Abstract
Tolperisone is a nonopioid, centrally acting muscle relaxant in clinical development in the USA for the treatment of symptoms associated with acute, painful muscles spasms of the back. CLN-301, RESUME-1, is a 14-day double-blind, randomized, placebo-controlled, parallel-group Phase III study of the efficacy and safety of tolperisone administered orally three-times daily in 1000 male and female subjects at approximately 70 clinical sites in the USA experiencing back pain due to or associated with muscle spasm of acute onset. Tolperisone is a promising therapeutic for managing acute, painful muscle spasms of the back as it appears to lack the off-target CNS effects often seen with conventional skeletal muscle relaxants.
Clinical Trials registration number: NCT04671082
Tolperisone is a nonopioid, centrally acting muscle relaxant in clinical development in the USA for the treatment of symptoms associated with acute and painful muscles spasms of the back. Tolperisone lacks off-target CNS effects and is not associated with somnolence and cognitive function impairment [Citation1–10]. In contrast, use of other centrally acting skeletal muscle relaxants (SMRs) is confounded by their off-target CNS activity, which is associated with somnolence [Citation9,Citation10]. Additionally, a novel treatment for symptoms associated with acute muscle spasms has not been developed in nearly two decades, the most recent being an extended-release form of cyclobenzaprine. Tolperisone is a promising therapeutic for treating pain due to acute muscle spasms of the back as tolperisone is a nonopioid centrally acting muscle relaxant that is not associated with somnolence and cognitive impairment, has demonstrated clinical efficacy and has no CNS off-target binding.
Background & rationale
Acute muscle spasm
Acute muscle spasms of the back, defined as sustained involuntary contractions [Citation1,Citation11] of a muscle or muscle group that cannot relax [Citation2,Citation12,Citation13] and have been present for 6 weeks or less [Citation14], are commonly associated with lower back pain [Citation3]. During a lifetime, approximately 84% of US adults (175 million) experience muscle spasms [Citation15,Citation16] and approximately 90% of US adults (188 million) experience back pain [Citation17,Citation18]. Back pain has a global lifetime prevalence of approximately 38.9% [Citation14,Citation19] and is the fifth most frequent reason for seeking medical care in the USA [Citation14,Citation18,Citation20–22]. As back pain associated with muscle spasms self-limits from 2 to 4 weeks [Citation16,Citation23,Citation24], up to 90% of patients recover to full activity within 1 month [Citation25–27] and return to work within 3 months [Citation15,Citation18,Citation28]. Despite this, within 1 year approximately 73% of patients experience both symptom recurrence and limited functionality [Citation17,Citation18,Citation29]. Each year, approximately 50% of working adults experience back pain [Citation11,Citation27,Citation30,Citation31]; in adults aged <45 years, back pain is the most common cause of work-related disability [Citation17,Citation24,Citation32–34].
In most cases, the specific cause of the involuntary contractions is unknown [Citation2,Citation13] In the small proportion of cases with a known cause, acute muscle spasms commonly result from an acute partial muscle tear (i.e., strain) or partial or complete ligament rupture (i.e., sprain) [Citation2,Citation13]. Acute muscle spasms may vary in severity, and spasm episodes can last from seconds to several minutes [Citation2]. Typically, episodes of acute muscle spasm are self-limiting and last from 2 to 4 weeks [Citation3,Citation16,Citation35]; however, persistence of low-grade symptoms [Citation35] and symptom recurrence is common [Citation18,Citation29]. The prevalence of acute muscle spasms increases with age [Citation14,Citation31,Citation36–38]. Despite affecting a large proportion of US adults [Citation15,Citation16], there has been little research on the topic within the last few decades [Citation39].
