painless KL grade 2 osteoarthritis in the knee were allowed.
Treatments
The study included 56-week treatment and 24-week safety follow-up periods (). Using the sponsor’s interactive response technology, a computer-generated blocked randomization scheme assigned patients in a 1:1:1 ratio to SC tanezumab 5 mg + oral placebo, SC tanezumab 10 mg + oral placebo, or oral celecoxib + SC placebo in a double-blind manner. Tanezumab was provided in a prefilled syringe of 1 ml volume and administered every 8 weeks by medical staff into the patient’s abdomen or anterior thigh. Celecoxib was administered in capsules at a dosage of 200 mg/day (100 mg twice daily). Placebo was provided and administered in a manner matching SC tanezumab or oral celecoxib as appropriate.
Subcutaneous treatment was administered every 8 weeks and oral treatment was administered daily at a dosage of 200 mg/day (100 mg twice daily). Prior to receiving treatment at week 16, patients must have had a ≥30% reduction from baseline in LBPI score at week 16 and a ≥15% reduction from baseline in LBPI score at any week from week 1 to week 15 to continue the study.
LBPI: Low back pain intensity; SC: Subcutaneous.
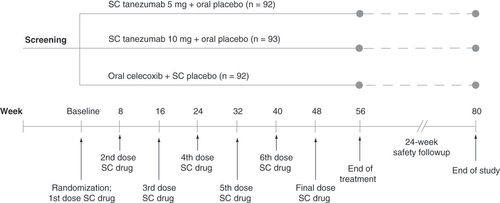
To continue past study week 16, patients were required to have a ≥15% reduction from baseline in weekly average LBPI score at any week from week 1 to 15 and a ≥30% reduction from baseline in weekly average LBPI score at week 16. Rescue medication (acetaminophen) was allowed ≤3 days/week at ≤3000 mg/day from baseline to week 16, daily at ≤3000 mg/day for weeks 16–64, and daily at ≤4000 mg/day after week 64. shows concomitant medication rules.
Table 1. Concomitant medication protocols.
Safety assessments
Safety assessments included TEAE (investigator-observed and patient-reported) reporting, laboratory testing, vital signs, 12-lead electrocardiography, orthostatic (supine/standing) blood pressure and neurological and physical examinations.
TEAEs suggestive of new or worsening peripheral neuropathy or abnormal peripheral sensation ( footnote) resulted in neurological consultation if they were reported as serious or severe, caused study withdrawal, or were ongoing at end of study. TEAEs of bradycardia, syncope, orthostatic hypotension, anhidrosis or hypohidrosis (which were part of a larger group of TEAEs that represent, but are not specific to, possible decreased sympathetic function) were referred to a cardiologist or neurologist to assess possible presence of a sympathetic autonomic neuropathy.
Patients received radiographs of the shoulders, knees and hips at screening, week 24, week 56, week 80 (or early termination), and any time the investigator identified a joint at risk for a joint safety event (eg, increased severe persistent pain in the joint). A central reader reviewed radiographs for study eligibility and identification of potential joint safety events that could warrant further evaluation or referral to an orthopedic surgeon. All potential joint safety events, including total joint replacement, were adjudicated by a blinded external adjudication committee. Events adjudicated as rapidly progressive osteoarthritis (RPOA) type 1 or 2 (defined in footnote), primary osteonecrosis, subchondral insufficiency fracture or pathologic fracture were included in a composite joint safety end point.
Efficacy assessments
At baseline and weeks 1, 2, 4, 8, 12, 16, 24, 32, 40, 48, 56 and 64, patients assessed average LBPI over the past 24 h on a scale from 0 = no pain to 10 = worst possible pain. Patients completed the 24-item Roland Morris Disability Questionnaire (RMDQ) to assess current state of function at baseline and weeks 2, 4, 8, 16, 24, 32, 40, 48, 56 and 64 (scores range from 0 = no disability to 24 = severe disability). The last pre-specified efficacy end point was week 56 (8 weeks after last SC dose).
Statistical analysis
Sample size was initially planned at approximately 390 patients. Considering study feasibility and the number of patients with 1-year exposure, minimum sample size was amended to approximately 200 (170–220) patients (though >220 was deemed acceptable from a safety perspective). Safety and efficacy analyses included all randomized patients who received ≥1 dose of SC study medication. Statistical analyses were performed using SAS v9.4 (SAS Institute Inc., NC, USA).
