Abstract
Aim: HTX-011 (ZYNRELEF™) is an extended-release, dual-acting local anesthetic containing bupivacaine and meloxicam. In bunionectomy and herniorrhaphy studies, HTX-011 resulted in less postoperative pain and less opioid consumption versus bupivacaine HCl. Here we evaluate HTX-011 in patients aged ≥65 years. Materials & methods: Patients received placebo, bupivacaine HCl or HTX-011 following surgery. End points included pain intensity, total opioid consumption, opioid-free patients and safety. Results: HTX-011-treated patients reported lower postoperative pain through 72 h versus bupivacaine HCl and placebo. Elderly patients administered HTX-011 used fewer opioids versus bupivacaine HCl, and a greater proportion remained opioid-free through 72 h. HTX-011 was well tolerated with a safety profile similar to bupivacaine HCl and placebo. Conclusion: HTX-011 maintained effectiveness and was well tolerated in elderly patients.
Clinical Trial registration numbers: NCT03295721 and NCT03237481
Older adults undergo surgery more frequently than any other age group and account for approximately half of all surgeries in the USA [Citation1,Citation2]. Inadequate control of postoperative pain in any patient can lead to adverse outcomes such as slow recovery, increased morbidity and higher costs [Citation3]. For the elderly, the result of uncontrolled pain can be far more significant because these patients are often already burdened with comorbidities and have age-related physical, functional and cognitive changes that make pain management more complex [Citation4].
The natural aging process leads to changes in the brain, brain neurotransmitters, and the central and peripheral nervous systems that can complicate the diagnosis and management of pain. When considering pain management therapy, opioids remain as one of the most commonly used classes of drugs for postsurgical pain, even though the elderly are particularly sensitive to opioid-induced respiratory and gastrointestinal adverse events (AEs) [Citation5,Citation6]. A meta-analysis evaluated 37,031 patients who underwent common surgical procedures and assessed risks of opioid-related AEs (ORAEs) as identified on discharge summary categorized into gastrointestinal, respiratory, genitourinary and CNS adverse effects [Citation7]. The study revealed that patients aged ≥65 years suffered ORAEs at a significantly higher rate than those aged <65 years (odds ratio: 2.11; p < 0.0001) [Citation7]. ORAEs often require an intervention to ease symptoms, which can lead to longer length of stay, increased hospital costs and higher readmission rates [Citation8]. The risks for ORAEs, pre-existing conditions, changes in muscle mass and metabolism, and alterations in production of neurotransmitters in older patients necessitate the reduction of opioid exposure compared with younger patients [Citation5,Citation9].
With these challenges in mind, a clinician must also consider that both uncontrolled pain and opioid use are risk factors for postoperative delirium [Citation10,Citation11]. Incidence of postoperative delirium can range from 4 to 65%, depending on the type of surgery [Citation12]. Postoperative delirium typically develops within the first few days following surgery and can result in significant morbidity and mortality [Citation13].
HTX-011 (ZYNRELEF) is an extended release solution of bupivacaine and meloxicam that is indicated in adults for soft tissue or periarticular instillation to produce postsurgical analgesia for up to 72 h after bunionectomy, open inguinal herniorrhaphy and total knee arthroplasty (TKA) [Citation14]. Each vial of HTX-011 contains a solution of 29.25 mg/ml bupivacaine and 0.88 mg/ml of meloxicam (a fixed ratio of 33 parts bupivacaine to one part meloxicam) [Citation14]. In HTX-011, bupivacaine and meloxicam are incorporated into a tri(ethylene glycol) poly(orthoester)-based polymer called Biochronomer®, which is specifically utilized for drug delivery applications. The unique, bioerodible polymer formulation enables controlled, simultaneous release of the active ingredients for approximately 72 h [Citation15,Citation16]. Low-dose meloxicam, a nonsteroidal anti-inflammatory drug, reduces local inflammation caused by surgery and normalizes the local pH, thereby potentiating the effect of bupivacaine [Citation16,Citation17]. The recommended dosages for HTX-011 (bupivacaine/meloxicam) are up to 60 mg/1.8 mg, 300 mg/9 mg and 400 mg/12 mg for bunionectomy, herniorrhaphy and TKA, respectively.