Tolperisone
Tolperisone is a nonopioid, centrally acting muscle relaxant in clinical development in the USA for the treatment of symptoms associated with acute and painful muscles spasms of the back. Tolperisone has been approved outside of the US since 1959 [Citation40,Citation41] and is widely used in parts of Europe, Asia, South America and Africa for the therapeutic treatment of poststroke spasticity and in some countries, acute and painful muscle spasms. Tolperisone is an oral formulation presented as film-coated tablets, and is being developed by Neurana Pharmaceuticals, Inc. for the treatment of symptoms associated with acute and painful back muscle spasms. There are two prominent effects of tolperisone’s mechanism of action. Tolperisone inhibits both mono- and polysynaptic spinal reflex transmission by both presynaptic and postsynaptic mechanisms via relatively selective reversible blockade at voltage-gated sodium channels and voltage-gated calcium channels [Citation40,Citation42–53]. Unlike other centrally acting SMRs, tolperisone does not appear to be associated with somnolence and cognitive function impairment [Citation1–10]. In the USA, a new drug substance manufacturing process has developed an ultrapure formulation of tolperisone that has reduced the impurity and degradant levels below International Council for Harmonization guidelines.
Tolperisone Phase I driving studies
Study CLN-115 (ClinicalTrials.gov, NCT03353922) was a randomized, placebo-controlled, multiple-dose, three-period crossover study of safety and cognitive effects of tolperisone [Citation10]. A total of 35 healthy male and female subjects received 3 days of each treatment: 150 mg three-times daily (TID) tolperisone, 10 mg TID cyclobenzaprine and placebo. Subjects completed the Cognitive Research Corporation’s Driving Simulator (CRCDS Mini-Sim), a validated driving test simulator of a monotonous 100-km highway route. Subjects were instructed to maintain lane position and speed, on day 1 at time to maximum plasma concentration (Tmax), on day 2 prior to morning dose and on day 3 at steady state after morning dose. A computer-administered digit-symbol substitution test (CogScreen symbol digit coding test) evaluated subjects on several driving parameters. The primary end point was Standard Deviation of Lateral Position (SDLP), a measure of weaving.
This study demonstrated that tolperisone (150 mg TID) did not impair driving performance which was based on three criteria: driving performance was not statistically different from placebo following dosing on day 1, day 2 or on day 3; under all three tolperisone dosing conditions, SDLP did not exceed the upper limit of the 95% CI consistent with the modeled effect of alcohol at 0.05% BAC and symmetry analysis showed that the distribution of the paired differences between placebo and tolperisone was not asymmetrical around zero. Results for secondary driving end points, measures of cognitive functioning and self-report measures also demonstrated no significant effects of tolperisone compared with placebo for all three tolperisone dosing conditions. Adverse events (AEs) with tolperisone were mild and were not related to driving outcomes. In contrast, patients who received cyclobenzaprine (10 mg TID) demonstrated significantly impaired driving performance (p < 0.01) on most end points compared with placebo. In this study, driving performance with tolperisone was similar to that observed with placebo at the time of maximum concentration, showed no impact on driving performance, no drowsiness effects following multiple doses, and no next day residual effects on driving performance.
Study CLN-116 (ClinicalTrials.gov, NCT04407377) was a randomized, placebo-controlled, multiple-dose, four-way cross-over study of safety, drowsiness and cognitive function in 39 male and female healthy volunteers. Treatment included tolperisone 200 mg TID (therapeutic), tolperisone 400 mg (supratherapeutic dose) TID, cyclobenzaprine 10 mg TID, and placebo; subjects received 3 days of each treatment. Each medication was given TID on days 1 and 2, and in the morning only on day 3 for a total of 7 doses (14 tablets). Approximately 3.5 h after the AM dose, cognitive function was assessed by Reaction Time and Rapid Visual Information Processing. Approximately 1 h after the second dose (PM) on day 1, subjects were administered the symbol digit coding (SDC) test, a cognitive performance test, and the Karolinska sleepiness scale (KSS), a sleepiness assessment, they were asked the driving readiness question, followed by the driving simulator examination. Right after driving, the visual analog scales were performed. This study was designed to test noninferiority of tolperisone doses relative to placebo, with a cyclobenzaprine test versus placebo to confirm the sensitivity of the simulator to detect treatment effects. The primary end point assessment was SDLP.