Frequencies of all-causality TEAEs, pre-specified TEAEs of abnormal peripheral sensation, and pre-specified TEAEs of the sympathetic nervous system were summarized through the 56-week treatment period. TEAEs were coded using Medical Dictionary for Regulatory Activities v22.0, with severity and causality assessed by investigators. Frequency of pre-specified joint safety events and the composite joint safety end point, along with 95% confidence interval (95% CI), was summarized through the full 80-week study (treatment + follow-up) period only, recognizing that joint events may have a delayed presentation.
Least squares mean (standard error) changes from baseline in LBPI and RMDQ scores were calculated from time of first assessment through week 56, using mixed analysis of covariance models with fixed effect of treatment group, baseline LBPI score as covariate (RMDQ analysis had an additional covariate of baseline RMDQ score) and study site as random effect. Missing data was handled using a multiple imputation approach dependent on the reason for missing data (Supplementary Table 1).
Results
Overall, 277 patients were randomized and received ≥1 dose of medication (). Patient demographics and other clinical characteristics were similar across groups. Most patients had predominantly nociceptive CLBP, based on Quebec Task Force and PainDetect scores ().
The diagram shows the flow of patients throughout the trial. Data are number (%) of patients.
*Two patients were screened but withdrew consent prior to randomization.
†If a patient withdrew due to efficacy reasons other than the specific criteria at week 16 (see Results section), the reason was classified as ‘insufficient clinical response’.
‡Patients completed the study if they completed the safety follow-up period, regardless of whether they completed the treatment phase.
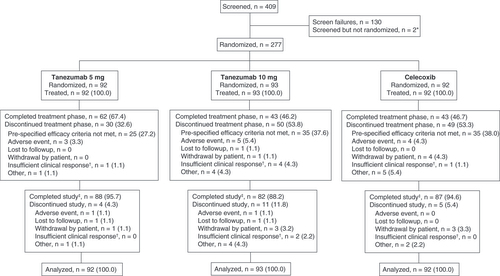
Table 2. Patient demographics and baseline characteristics.
The proportion of patients completing the 56-week treatment phase was 67.4% for tanezumab 5 mg, 46.2% for tanezumab 10 mg and 46.7% for celecoxib. The most common reason for study withdrawal during the treatment period was failure to meet protocol-specific efficacy criteria at week 16 (tanezumab 5 mg = 27.2%, tanezumab 10 mg = 37.6%, celecoxib = 38.0%). Overall, 95.7, 88.2 and 94.6% of patients completed the safety follow-up period in the tanezumab 5 mg, tanezumab 10 mg, and celecoxib groups, respectively. Patients withdrawn from treatment were placed directly into the 24-week safety follow-up period. They were considered to complete the safety period if they completed the full 24 weeks of follow-up, regardless of whether they completed the full 56-week treatment period.
TEAE rates during the 56-week treatment period were 63.0% for tanezumab 5 mg, 54.8% for tanezumab 10 mg and 67.4% for celecoxib (). Few TEAEs were serious or severe and the percentage of patients discontinuing treatment due to TEAEs was low across all groups. Nasopharyngitis, fall, arthralgia, contusion, hypoesthesia and back pain were most frequently reported in the tanezumab groups. There was no obvious difference in the frequency of any common TEAE (those occurring in ≥3% of patients in any group) for either tanezumab group compared with celecoxib. The pattern of TEAEs was generally similar over the full 80-week (treatment + follow-up) study period (Supplementary Table 2). However, more patients reported a TEAE with tanezumab 5 mg (76.1%) than with tanezumab 10 mg (67.7%) or celecoxib (72.8%) over the full study. This was due, at least partly, to a few additional reports of arthralgia (tanezumab 5 mg n = 6; tanezumab 10 mg n = 2; celecoxib n = 1) and periarthritis (painful swelling around, as opposed to within, the joint; tanezumab 5 mg n = 4; tanezumab 10 mg n = 1; celecoxib n = 0) during the follow-up period in the tanezumab 5 mg group compared with other groups. No deaths were reported.
Table 3. Summary of all-causality TEAEs through week 56 (treatment period).