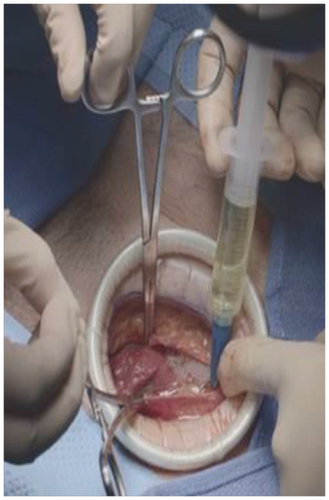
HTX-011 is administered via needle-free application as a single dose into the surgical site before wound closure (). Upon application, the active ingredients begin to release, with approximately 50% of the total dose released by 24 h and >90% released by 72 h [Citation18]. The sustained release of active ingredients from the polymer results in systemic plasma levels of bupivacaine and meloxicam for up to 120 h in bunionectomy and herniorrhaphy, and up to 140 h in TKA. After bupivacaine and meloxicam have been released from the polymer and are absorbed systemically, their distribution, metabolism and elimination are expected to be the same as other formulations of bupivacaine HCl solution and oral meloxicam [Citation14,Citation19,Citation20]. After the release of active ingredients, the polymer is hydrolyzed to small water-soluble polymer fragments that are primarily eliminated via the kidneys [Citation16].
HTX-011 is applied without a needle into the surgical site following final irrigation and suctioning, and prior to suturing of each layer.
Greater pain relief and opioid reduction have been demonstrated in two previously published Phase III studies in patients aged ≥18 years undergoing bunionectomy and herniorrhaphy, where HTX-011 significantly reduced pain intensity and opioid use through 72 h compared with saline placebo or standard-of-care bupivacaine HCl [Citation17,Citation21]. This article specifically aims to evaluate the efficacy and safety of HTX-011 in the subpopulation of patients aged ≥65 years undergoing bunionectomy and herniorrhaphy.
Materials & methods
Study design & procedures
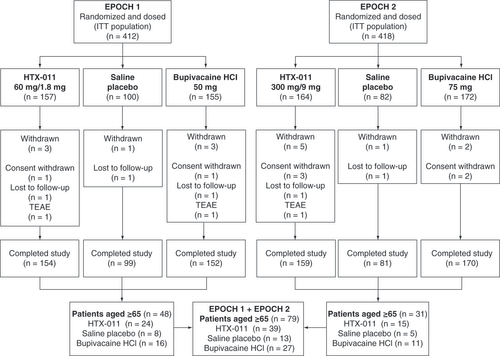
Data for this analysis were obtained from two Phase III studies, EPOCH 1 and EPOCH 2 (NCT03295721 and NCT03237481), that were designed to assess the efficacy and safety of HTX-011 for the treatment of postoperative pain following bunionectomy and herniorrhaphy, respectively ( & ). The study protocols were approved by Aspire IRB (Santee, CA, USA) and other institutional review boards and the studies were conducted in accordance with the International Council for Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to any study-related procedures.
Table 1. Studies and end points included for analyses of patients aged ≥65 years.Table Footnote†
EPOCH 1 and EPOCH 2 had similar study designs but were conducted in different surgical models. Both were randomized, double-blind, active- and saline placebo-controlled, multicenter studies. EPOCH 1 was conducted in subjects undergoing primary unilateral, distal, first metatarsal bunionectomy with osteotomy and internal fixation. EPOCH 2 was conducted in subjects undergoing unilateral open inguinal herniorrhaphy with mesh. Surgeries were performed under regional anesthesia (a lidocaine Mayo block) in EPOCH 1 and under general anesthesia in EPOCH 2. All patients were randomized to receive either saline placebo via injection; bupivacaine HCl via injection; or a single, intraoperative dose of HTX-011 applied without a needle to the surgical site prior to wound closure. Surgical staff were not blinded, but once transferred to the postanesthesia care unit, the investigator and site staff involved in all efficacy and safety assessments were blinded to treatment group. The dose of HTX-011 was different for each study due to differences in surgical procedures and the size of the surgical site. Following surgery, patients remained in the hospital for 72 h for efficacy and safety assessments. Blood samples were obtained at 4, 18, 24 and 48 h after study drug administration to determine bupivacaine and meloxicam concentrations, and pharmacokinetic parameters were derived using nonlinear mixed effects modeling. Patients who requested treatment for pain were provided rescue medication that included either acetaminophen or an opioid (intravenous morphine or oral oxycodone) during the 72-h postoperative observation period [Citation17,Citation21].