This study demonstrated that tolperisone was not associated with driving impairment, sleepiness or cognitive impairment based on the following: SDLP and all other driving end points (including frequency, severity and duration of lane exceedances) did not significantly differ in subjects receiving tolperisone 200 and 400 mg TID versus placebo; SDLP was significantly worse for cyclobenzaprine versus placebo on days 1 (p = 0.0002), 2 (p = 0.0015) and 3 (p = 0.0023); frequency of lane exceedances was significantly worse for cyclobenzaprine versus placebo on days 1 (p = 0.0005), 2 (p = 0.0233) and 3 (p = 0.0383); there were no significant differences in self-reported sleepiness as per KSS on days 1–3 or ESS on day 3 in subjects receiving tolperisone 200 and 400 mg TID versus placebo; subjects who received cyclobenzaprine reported sleepiness that was significantly increased versus placebo (KSS, p = 0.0057 on day 1; ESS, p = 0.0306 on day 3). Majority of TEAEs were mild to moderate in severity. Somnolence was reported by 0 and 2 (5.3%) of subjects in the tolperisone 200 and 400 mg TID groups, respectively, and this was comparable with that reported by the one placebo subject (2.8%) and lower than the nine subjects in the cyclobenzaprine group (25.0%). Overall, therapeutic (200 mg) and supratherapeutic (400 mg) doses of tolperisone appeared to be well-tolerated and were not associated with driving impairment, sleepiness or cognitive impairment.
Tolperisone Phase II dosing study
CLN-201, STAR study (ClinicalTrials.gov, NCT03802565) was a double-blind, randomized, placebo-controlled, parallel-group, dose-ranging Phase II study of the safety and efficacy of the ultrapure formulation of tolperisone in subjects experiencing back pain due to or associated with muscle spasm in the back [Citation54]. Treatment with tolperisone at doses of 50, 100, 150 and 200 mg TID taken orally for 14 days for relief of pain due to acute back muscle spasm () was found to be efficacious at three of the four dose groups with the largest separation seen at the 200 mg TID group versus placebo. Most subjects in both the tolperisone and placebo groups completed the study. Among the tolperisone dose groups, only four subjects (1.2%) discontinued due to AEs, with no subjects discontinuing due to lack of efficacy.
ePRO: Electronic patient reported outcomes; TID: Three times a day.
Image provided by and redrawn from Neurana Pharmaceuticals, Inc.
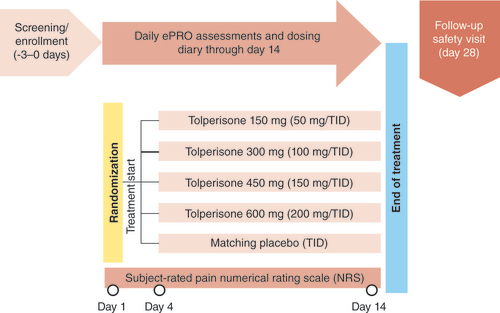
Tolperisone was well tolerated with AEs reported in 18.1% of subjects in tolperisone groups compared with 14.1% of subjects in the placebo group. Somnolence was reported by 0, 3 (3.4%), 0, and 1 (1.2%) subject(s) in 50, 100, 150 and 200 mg TID tolperisone dose groups, respectively, versus 2 (2.6%) subjects in the placebo group. All somnolence events were mild or moderate and none led to subject discontinuation. TEAEs were reported in 45 (13.4%) and 5 (6.4%) subjects in tolperisone and placebo groups, respectively, with headache for 21 subjects (6.2%) and diarrhea for 7 subjects (2.1%). The occurrence of TEAEs that resulted in discontinuation related to study drug was comparable between the total tolperisone group with 5 subjects (1.5%) and the placebo group with 1 subject (1.3%). There were no significant differences between any of the tolperisone dose groups and placebo in subject-reported sleepiness as measured in the clinic on day 4. There were no serious AEs or deaths reported during the study. Linear test of trend on the least-squares mean difference (treatment-placebo) for the mean change from baseline in day 14 numeric rating scale (NRS) score of pain ‘right now’ (the primary efficacy end point) approached statistical significance (p = 0.0539). The greatest numerical difference and significance were observed for tolperisone 200 mg TID (p = 0.0040) in an analysis of pairwise estimates (treatment-placebo). Several secondary end points trended toward significance for the tolperisone 200 mg group versus placebo.
The STAR study was designed to select a tolperisone dose for the RESUME-1 study, and the greatest effect in terms of relief of pain associated with acute muscle spasm was observed at the highest dose evaluated of 200 mg TID. The primary efficacy end point (i.e., subject-rated pain ‘right now’ on day 14) was significantly lower (p = 0.0040) at the tolperisone 200 mg TID dose versus placebo, and the numerically lowest LSM pain scores were achieved with the highest tolperisone dose. The selected tolperisone doses for the CLN-301, RESUME-1 study are 50, 100 and 200 mg TID.