The frequency of TEAEs of abnormal peripheral sensation during the 56-week treatment period was 9.8% for tanezumab 5 mg, 4.3% for tanezumab 10 mg and 4.3% for celecoxib (). The most common event across all groups was hypoesthesia. Nearly all events were mild (except 1 instance of moderate sciatica in the celecoxib group) and did not result in treatment discontinuation (except 1 instance of hypoesthesia in the celecoxib group). Compared with the treatment period, there were only a few additional TEAEs of abnormal peripheral sensation reported over the full 80-week (treatment + follow-up) study period (Supplementary Table 3). These included four patients reporting hypoesthesia (tanezumab 5 mg = 1, tanezumab 10 mg = 1, celecoxib = 2) and 1 patient reporting sciatica (celecoxib = 1).
Table 4. Summary of TEAEs of abnormal peripheral sensation through week 56 (treatment period).
The frequency of TEAEs of possible decreased sympathetic function during the 56-week treatment period was 3.3% for tanezumab 5 mg, 4.3% for tanezumab 10 mg and 7.6% for celecoxib (). No individual event was reported by >2 patients in any group except diarrhea (3 patients in the celecoxib group). Nearly all events were mild (except 1 instance of moderate diarrhea in the celecoxib group) and did not result in treatment discontinuation (except 1 instance of orthostatic hypotension in the celecoxib group). Compared with the treatment period, there were only a few additional TEAEs of possible decreased sympathetic function reported over the full 80-week study period (Supplementary Table 4). These included diarrhea, nausea and orthostatic hypotension in the tanezumab 5 mg group (1 patient each), syncope in the tanezumab 10 mg group (1 patient), and nausea, abdominal discomfort and dizziness postural in the celecoxib group (1 patient each). Five patients had a consultation related to possible decreased sympathetic function; 2 with bradycardia in the tanezumab 5 mg group, 1 with syncope in the tanezumab 10 mg group, 1 with bradycardia in the celecoxib group and 1 with orthostatic hypotension in the celecoxib group. None were deemed to have a sympathetic neuropathy.
Table 5. Summary of TEAEs of possible decreased sympathetic function through week 56 (treatment period).
During the 80-week study, three patients were included in the composite joint safety end point (). One patient in the tanezumab 5 mg group was adjudicated with RPOA type 1 of the right (onset on study day 390, KL grade 1 at screening) and left (onset on study day 408, KL grade 2 at screening) knees. One patient in the tanezumab 10 mg group was adjudicated with subchondral insufficiency fracture of the left knee (onset on study day 296; KL grade 2 at screening). One patient in the tanezumab 10 mg group was adjudicated with RPOA type 2 of the left hip (onset on study day 218; KL grade 1 at screening) that led to the only total joint replacement reported in the study.
Table 6. Summary of joint safety through week 80 (treatment + follow-up period).
There was no evidence of an effect of tanezumab on safety related to vital signs, electrocardiography measures or safety laboratory tests (data not shown).
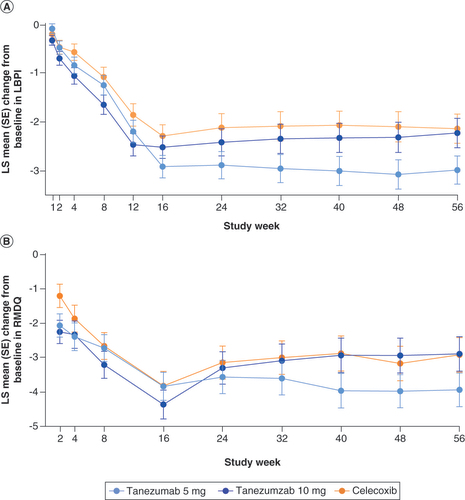
Sustained improvements in LBPI (A) and RMDQ (B) scores were evident in all treatment groups over the 56-week treatment period.
The graphs show LS mean change from baseline in (A) LBPI and (B) RMDQ scores throughout the trial. No formal statistical hypothesis testing was performed.
LBPI: Low back pain intensity; LS: Least squares; RMDQ: Roland Morris disability questionnaire; SE: Standard error.
Discussion
This study demonstrates that 56-week treatment with tanezumab is well tolerated in most patients and may suggest efficacy in Japanese adults with moderate-to-severe CLBP who experience some benefit from a stable regimen of NSAIDs but require additional pain relief.