Patient population
Eligible patients for this analysis included those aged ≥65 years from the EPOCH 1 and EPOCH 2 studies. All patients had an American Society of Anesthesiologists Physical Status of I, II or III [Citation17,Citation21]. Patients with pre-existing, concurrent, acute or chronic painful physical/restrictive conditions that could confound postoperative assessments were excluded, as were those with known or suspected daily use of opioids for ≥7 consecutive days within 6 months prior to surgery, use of nonsteroidal anti-inflammatory drugs within 10 days, use of long-acting opioids within 3 days, any opioids within 24 h, bupivacaine within 5 days and systemic corticosteroids within 10 days prior to administration of study drug [Citation17,Citation21]. Patients were excluded if they had a history of severe kidney function impairment as defined by creatinine clearance (Cockcroft Gault) of <30 ml/min, being on dialysis and/or having a serum creatinine level of >2 × upper limit of normal [Citation17,Citation21].
End points & outcome measures
Efficacy outcomes were measured across HTX-011 studies using pain intensity as per the numerical rating scale (NRS). The primary end point of both Phase III trials was the mean area under the curve (AUC) for the first 72 h following surgery (AUC0–72) of the NRS pain intensity curve for HTX-011 compared with placebo () [Citation17,Citation21]. Key secondary end points included mean AUC0–72 of the NRS compared with bupivacaine HCl, mean total opioid consumption (in intravenous morphine milligram equivalents [MMEs]) through 72 h for HTX-011 compared with saline placebo and bupivacaine HCl, and the proportion of patients opioid-free through 72 h for HTX-011 compared with bupivacaine HCl. Safety end points include the incidence and severity of AEs as well as ORAEs [Citation17,Citation21].
Statistical methods
All patients who were treated with a study drug were included for analyses. The randomized treatment assignment was used for efficacy analyses, whereas the actual treatment received was used for safety analyses. Data were analyzed by treatment and by study. Missing pain intensity scores, which were very low due to the 72-h hospitalization following surgery, were imputed via last observation carried forward, whereas scores for patients not completing 72 h of evaluation were imputed by worst observation carried forward. All other data were analyzed using observed data. To adjust for the analgesic effect of opioid rescue medication, pain intensity scores were replaced by the highest observed score at any time before rescue medication use to adjust for opioid administration. Efficacy end points were summarized using descriptive statistics. No statistical testing was performed given the small sample size per treatment group. The treatment difference and associated 95% CI were estimated using ANOVA with randomized treatment as the main effect for NRS AUC0–72, Hodges–Lehmann estimation for total MME0–72 and Farrington–Manning score statistics for opioid free0–72 (subjects who had total MME opioid dose equal to 0 from 0 to 72 h). Safety end points were analyzed using descriptive statistics based on actual treatment received.
Results
Study populations
Across the two Phase III studies, 79 patients aged ≥65 years were treated with study drug; 39, 13 and 27 patients received HTX-011, saline placebo and bupivacaine HCl, respectively (). Patient demographics are generally similar across treatment groups and predominantly female in EPOCH 1 (bunionectomy) and male in EPOCH 2 (herniorrhaphy), reflecting the demographics of the surgical conditions.
Table 2. Baseline demographics for patients aged ≥65 years in EPOCH 1 and EPOCH 2.
Pharmacokinetics
Plasma concentrations were obtained through the first 48 h and revealed similar exposure to bupivacaine or meloxicam for patients aged ≥65 years compared with those aged <65 years ().
Table 3. Summary of pharmacokinetic parameters.