Tolperisone Phase III, RESUME-1 study
CLN-301, RESUME-1 (ClinicalTrials.gov, NCT04671082) study, is a double-blind, randomized, placebo-controlled, multicenter Phase III trial, evaluates the efficacy and safety of tolperisone administered in subjects with pain due to acute back muscle spasms. The study is sponsored by Neurana Pharmaceuticals, Inc. (CA, USA).
Design
Study design
RESUME-1 is a 14-day double-blind, randomized, placebo-controlled, parallel-group Phase III study of the efficacy and safety of tolperisone or placebo administered as multiple doses TID in approximately 1000 male and female subjects at approximately 70 clinical sites in the USA experiencing back pain due to or associated with muscle spasm of acute onset. The tolperisone group will receive 50, 100 or 200 mg administered TID for 14 days, with a safety check-in telephone call at 21 days (7 days post last dose) as follow-up (). Subjects randomized to the placebo group will receive matching placebo tablets TID also for 14 days with a safety check-in telephone call at day 21 (7 days post last dose) as follow-up. Subjects will be randomized in a 1:1:1:1 ratio. Subject participation will be up to 4 weeks.
Image provided by and redrawn from Neurana Pharmaceuticals, Inc.
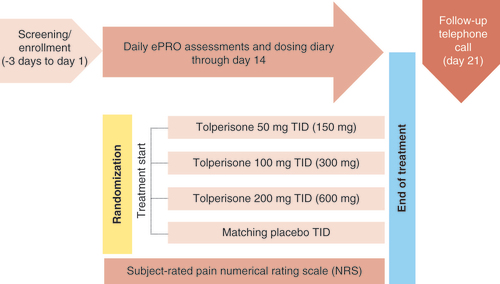
Subjects will be screened for eligibility for participation in the study at the screening visit after reviewing and signing the informed consent form. Subjects meeting all inclusion/exclusion criteria will then be randomized into the study to begin dosing (day 1). Subjects are instructed to take study drug TID (at 6–8 am, 12–2 pm and 6–8 pm) through day 14 and complete their dosing/rescue medication diary and daily electronic patient-reported outcomes assessments on the smartphone. Subjects will return to the clinic to complete procedures on day 4 (±1 day; visit 2) and day 14 (±1 day, end of treatment [EOT] visit; visit 3). Subjects must also allow a safety follow-up call on day 21 (±1 day, follow-up safety 7 days post last dose, day 21 telephone call). For select consenting subjects, an additional pharmacokinetic blood sample will be collected on day 4 (visit 2).
Primary & secondary objectives
The primary objective of the RESUME-1 study is to assess the efficacy and safety of 50, 100 and 200 mg TID tolperisone for relief of pain due to acute back muscle spasms. Key secondary objectives include assessing the tolerability of tolperisone in subjects with pain due to acute back muscle spasm, determining the onset of action of tolperisone in the treatment of pain due to acute back muscle spasm, and determining the need for rescue medication when treated with tolperisone for pain due to acute back muscle spasm.
Key eligibility criteria
Eligible patients must meet the following key inclusion criteria: ambulatory male or female, 18–64 years of age, inclusive, for the entire duration of the study (from screening through day 21); current acute back pain due to acute muscle spasm starting within 7 days prior to study entry (day 1) and at least 8 weeks following resolution of the last episode of acute back pain; minimum pain score required on the subject ‘right now’ rating of pain intensity NRS of 0–10 points at baseline; willingness to discontinue all previous or ongoing treatment of pain or muscle spasm on study entry at day 1 through the EOT including medication, acupuncture, chiropractic adjustment, massage, transcutaneous electrical nerve stimulation (TENS) or physiotherapy; and pain must be localized from the neck (C-3 or lower) to the inferior gluteal folds and spasm assessed during the screening physical examination.