Overall TEAE rates during the treatment period were not dose dependent in the tanezumab-treated groups (5 mg = 63.0%; 10 mg = 54.8%). Nasopharyngitis, fall, arthralgia, contusion, hypoesthesia and back pain were the most commonly reported events in the tanezumab groups, but did not occur at greater frequency than in the celecoxib group. Overall TEAE rates were slightly higher with celecoxib (67.4%) than tanezumab, though no single TEAE was markedly more common relative to other events in the celecoxib or tanezumab groups. Common TEAEs in the celecoxib group included nasopharyngitis, arthralgia, back pain, pyrexia, fall, intervertebral disc protrusion, hypoaesthesia, diarrhea, gastroenteritis, pain in extremity, hypertension and musculoskeletal pain. Some of these are expected with celecoxib (diarrhea, gastroenteritis, hypertension), while others may reflect use of comprehensive neurological (hypoaesthesia) and musculoskeletal (arthralgia, back pain, musculoskeletal pain) assessments in the study. Serious TEAEs were more frequent with tanezumab 10 mg (9.7%), than tanezumab 5 mg (4.3%) or celecoxib (2.2%), but treatment discontinuations due to TEAEs were similar across groups.
TEAEs of abnormal peripheral sensation were also not dose dependent in the tanezumab-treated groups (5 mg = 9.8%; 10 mg = 4.3%). However, all of these events were mild and none resulted in treatment discontinuation in tanezumab-treated patients. Hypoesthesia was most common across all groups. The frequency of TEAEs of abnormal peripheral sensation observed with celecoxib (4.3%) is higher than expected since these events are not commonly associated with celecoxib, and may be due to the use of comprehensive neurological examinations (more likely to detect these TEAEs) and patient/physician expectation (knowledge that these TEAEs have been reported in previous trials of tanezumab) [Citation26].
TEAEs of possible decreased sympathetic function were reported infrequently during the treatment period (5 mg = 3.3%, 10 mg = 4.3%, celecoxib = 7.6%). The increased rate observed with celecoxib was largely due to three patients with diarrhea, an event commonly associated with celecoxib; no other event occurred in ≥1 celecoxib-treated patient [Citation27]. Diarrhea and other events in the category of TEAEs of possible decreased sympathetic function, however, can have etiologies unrelated to sympathetic dysfunction and do not necessarily represent a sympathetic safety concern. Other than bradycardia (5 mg = 2.2%) and orthostatic hypotension (10 mg = 2.2%), no TEAE of possible decreased sympathetic function occurred in ≥1 tanezumab-treated patient. All events of possible decreased sympathetic function were mild and none resulted in treatment discontinuation.
Joint safety events have been observed across several trials of anti-NGF antibodies [Citation28]. Independent expert adjudication of these events revealed that they are predominantly events of RPOA [Citation29]. Tanezumab was associated with infrequent joint safety events (5 mg = 1.1%, 10 mg = 2.2%); none occurred with celecoxib. These events included RPOA type 1 (both knees; 5 mg group), RPOA type 2 (hip; 10 mg), and subchondral insufficiency fracture (knee; 10 mg) and, as in previous studies of tanezumab, occurred in some joints with only possible-to-mild (KL grade 1–2) radiographic evidence of osteoarthritis at screening. RPOA type 1 was defined as a significant loss of joint space width ≥2 mm within approximately 1 year, without gross structural failure [Citation25]. RPOA type 2 was defined as abnormal bone loss or destruction, including limited or total collapse of at least one subchondral surface, which is not normally present in conventional end-stage osteoarthritis [Citation25]. Subchondral insufficiency fracture was defined as focal bone defect, or loss of sphericity of the articular surface and/or focal radiolucency in the subchondral trabecular bone, with or without adjacent cortical defect. The mechanism underlying the RPOA, and other joint safety events, observed in trials of anti-NGF antibodies is unknown.
Despite differences in study design and patient characteristics, the safety profile of tanezumab observed in Japanese patients with CLBP was mostly consistent with that observed in a large (n = 1825), recently completed, 80-week, global study of SC tanezumab for moderate-to-severe CLBP and no new clinically relevant events were identified [Citation20]. However, increased rates of overall TEAEs and TEAEs of abnormal peripheral sensation observed with tanezumab 5 mg compared with 10 mg in the current study are not consistent with previous tanezumab studies [Citation20]. These observations are likely an anomaly of the current study rather than a true difference in Japanese versus western populations. It is possible the relatively low number of patients randomized (92–93/group) confounded assessment of TEAE rates, particularly infrequent peripheral, sympathetic and joint events. Treatment discontinuations, including discontinuation for not meeting pre-specified efficacy criteria, may also have disproportionately reduced the number of TEAEs observed among the treatment groups.