Pain intensity
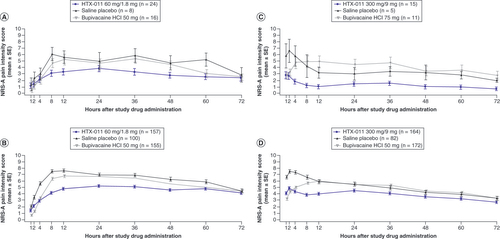
HTX-011 resulted in lower pain scores, with a clear separation in pain curves throughout the 72 h for patients who received HTX-011 compared with those receiving saline placebo or bupivacaine HCl (A–D & ). This result is consistent in both EPOCH 1 and EPOCH 2 for the overall populations (B & D) as well as for the ≥65 populations (A & C). The cumulative pain score as measured by AUC0–72 demonstrates overall lower pain scores in the elderly population compared with the overall population. HTX-011-treated patients reported the lowest overall pain scores in the overall population as well as for patients aged ≥65 years compared with patients given bupivacaine HCl and saline placebo in both studies. HTX-011 demonstrated consistent pain reduction compared with bupivacaine HCl or saline placebo in both studies across all age groups. Notably, the magnitude of pain reduction was greatest in patients aged ≥65 years compared with those aged <65 years and the overall population ().
NRS: Numeric Rating Scale of pain intensity; SE: Standard error.
The treatment difference and 95% CI for AUC were estimated using ANOVA with randomized treatment as main effect. The treatment difference and 95% CI for total opioid consumption were estimated using Hodges–Lehmann estimation. The treatment difference and 95% CI for opioid-free patients were estimated using the Farrington–Manning score statistic.
% Opioid free0–72: Subjects who had total MME opioid dose = 0 from 0 to 72 h; AUC: Area under the curve; AUC0–72: AUC through 72 h; MME: Morphine milligram equivalent; MME0–72: MME through 72 h.
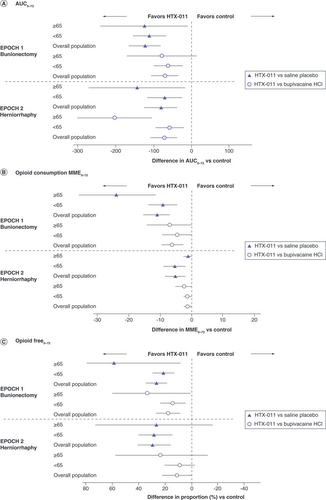
Table 4. Summary of efficacy end points: pain intensity, opioid-free and opioid consumption during the first 72 h.
Total opioid consumption through 72 h was lower overall in elderly patients compared with the overall population in both studies. Patients receiving HTX-011 had substantially lower opioid consumption compared with those receiving placebo and bupivacaine HCl for those aged ≥65 years, <65 years and in the overall population ( & ) [Citation17,Citation21]. In both studies, patients aged ≥65 years receiving HTX-011 demonstrated a greater percent reduction in opioid consumption than bupivacaine HCl or saline placebo compared with the overall population (). HTX-011 enabled a greater proportion of subjects to recover opioid-free compared with bupivacaine and placebo in the overall population in both studies ( & ). This opioid-sparing effect of HTX-011 was enhanced in the elderly population. Following bunionectomy, 58% of patients aged ≥65 years who received HTX-011 were opioid free through 72 h compared with 29% of the overall population. Following herniorrhaphy, 87% of patients aged ≥65 years who received HTX-011 were opioid free through 72 h compared with 51% of overall population ().
Safety
Overall HTX-011 was well tolerated, and the safety profile was similar regardless of age. The safety profile in each procedure was similar when comparing across the subsets of patients aged ≥65 years, <65 years and the overall adult Phase III study populations (≥18 years) (). Most patients receiving HTX-011 reported ≥1 AE; rates were similar for patients aged ≥65 years, <65 years and the overall population in the bunionectomy study. For patients receiving HTX-011 in the herniorrhaphy study, all patients aged ≥65 years reported ≥1 AE compared with the overall population (73.0%) and patients aged <65 years (70.3%). For all patients aged ≥65 years, the majority of AEs were mostly mild or moderate in severity and none led to study withdrawal. The occurrence of AEs possibly related to the study drug for bunionectomy was similar for patients aged ≥65 years, <65 years and the overall population (12.5, 19.5 and 18.5%, respectively). Similarly, the occurrence of AEs possibly related to study drug for herniorrhaphy was comparable for patients aged ≥65 years, <65 years and the overall population (20.0, 25.7 and 25.2%, respectively). The one severe AE in patients aged ≥65 years receiving HTX-011 was a patient who had an exacerbation of chronic obstructive pulmonary disease and was considered by the investigator as unlikely related to study drug. No local anesthetic systemic toxicity events occurred. Serious AEs were rare, and none was considered related to the study drug.