Patients are excluded if they meet any of the following key exclusion criteria: presence of acute or chronic back pain for the previous 8 days or longer, where back pain is present on more days than not; presence of neurogenic pain in the back, neck, upper or lower extremities, including pain from (or suspected from) nerve root compression or injury (radicular pain or ‘pinched nerve’) or neuropathic pain with evidence of these types of exclusionary pain including radiation of pain that radiates beyond the back, chronic pain and pain associated with abnormal sensation or loss of sensation in the back or extremities; presence of pain anywhere other than the target back pain that is bothersome, interferes with activity, or for which pain relief is taken; history of any neck, back or pelvic surgery; history within the previous 3 years of: spinal fracture or spinal infection, inflammatory arthritis, degenerative spine disease, or any other back or spine condition that may reasonably contribute to current back pain; subjects who are currently taking medications that are moderate to potent inhibitors of cytochrome P450 (CYP) isozymes CYP2D6 and are unable or unwilling to stop taking the medication for the duration of the clinical study, which are likely to cause drug interactions with tolperisone HCl (e.g., medications such as paroxetine and fluvoxamine); and subjects who are unable or unwilling to stop using any medication or dietary supplement to promote sleep, including over-the-counter sleep medications, during their participation in the study.
Planned sample size & study period
RESUME-1 is a multicenter study that will be conducted at approximately 70 clinical sites in the USA. Approximately 1000 male and female subjects aged 18 to 64 years, inclusive, with pain due to acute muscle spasm of the back at the time of informed consent will be randomized into the study. Study drug treatment will be administered for 14 days. The total duration of study participation will be up to 24 days, including screening and follow-up.
Study procedure
Subjects will be screened for eligibility for participation in the study at the screening visit after reviewing and signing the informed consent form. Subjects meeting all inclusion/exclusion criteria will then be randomized into the study to begin dosing (day 1). It is preferred to complete all screening and baseline assessments on the same day the subject presents at the study site to minimize drop-out between screening and randomization. However, to accommodate real-world logistical issues and time constraints of either the subject or study site, the site has the flexibility to allow completion of screening assessments within 3 days (-3 days) prior to day 1 baseline assessments, randomization, deployment of smartphone and first dose (in that order).
After completing all screening and baseline assessments by/on day 1, subjects will receive all study drug and rescue medication (acetaminophen 500 mg) and will take their study drug (first dose) while at the study site and be observed recording the dose on a smartphone application. Depending on the time of their clinic visit, subjects should be instructed to continue their TID dosing at home with either the midday dose (12–2 pm) or the evening dose (6–8 pm). Subjects will be instructed to continue taking study drug TID (at 6–8 am, 12–2 pm and 6–8 pm) through day 14 and complete their dosing/rescue medication diary and daily electronic patient-reported outcomes assessments on the smartphone.
Subjects will return to the clinic to complete the procedures listed in the Schedule of Procedures (Section 30) on day 4 (±1 day; visit 2) and day 14 (±1 day, EOT visit; visit 3). Subjects must also allow a safety follow-up call on day 21 (±1 day, follow-up safety 7 days post last dose, day 21 telephone call). For all subjects, safety laboratory assessments will be performed at screening/baseline (visit 1, -3 days to day 1) and day 14 (visit 3). For select consenting subjects, an additional pharmacokinetic blood sample will be collected on day 4 (visit 2).
Primary end point & secondary end points
The primary end point of the RESUME-1 study is subject-rated pain ‘right now’ due to acute back spasm using a NRS (0–10 scale, from no pain to worst pain imaginable) on day 14.
The key secondary end point is subject-rated pain ‘right now’ due to acute back spasm using an NRS on day 4. Other secondary end points include subject-rated average pain ‘last hour’ due to acute back spasm using an average daily (AM/PM) NRS over days 1–4, subject-rated average pain ‘last hour’ due to acute back spasm using an average daily (AM/PM) NRS over days 1–7, and time to relief of pain due to acute back spasm, defined as first occurrence of a subject-rated NRS equal to 2 or lower from subject-rated average pain ‘last hour’ assessed on days 1–14.
Efficacy analysis
Subject-rated pain ‘right now’ due to acute back spasm will be assessed during clinical visits on day 1 (baseline), day 4 and day 14 using an NRS. The primary efficacy end point is change from baseline in pain right now on day 14. It will be analyzed using a mixed model with repeated measures. The estimated treatment effects and the treatment difference will be presented. The two-sided 95% CI of the treatment difference will also be presented.