Both doses of tanezumab improved pain and function scores relative to baseline through week 56, suggesting a long-term efficacy benefit. Celecoxib also improved pain and function throughout the study. Though differences in the extent of improvement were often observed between tanezumab and celecoxib groups, such differences were small at some time points and absent at others. However, since the study was primarily designed to assess long-term tanezumab safety, interpretation of these findings is limited and no prespecified efficacy hypotheses were tested. The study was not powered to detect differences in efficacy between groups. Additionally, a large proportion of patients (34.3%) were discontinued at week 16 due to inadequate response. Use of adjunctive analgesics and rescue medication, particularly after week 16, may also confound evaluation of study treatment effects.
As in the large, global CLBP study, both 5 and 10 mg tanezumab provided sustained improvements in pain and function in the current study [Citation20]. In contrast to the previous study, tanezumab 5 mg provided numerically greater improvements in pain and function after week 16 and had fewer patients being discontinued at week 16 for not meeting protocol-specific efficacy criteria (5 mg = 27.2%; 10 mg = 37.6%) than tanezumab 10 mg in the current study. The limitations described above, in addition to higher incidences of serious TEAEs and treatment discontinuations in the tanezumab 10 mg group, may have contributed to this outcome and it is likely not the result of a differential response to tanezumab in Japanese patients relative to other populations.
Since the primary objective of the study was to assess safety, elements of trial design make it difficult to compare the efficacy of tanezumab observed in this study to the efficacy of other agents for CLBP. These elements include a lack of placebo-comparator, discontinuation of inadequate responders at week 16, and a very specific patient population (Japanese adults experiencing some analgesic benefit from a stable regimen of NSAIDs). Despite these limitations, some general comparisons can be made. First, long-term improvements (up to 56 weeks) in pain and function were observed. This is notable since many treatments for CLBP are associated with short-term (if any) effects on pain [Citation7,Citation9]. In addition, since there are few studies of or near the 56-week treatment duration in this study, little is known about the maintenance of effect of other CLBP treatments [Citation7,Citation9]. Sustained improvement in function (as assessed via the RMDQ) observed in this study are particularly notable as this is not typically observed in trials of other commonly used agents to treat CLBP such NSAIDs and, in particular, of opioids [Citation9,Citation30].
Conclusion
The overall safety profile of SC tanezumab observed in this 80-week (56-week treatment; 24-week follow-up) study of Japanese patients with moderate-to-severe CLBP was mostly consistent with previous studies and confirms the safety and efficacy profile of SC tanezumab in patients with CLBP. However, overall TEAEs and TEAEs of abnormal peripheral sensation occurred more frequently in the tanezumab 5 mg treatment arm than in the 10 mg arm. The number of patients experiencing a pre-specified joint safety event was 1 in the tanezumab 5 mg group, 2 in the tanezumab 10 mg group and 0 in the celecoxib group; all with possible-to-mild radiographic evidence of osteoarthritis in the affected joint at screening. The study was not designed or powered to assess efficacy; however, both tanezumab and celecoxib demonstrated long-term improvements in pain and function relative to baseline. These findings suggest that tanezumab may warrant further investigation for CLBP.
Low back pain is a major contributor to disability in Japan and a need remains for safe and effective pharmacological agents that improve patient quality of life.
Growing evidence supports a role for NGF in chronic low back pain.
An 80-week (56-week treatment; 24-week follow-up), randomized, double-blind, celecoxib-controlled, Phase III study was conducted to evaluate the long-term safety and efficacy of subcutaneous tanezumab (a monoclonal antibody against nerve growth factor) in Japanese patients with moderate-to-severe chronic low back pain.
The safety profile of tanezumab in Japanese patients was mostly consistent with previous studies of subcutaneous tanezumab for chronic low back pain, though overall treatment-emergent adverse events (tanezumab 5 mg = 63.0%, tanezumab 10 mg = 54.8%, celecoxib = 67.4%) and treatment-emergent adverse events of abnormal peripheral sensation (tanezumab 5 mg = 9.8%, tanezumab 10 mg = 4.3%, celecoxib = 4.3%) were observed more frequently with 5 mg than 10 mg tanezumab during the treatment period.