Table 5. Summary of adverse events.
ORAEs in HTX-011-treated patients aged ≥65 years were common and occurred in 41.7% after bunionectomy compared with 43.9% of the overall population and 44.4% of patients aged <65 years. ORAEs in HTX-011-treated patients aged ≥65 years occurred in 20.0% after herniorrhaphy compared with 32.5% in the overall population and 33.8% in patients aged <65 years. Constipation and nausea were the most commonly observed ORAEs.
Discussion
In two Phase III studies in bunionectomy and herniorrhaphy, HTX-011 demonstrated superior pain control and reduction in opioid use through 72 h compared with bupivacaine HCl and saline placebo [Citation17,Citation21]. This subgroup analysis demonstrates that the efficacy and safety of HTX-011 observed in the overall Phase III population persists among patients aged ≥65 years. According to the prescribing information of other long-acting local anesthetics, the clinical efficacy and safety is similar in elderly and younger patients, which is consistent with these data [Citation22,Citation23]. However, details regarding subgroup analyses from other long-acting local anesthetic Phase III data are not available. To our knowledge, this is the first analysis of a local anesthetic that provides detailed efficacy and safety data including pain curves, opioid consumption, AEs and ORAEs in subsets of patients ≥65 and <65 years compared with the overall Phase III study populations.
Compared with the overall population, this subgroup of older adults displayed lower mean pain scores (NRS AUC0–72 values) and lower mean opioid consumption (MME0–72) through 72 h across all treatment arms [Citation17,Citation21]. These differences in pain intensity and opioid consumption among older adults could reflect reduced neurological functionality and pain sensation as discussed earlier, or an altered sensitivity to analgesics due to physiologic changes that occur as a person ages [Citation4,Citation24]. Although the physiological changes that impact pain sensation and analgesic sensitivity in the elderly population are becoming better understood, data that evaluate these changes with regard to clinical outcomes in the postoperative pain setting are lacking. Pain data in elderly patients are largely limited to chronic and cancer pain management. Therefore, this study provides a better understanding of pain scores and opioid consumption following surgery in the elderly population.
Despite the lower pain experienced in this elderly population, HTX-011 demonstrated greater pain reduction through 72 h compared with both saline placebo and bupivacaine HCl in both bunionectomy and herniorrhaphy. In fact, the magnitude of pain reduction compared with saline placebo and bupivacaine HCl was greater in the subgroup of patients aged ≥65 years compared with the overall population.
Notably, the greater pain reduction observed with HTX-011 was achieved while consuming fewer opioids compared with bupivacaine HCl and saline placebo. This opioid-sparing effect was enhanced in the elderly population. Patients aged ≥65 years receiving HTX-011 consumed fewer rescue opioids than patients in the overall population during the 72-h postoperative period after bunionectomy (7.7 vs 18.9 MME) or herniorrhaphy (1.7 vs 10.9 MME). Given the potential for serious adverse effects associated with even low doses of opioids in the elderly, the ability to achieve an opioid-free postoperative recovery is clinically meaningful. In the overall population, HTX-011 resulted in significantly more patients being opioid free through 72 h compared with bupivacaine HCl and saline placebo in both Phase III studies. In the subgroup of elderly patients, HTX-011 again resulted in a greater proportion of patients being opioid free compared with bupivacaine HCl and saline placebo, and substantially increased the proportion of subjects who were opioid free compared with the overall population. In the bunionectomy and herniorrhaphy studies, 58 and 87% of patients aged ≥65 years remained opioid free if treated with HTX-011 compared with 29 and 51% in the overall population, respectively. This reduction in opioid use may be important to older adults who are both more vulnerable to oversedation, decreased cognition, postoperative delirium, physical challenges, risk of falls and ORAEs. Additionally, co-prescribing of opioids and psychotropic drugs is a growing concern; the US FDA recently issued a boxed warning for particular caution in prescribing opioids and benzodiazepines together for concern of risk of respiratory depression and death [Citation25]. A nonopioid postoperative analgesic regimen that provides safe and effective pain management could reduce the risk burden of opioid-related complications in older adults needing surgery.