The key secondary end point is change from baseline in subject-rated pain ‘right now’ due to acute back spasm using an NRS on day 4. This end point will be analyzed using same methods as the primary end point.
Safety analysis
Safety measures will be summarized using descriptive statistics and listed for each subject. All AEs will be coded from the verbatim text to the lower-level term and mapped to preferred term and primary system organ class using the latest version of the Medical Dictionary for Regulatory Activities. TEAEs, defined as AEs occurring after the initiation of treatment, will be summarized by body system, preferred term and treatment. Summaries of TEAE by intensity and relationship to study drug will also be produced. Serious AEs and AEs that lead to study discontinuation, if any, will be tabulated. All AEs, including nontreatment-emergent AEs, will be presented in subject listings.
To evaluate the treatment effects on drowsiness, the percentage of subjects reporting any of the following will be summarized: somnolence, excessive daytime sleepiness, drowsy on awakening, daytime sleepiness, less alert on arising and groggy on awakening.
Statistical analyses
Assuming approximately 6% of subjects will dropout prior to day 14, a total sample size of 1000 subjects (250 per treatment arm) will need to be randomized. Three populations are prespecified. The Safety Population will include all subjects who received at least one dose of study drug. All safety assessments will be based on this Safety Population (according to treatment they actually received). The intent-to-treat (ITT) population will include all randomized subjects who received at least one dose of study drug. The primary efficacy analyses will be based on this ITT population (according to planned treatment). The per-protocol population will include all ITT subjects without major protocol deviations. The criteria of major protocol deviations will be documented prior to database lock.
Conclusion
Tolperisone is a nonopioid, centrally acting muscle relaxant in clinical development in the USA for the treatment of symptoms associated with acute and painful muscles spasms of the back. In contrast to other centrally acting SMRs, tolperisone lacks off-target CNS effects and is not associated with somnolence and cognitive function impairment [Citation2,Citation5,Citation7,Citation47–53]. This is promising as centrally acting SMRs use is complicated by their off-target CNS activity, which is associated with somnolence [Citation52,Citation53]. Additionally, a novel treatment for acute muscle spasms has not been developed in nearly two decades, the most recent being an extended-release form of cyclobenzaprine. A muscle relaxant such as tolperisone that is a nonopioid, not associated with somnolence and cognitive impairment, with demonstrated clinical efficacy, and no CNS off-target binding and effects are promising as a therapeutic for managing painful, acute muscle spasms of the back. The maximum proposed clinical dose is 200 mg TID for 14 days. Following RESUME-1, the upcoming Phase III study, CLN-302 (RESUME-2) will utilize the results of RESUME-1 to determine dosing and serve as a confirmatory trial to RESUME-1.
Infographic
Download PDF (1.3 MB)Supplementary data
An infographic accompanies this paper and is included at the end of the references section in the PDF version. To view or download this infographic in your browser please click here: www.tandfonline.com/doi/suppl/10.2217/pmt-2021-0041
Financial & competing interests disclosure
SA Vaughan, K Torres and R Kaye are employees of Neurana Pharmaceuticals, which provided financial support for this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Pratzel HG , AlkenRG , RammS. Efficacy and tolerance of repeated oral doses of tolperisone hydrochloride in the treatment of painful reflex muscle spasm: results of a prospective placebo-controlled double-blind trial. Pain67(2–3), 417–425 (1996).
- Frydrych V , OderdaG. Skeletal muscle relaxants drug class review 12:20.04 centrally acting skeletal muscle relaxants. University Utah College Pharm.12(20), 1–40 (2016).
- Rao R , PanghateA , ChandanwaleAet al. Clinical comparative study: efficacy and tolerability of tolperisone and thiocolchicoside in acute low back pain and spinal muscle spasticity. Asian Spine J.6(2), 115–122 (2012).
- Dulin J , KovacsL , RammSet al. Evaluation of sedative effects of single and repeated doses of 50 mg and 150 mg tolperisone hydrochloride. Results of a prospective, randomized, double-blind, placebo-controlled trial. Pharmacopsychiatry31, 137–142 (1998).
- Stamenova P , KoytchevR , KuhnKet al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of tolperisone in spasticity following cerebral stroke. Eur. J. Neurol.12(6), 453–461 (2005).