The number of patients experiencing a pre-specified joint safety event during the 80-week study was 1 in the tanezumab 5 mg group, 2 in the tanezumab 10 mg group, and 0 in the celecoxib group; all with possible-to-mild radiographic evidence of osteoarthritis in the affected joint at screening.
Although the study was not designed or powered to assess efficacy, both tanezumab and celecoxib demonstrated long-term improvements in pain and function relative to baseline.
These findings suggest that tanezumab may warrant further investigation for chronic low back pain.
Author contributions
S Konno, T Nikaido, JD Markman, M Ohta, T Machida, N Isogawa, H Yoshimatsu, L Viktrup, MT Brown, CR West and KM Verburg were involved in study conception or design and acquisition or analysis of data. All authors were involved in interpretation of data, revising the paper for critically important content, provided final approval for submission and agree to be accountable for all aspects of the work.
Ethical conduct of research
The study was conducted (in compliance with ethical principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines) from May 2016 to June 2019 at 58 sites in Japan. The protocol was approved by Institutional Review Boards or Independent Ethics Committees for each site and all patients provided informed consent.
Supplemental Document 1
Download MS Word (21.8 KB)Supplemental Document 2
Download MS Word (34.4 KB)Supplemental Document 3
Download MS Word (25.8 KB)Supplemental Document 4
Download MS Word (27 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pmt-2021-0040
Financial & competing interests disclosure
The study was sponsored by Pfizer and Eli Lilly and Company. S Konno has received grants or personal fees from Medtronic Japan Company Ltd, Hoken Kagaku Inc, Asahi KASEI Pharma Corporation, Mochida Pharmaceutical Company Ltd, Daiichi Sankyo Company Ltd, Shionogi & Company Ltd, Hisamitsu Pharmaceutical Company Inc, Taisho Pharmaceutical Company Ltd, Takeda Pharmaceutical Company Ltd, Nippon Shinyaku Company Ltd, Eisai Company Ltd, Chugai Pharmaceutical Company Ltd, Taiho Pharmaceutical Company Ltd, Nippon Zoki Pharmaceutical Company Ltd, Astellas Amgen BioPharma K.K., Ono Pharmaceutical company Ltd, Eli Lilly Japan K.K., Pfizer, Taisho Pharma Company Ltd, Mochida Pharmaceutical Company Ltd, Astellas Pharma Inc, Janssen Pharmaceutical K.K., Johnson & Johnson; and served as Coordinating Investigator for conduct of the reported study. T Nikaido has served as a lecturer for Pfizer Japan Inc, Daiichi Sankyo Company, Eisai Company and Shionogi Company; and served as Principle Investigator for conduct of the reported study at the Fukushima Medical University. JD Markman has served on an advisory board for Clexio Biosciences, Esteve Pharmaceuticals, Flexion Therapeutics, Quark Pharmaceuticals, Quartet Medicine, Collegium Pharmaceutical, Purdue Pharma, Biogen, Novartis, Aptinyx, Nektar, Allergan, Grünenthal, Eli Lilly and Company, Depomed, Janssen Pharmaceuticals, Teva Pharmaceutical Industries, KemPharm, Abbott Laboratories, Plasma Surgical, Chromocell, Convergence Pharmaceuticals, Inspirion, Pfizer, Sanofi, Daiichi Sankyo, SK lifesciences, and Trevena; has served as a consultant to Trigemina, Editas Medicine, and Plasma Surgical; has served on data safety monitoring boards for Novartis, Allergan, and Regenacy; and has served on the board of Flowonix Medical. M Ohta, T Machida, N Isogawa and H Yoshimatsu are employees of Pfizer R&D Japan. L Viktrup is an employee of Eli Lilly & Company. MT Brown, CR West and KM Verburg are employees of, and own stock and/or options in, Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by M Soulsby of Engage Scientific Solutions and was funded by Pfizer and Eli Lilly and Company.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data, NCT02725411. Upon request, and patient to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer and Lilly will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Additional information
Funding
References
- Institutes for Health Metrics and Evaluation . Global burden of disease (GBD) compare. (2019). https://vizhub.healthdata.org/gbd-compare/
- Iizuka Y , IizukaH , MiedaTet al. Prevalence of chronic nonspecific low back pain and its associated factors among middle-aged and elderly people: an analysis based on data from a musculoskeletal examination in Japan. Asian Spine J11(6), 989–997 (2017).