In patients aged ≥65 years, pharmacokinetics of medications is influenced by a number of factors. As seen in , only modest changes occurred in maximum concentration, maximum time, and AUC for bupivacaine and meloxicam. Thus, the overall patient exposure remains similar in both age groups.
HTX-011 was well tolerated in the overall population. Older adults experienced AEs at similar rates across age populations, indicating the safety of HTX-011 regardless of age. Overall, most patients aged ≥65 years experienced ≥1 AE in the HTX-011 group; however, no serious AEs were considered related to HTX-011 in either study. There was no difference in rates of wound or bone healing findings with HTX-011 compared with saline placebo or bupivacaine HCl, which is consistent with Phase III studies in the overall population [Citation17,Citation21]. Similar rates of ORAEs were observed in the ≥65 population compared with the overall population and the <65 population. This result likely represents the fact that many ORAEs overlap with AEs that are common in the postoperative setting, such as nausea and constipation, given that the elderly population had notably less opioid consumption during the first 72 h compared with the overall population.
In addition to the careful consideration toward postoperative pain management in the elderly, attention must be given to their pre-existing conditions and physiological changes [Citation1]. These changes may be decreased cardiac output, reduced muscle mass and higher amounts of fat, reductions in kidney or liver function, and decrease in plasma volume and plasma protein, any of which can alter the pharmacological effects of drugs [Citation1]. HTX-011 is administered once and does not require any dosage adjustment for renal function, although it has not been studied in patients with severe renal impairment (creatinine clearance of ≤30 ml/min). However, the fact that older adults are more likely to have impaired renal function that may affect bupivacaine pharmacokinetics should be taken into account when considering the dose of HTX-011. HTX-011 contains low-dose meloxicam, and in studies in TKA the dose of HTX-011 administered was 400 mg bupivacaine/12 mg meloxicam [Citation26]. At this dose the daily exposure to meloxicam would be lower than the lowest recommended adult oral dose of 7.5 mg, as confirmed by in vitro data demonstrating that approximately 5 mg of meloxicam in HTX-011 is released in the first 24 h [Citation20,Citation27]. HTX-011 has also been studied in open-label trials where ibuprofen or celecoxib had been given as part of a scheduled multimodal analgesia regimen without any evidence of cardiovascular, gastrointestinal or renal toxicity [Citation28,Citation29].
The strength of the current analysis is the data that come from two well-controlled, randomized, multicenter, Phase III clinical studies. Each study used both placebo and active comparator (standard-of-care bupivacaine HCl) for primary and key secondary end points. However, there are limitations to this current analysis. First, the sample sizes for the ≥65 cohort are small and are limited to the two studies; however, results from this subpopulation demonstrated greater pain reduction and less opioid use compared with both saline placebo and bupivacaine HCl, which is consistent with the results in the overall Phase III populations. Second, the studies were conducted in only two surgical models: bunionectomy, which comprises primarily female patients; and herniorrhaphy, which consists of primarily male patients. However, these models are considered representative of those surgical populations and bony and soft tissue surgeries, respectively, supporting extrapolation of the results to other surgeries [Citation30].
Conclusion
Poor pain control can have deleterious effects on the elderly in particular because these patients are already more likely to have preexisting conditions, concurrent diseases and declining cognition that can impact the outcome of surgery [Citation4]. For this reason, effective nonopioid alternatives for controlling pain are needed, because the use of traditional opioid therapy can have serious negative consequences in elderly patients. The current analysis in the elderly population shows that HTX-011 was well tolerated and demonstrated reduction in both pain and the need for opioids, which for the elderly population could have positive implications during and beyond their postoperative course.
Postoperative pain management in elderly adults aged ≥65 years can be complex, making nonopioid alternatives a much-needed option.