- Prabhoo R , KenyS , PrabhooT , SinghA , RanaR. A Phase IV observational multi-centre open-label study on efficacy and safety of tolperisone 150 mg in patients with painful muscle spasm associated with degenerative or inflammatory diseases of the musculoskeletal system. J. Assoc. Physicians India59, 33–37 (2011).
- European Medicines Agency . Assessment report for tolperisone-containing medicinal products. European Medicines Agency (2013).
- Agarwal S , PatelT , ShahN , PatelBM. Corrigendum to “comparative study of therapeutic response to baclofen vs tolperisone in spasticity” [Biomed. Pharmacother. 87 (2017) 628–635]. Biomed. Pharmacother.92, 1140 (2017).
- Fudin J , RaoufM. A review of skeletal muscle relaxants for pain management. Pract. Pain Manag.16(5), 1–15 (2016).
- Caron J , KayeR , WesselT , HalsethA , KayG. An assessment of the centrally acting muscle relaxant tolperisone on driving ability and cognitive effects compared to placebo and cyclobenzaprine. J. Clin. Pharm. Ther.45, 774–782 (2020).
- Fischer AA , ChangCH. Electromyographic evidence of paraspinal muscle spasm during sleep in patients with low back pain. Clin. J. Pain1, 147–154 (1985).
- Deyo RA , WeinsteinJN. Low back pain. N. Engl. J. Med.344(5), 363–370 (2001).
- Toth PP , UrtisJ. Commonly used muscle relaxant therapies for acute low back pain: a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone. Clin. Ther.26(9), 1355–1367 (2004).
- Bratton RL . Assessment and management of acute low back pain. Am. Fam. Physician60(8), 2299–2308 (1999).
- Chang E , GhoshN , YanniD , LeeS , AlexandruD , MozaffarT. A review of spasticity treatments: pharmacological and interventional approaches. Crit. Rev. Phys. Rehabil. Med.25(1–2), 11–122 (2013).
- Chou R . In the clinic. Low back pain. Ann. Intern. Med.160(11), ITC6-1 (2014).
- Frymoyer JD . Back pain and sciatica. N. Engl. J. Med.318(5), 291–300 (1988).
- Patel AT , OgleAA. Diagnosis and management of acute low back pain. Am. Fam. Physician61(6), 1779–1790 (2000).
- Hoy D , BainC , WilliamsGet al. A systematic review of the global prevalence of low back pain. Arthritis Rheum.64(6), 2028–2037 (2012).
- Hart LG , DeyoRA , CherkinDC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine20, 11–19 (1995).
- Kuritzky L , CarpenterD. The primary care approach to low back pain. Prim. Care Rep.1, 29–38 (1995).
- Schappert SM . Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997. Vital Health Stat. 13143, i-39 (1999).
- Chou R , QaseemA , SnowVet al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med.147(7), 478–491 (2007).
- McCarberg BH , RuoffGE , Tenzer-IglesiasP , WeilAJ. Diagnosis and treatment of low-back pain because of paraspinous muscle spasm: a physician roundtable. Pain Med.12(Suppl. 4), S119–S127 (2011).
- White KL , WilliamsTF , GreenbergBG. The ecology of medical care. N. Engl. J. Med.265, 885–892 (1961).
- Staiger TO , PaauwDS , DeyoRA , JarvikJG. Imaging studies for acute low back pain: when and when not to order them. Postgrad. Med.105, 161–172 (1999).
- Browning R , JacksonJL , O’MalleyPG. Cyclobenzaprine and back pain: a meta-analysis. Arch. Intern. Med.161(13), 1613–1620 (2001).
- Andersson GB , SvenssonHO , OdenA. The intensity of work recovery in low back pain. Spine8, 880–884 (1983).
- Von Korff M , SaundersK. The course of back pain in primary care. Spine21(24), 2833–2837 (1996).
- Nachemson AL . Newest knowledge of low back pain. A critical look. Clin. Orthop. Relat. Res.279, 8–20 (1992).
- Wipf JE , DeyoRA. Low back pain. Med. Clin. North Am.79(2), 231–246 (1995).
- Lim S , VosT , FlaxmanAet al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet380, 2224–2260 (2012).
- Vos T , FlaxmanAD , NaghaviMet al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet380, 2163–2196 (2012).