- Fujii T , MatsudairaK. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur. Spine J.22(2), 432–438 (2013).
- Kamada M , KitayuguchiJ , LeeIMet al. Relationship between physical activity and chronic musculoskeletal pain among community-dwelling Japanese adults. J. Epidemiol.24(6), 474–483 (2014).
- Takahashi A , KitamuraK , WatanabeYet al. Epidemiological profiles of chronic low back and knee pain in middle-aged and elderly Japanese from the Murakami cohort. J. Pain Res.11, 3161–3169 (2018).
- Montgomery W , SatoM , NagasakaY , VietriJ. The economic and humanistic costs of chronic lower back pain in Japan. Clinicoecon Outcomes Res9, 361–371 (2017).
- Qaseem A , WiltTJ , McleanRM , ForcieaMA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline From the American College of Physicians. Ann. Intern. Med.166(7), 514–530 (2017).
- Deyo RA , DworkinSF , AmtmannDet al. Report of the NIH Task Force on research standards for chronic low back pain. J. Pain15(6), 569–585 (2014).
- Chou R , DeyoR , FriedlyJet al. Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med.166(7), 480–492 (2017).
- The Committee for Clinical Practice Guidelines for Chronic Pain . Clinical practice guidelines for chronic pain. (2019). http://plaza.umin.ac.jp/~jaspain/pdf/consortium_20180913en.pdf
- Ng SC , ChanFK. NSAID-induced gastrointestinal and cardiovascular injury. Curr. Opin. Gastroenterol.26(6), 611–617 (2010).
- Enthoven WT , RoelofsPD , DeyoRA , Van TulderMW , KoesBW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst. Rev.2(2), Cd012087 (2016).
- Barker PA , MantyhP , Arendt-NielsenL , ViktrupL , TiveL. Nerve growth factor signaling and its contribution to pain. J. Pain Res.13, 1223–1241 (2020).
- Schmelz M , MantyhP , MalfaitAMet al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain160(10), 2210–2220 (2019).
- Markman JD , Czerniecka-FoxxK , KhalsaPSet al. AAPT diagnostic criteria for chronic low back pain. J. Pain21(11–12), 1138–1148 (2020).
- Sugiura A , OhtoriS , YamashitaMet al. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976)33(19), 2047–2051 (2008).
- Taguchi T , HoheiselU , MenseS. Dorsal horn neurons having input from low back structures in rats. Pain138(1), 119–129 (2008).
- Freemont AJ , PeacockTE , GoupilleP , HoylandJA , O’brienJ , JaysonMI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet350(9072), 178–181 (1997).
- Freemont AJ , WatkinsA , LeMaitre Cet al. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol.197(3), 286–292 (2002).
- Markman JD , BolashRB , McalindonTEet al. Tanezumab for chronic low back pain: a randomized, double-blind, placebo- and active-controlled, Phase III study of efficacy and safety. Pain161(9), 2068–2078 (2020).
- Katz N , BorensteinDG , BirbaraCet al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain152(10), 2248–2258 (2011).
- Kivitz AJ , GimbelJS , BramsonCet al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain154(7), 1009–1021 (2013).
- Schnitzer TJ , EastonR , PangSet al. Effect of Tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA322(1), 37–48 (2019).
- Berenbaum F , BlancoFJ , GuermaziAet al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis.79(6), 800–810 (2020).
- Miller CG , GuermaziA , RoemerF. The current status of imaging in anti-NGF clinical trials. Osteoarthritis Cartilage23(Suppl. 1), S3–S7 (2015).
- Tive L , BelloAE , RadinDet al. Pooled analysis of tanezumab efficacy and safety with subgroup analyses of phase III clinical trials in patients with osteoarthritis pain of the knee or hip. J. Pain Res.12, 975–995 (2019).
- Celecoxib USPI (February 10). http://labeling.pfizer.com/showlabeling.aspx?id=793
- Hochberg MC . Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage23(Suppl. 1), S18–S21 (2015).
- Hochberg MC , TiveLA , AbramsonSBet al. When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the Tanezumab Clinical Development Program. Arthritis Rheumatol68(2), 382–391 (2016).
- Ashworth J , GreenDJ , DunnKM , JordanKP. Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6-month follow-up?Pain154(7), 1038–1044 (2013).