HTX-011 (ZYNRELEF™) is an extended-release, dual-acting local anesthetic containing bupivacaine and low-dose meloxicam incorporated in a polymer formulation.
In Phase III studies of bunionectomy and herniorrhaphy, HTX-011 resulted in significantly less postoperative pain, less opioid consumption and more patients recovering opioid free compared with standard-of-care bupivacaine HCl.
This post hoc analysis evaluated the efficacy and safety of HTX-011 in the subpopulation of patients aged ≥65 years from the Phase III studies.
Here, HTX-011-treated patients reported lower postoperative pain scores through 72 h regardless of age compared with bupivacaine HCl and saline placebo.
Elderly patients administered HTX-011 used fewer opioids in bunionectomy and herniorrhaphy compared with bupivacaine HCl (7.7 morphine milligram equivalents [MMEs] vs 15 and 1.7 MME vs 3.5 MME, respectively) and a greater proportion remained opioid free through 72 h (58 vs 25% and 87 vs 64%, respectively).
HTX-011 was well tolerated in patients aged ≥65 years, with a safety profile similar to that for bupivacaine HCl and saline placebo.
HTX-011 administration in patients aged ≥65 years resulted in less postoperative pain, less opioid consumption and more patients recovering opioid free compared with standard-of-care bupivacaine HCl.
Author contributions
PS Hawn was responsible for study conception and design. A Yamamoto and J Hu were responsible for acquisition of data. All authors participated in data analysis and drafting and revision of the manuscript.
Ethical conduct of research
The study protocols were approved by Aspire IRB (Santee, CA, USA) and other institutional review boards and were conducted in accordance with the International Council for Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to any study-related procedures.
Acknowledgments
The authors thank the patients, investigators and study site personnel for their valuable contributions to this work.
Financial & competing interests disclosure
This study was funded by Heron Therapeutics, Inc. (San Diego, CA, USA). T Yip has nothing to disclose. J Hu is an employee of Heron Therapeutics, Inc., and reports personal fees during the conduct of the study and outside the submitted work. A Yamamoto is an employee of Heron Therapeutics, Inc. G Oderda is a consultant for Heron Therapeutics, Inc. PS Hawn was an employee of Heron Therapeutics at the time of manuscript submission. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial and submission assistance was provided by S Dilks and L Raman of ApotheCom (San Francisco, CA, USA), and was funded by Heron Therapeutics, Inc. (San Diego, CA, USA).
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of these shared data is in accordance with the terms (if any) agreed upon their receipt. The source of these data is: ClinicalTrials.gov, NCT03295721 and NCT03237481. Data are available on reasonable request.
Additional information
Funding
References
- Falzone E , HoffmannC , KeitaH. Postoperative analgesia in elderly patients. Drugs Aging30(2), 81–90 (2013).
- Ko FC . Preoperative frailty evaluation: a promising risk-stratification tool in older adults undergoing general surgery. Clin. Ther.41(3), 387–399 (2019).
- Gan TJ . Poorly controlled postoperative pain: prevalence, consequences, and prevention. J. Pain. Res.10, 2287–2298 (2017).
- Halaszynski TM . Perioperative pain management in the elderly surgical patient. Univers J. Med. Sci.1(2), 36–49 (2013).
- Mckeown JL . Pain management issues for the geriatric surgical patient. Anesthesiol. Clin.33(3), 563–576 (2015).
- Oderda GM , SenagoreAJ , MorlandKet al. Opioid-related respiratory and gastrointestinal adverse events in patients with acute postoperative pain: prevalence, predictors, and burden. J. Pain. Palliat. Care Pharmacother.33(3-4), 82–97 (2019).
- Kessler ER , ShahM , GruschkusSK , RajuA. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy33(4), 383–391 (2013).
- Oderda G . Challenges in the management of acute postsurgical pain. Pharmacotherapy32(Suppl. 9), S6–S11 (2012).
- Rajan J , BehrendsM. Acute pain in older adults: recommendations for assessment and treatment. Anesthesiol. Clin.37(3), 507–520 (2019).
- Clegg A , YoungJB. Which medications to avoid in people at risk of delirium: a systematic review. Age. Ageing40(1), 23–29 (2011).