- Ferguson SA , MerryweatherA , TheseMSet al. Prevalence of low back pain, seeking medical care, and lost time due to low back pain among manual material handling workers in the United States. BMC Musculoskelet. Disord.20, 243 (2019).
- Atlas SJ , DeyoRA. Evaluating and managing acute low back pain in the primary care setting. J. Gen. Intern. Med.16(2), 120–131 (2001).
- Deyo RA , CherkinDC , ConradD , VolinnE. Cost, controversy, crisis: low back pain and health of the public. Annu. Rev. Pub. Health12, 141–156 (1991).
- Borenstein DG . Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr. Opin. Rheumatol.9, 144–150 (1997).
- Kent PM , KeatingJL. The epidemiology of low back pain in primary care. Chiropr. Osteopat.13, 13 (2005).
- Pransky G , BuchbinderR , HaydenJ. Contemporary low back pain research – and implications for practice. Best Pract. Res. Clin. Rheumatol.24(2), 291–298 (2010).
- Tekes K . Basic aspects of the pharmacodynamics of tolperisone, a widely applicable centrally acting muscle relaxant. Open Med. Chem. J.8, 17–22 (2014).
- Nádor K , PorszászJ. Pharmakologische und pharmakochemische studien über β- aminoketone. Arzneim. Forsch.8, 313 (1958).
- Pórszász J , NádorK , GibiszerK , BarankayT. The pharmacology of Mydeton (Mydocalm,1-piperidino-2-methyl-3-p-tolylpropanone-3), a new interneurone blocking compound. Acta Physiol. Hung.18, 149–170 (1960).
- Novales-Li P , SunXP , TakeuchiH. Suppression of calcium current in a snail neurone by eperisone and its analogues. Eur. J. Pharmacol.168(3), 299–305 (1989).
- Ono H , FukudaH , KudoY. Mechanisms of depressant action of muscle relaxants on spinal reflexes: participation of membrane stabilizing action. J. Pharmacobiodyn.7(3), 171–176 (1984).
- Fujioka M , KuriyamaH. Eperisone, an antispastic agent, possesses vasodilating actions on the guinea-pig basilar artery. J. Pharmacol. Exp. Ther.235(3), 757–763 (1985).
- Farkas S , TarnawaI , BerzsenyiP. Effects of some centrally acting muscle relaxants on spinal root potentials: a comparative study. Neuropharmacology28(2), 161–173 (1989).
- Novales-Li P . Piperidinopropiophenone derivatives as calcium antagonists in neuronal cells. Philip. J. Sci.122(3), 289–290 (1993).
- Sakitama K , OzawaY , AotoN , NakamuraK , IshikawaM. Pharmacological properties of NK433, a new centrally acting muscle relaxant. Eur. J. Pharmacol.273(1–2), 47–56 (1995).
- Okada H , HondaM , OnoH. Method for recording spinal reflexes in mice: effects of thyrotropin-releasing hormone, DOI, tolperisone and baclofen on monosynaptic spinal reflex potentials. Jpn J. Pharmacol.86(1), 134–136 (2001).
- Kocsis P , FarkasS , FodorLet al. Tolperisone-type drugs inhibit spinal reflexes via blockade of voltage-gated sodium and calcium channels. J. Pharmacol. Exp. Ther.315(3), 1237–4 (2005).
- Hofer D , LohbergerB , SteineckerB , SchmidtK , QuasthoffS , SchreibmayerW. A comparative study of the action of tolperisone on seven different voltage dependent sodium channel isoforms. Eur. J. Pharmacol.538(1–3), 5–14 (2006).
- Quasthoff S , MockelC , ZieglgansbergerWet al. Tolperisone: a typical representative of a class of centrally acting muscle relaxants with less sedative side effects. CNS Neurosci. Ther.14, 107–119 (2008).
- Sakaue A , HondaM , TanabeM , OnoH. Antinociceptive effects of sodium channel-blocking agents on acute pain in mice. J. Pharmacol. Sci.95(2), 181–188 (2004).
- Nalamachu S , PergolizziJ , KayeR. Tolperisone for the treatment of acute muscle spasm of the back: results from the dose-ranging Phase II STAR Study (NCT03802565). J. Pain Res.13, 3059–3069 (2020).