- Leung JM , SandsLP , LimE , TsaiTL , KinjoS. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium?Am. J. Geriatr. Psychiatry21(10), 946–956 (2013).
- Rudolph JL , MarcantonioER. Review articles: postoperative delirium: acute change with long-term implications. Anesth. Analg.112(5), 1202–1211 (2011).
- Strom C , RasmussenLS , SieberFE. Should general anaesthesia be avoided in the elderly?Anaesthesia69(Suppl. 1), 35–44 (2014).
- ZYNRELEF™, prescribing information. Heron Therapeutics, Inc., CA, USA (2021).
- Balocco AL , Van ZundertPGE , GanSS , GanTJ , HadzicA. Extended release bupivacaine formulations for postoperative analgesia: an update. Curr. Opin. Anaesthesiol.31(5), 636–642 (2018).
- Ottoboni T , QuartB , PawasauskasJ , DastaJF , PollakRA , ViscusiER. Mechanism of action of HTX-011: a novel, extended-release, dual-acting local anesthetic formulation for postoperative pain. Reg. Anesth. Pain. Med. doi:10.1136/rapm-2019-100714 (2019) ( Epub ahead of print).
- Viscusi E , GimbelJS , PollackRA , HuJ , LeeGC. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in bunionectomy: phase III results from the randomized EPOCH 1 study. Reg. Anesth. Pain. Med. doi:10.1136/rapm-2019-100531 (2019) ( Epub ahead of print).
- Hardman D , LukeCB , SalehJ , OttoboniT. HTX-011: predictable release rates of bupivacaine and meloxicam for 72 hours. Presented at: Anesthiesiology 2020.2–5 October 2020.
- Marcaine®, prescribing information. Hospira, Inc., IL, USA (2011).
- Mobic®, package insert. Boehringer Ingelheim Pharmaceuticals, Inc., CT, USA (2016).
- Viscusi E , MinkowitzH , WinkleP , RamamoorthyS , HuJ , SinglaN. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in herniorrhaphy: results from the Phase III EPOCH 2 study. Hernia23(6), 1071–1080 (2019).
- Exparel®, prescribing information. Pacira Pharmaceuticals, CA, USA (2018).
- Xaracoll®, prescribing information. Innocoll Pharmaceuticals Limited, Athlone, Ireland (2020).
- Aubrun F , MarmionF. The elderly patient and postoperative pain treatment. Best Pract. Res. Clin. Anaesthesiol.21(1), 109–127 (2007).
- US Food and Drug Administration . FDA Drug Safety Communication: FDA requiring Boxed Warning updated to improve safe use of benzodiazepine drug class. Includes potential for abuse, addiction, and other serious risks. (2020). https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
- Lachiewicz PF , LeeGC , PollakRA , LeimanDG , HuJ , SahAP. HTX-011 reduced pain and opioid use after primary total knee arthroplasty: results of a randomized phase 2b trial. J. Arthroplasty35(10), 2843–2851 (2020).
- Luke C , SalehJ , HardmanD , OttoboniT. HTX-011: predictable release rates of bupivacaine and meloxicam for 72 hours. Presented at: American Society of Regional Anesthesia and Pain Medicine (ASRA) Worldwide 2020: 19th Annual Pain Medicine Meeting.19–22 November 2020.
- Pollak R , CaiD , GanTJ. Opioid-free recovery from bunionectomy with HTX-011, a dual-acting local anesthetic combining bupivacaine and meloxicam, as the foundation of non-opioid multimodal analgesia. J. Am. Podiatr. Med. Assoc.111(3), Article_15 (2021).
- Singla N , WinkleP , BertochT , HuJ , BeatonA , RedanJ. Opioid-free recovery after herniorrhaphy with HTX-011 as the foundation of a multimodal analgesic regimen. Surgery168(5), 915–920 (2020).
- US Food and Drug Administration . Draft Guidance for Industry on Analgesic Indications: Developing Drug and Biological Products; Availability [Docket No. FDA-2014-D-0091]. (2014). https://www.federalregister.gov/documents/2014/02/06/2014-02557/draft-guidance-for-industry-on-analgesic-indications-developing-drug-and-biological-products