Abstract
Aim: Chronic postsurgical pain (CPSP) is a common complication of surgery. This study was conducted to evaluate the efficacy and safety of paresthesia-free, 10-kHz spinal cord stimulation (SCS) as a treatment for CPSP. Patients & methods: Subjects in this prospective, single-arm study had an average pain intensity of ≥5 cm on a 10-cm visual analog scale. The subjects who had pain relief of ≥50% (response) with temporary trial stimulation were permanently implanted with 10-kHz SCS and assessed for 1 year. Results: At 12 months, 94% of subjects were responders to 10-kHz SCS, and 88% had pain remission (visual analog scale ≤2.5 cm). Conclusion: The pain relief was durable in CPSP subjects and the safety profile of 10-kHz SCS was as expected.
Clinical Trial registration number: VT005076953 (Privacy Commission of Belgium)
Chronic postsurgical pain (CPSP) is defined by WHO and the International Association for the Study of Pain as pain developing after a surgical procedure and lasting for at least 3 months and not attributable to any other condition [Citation1]. The incidence of CPSP varies widely by surgical procedure, with CPSP reported after 12% of hip replacements, 20–50% of breast surgeries and following up to 85% of amputations [Citation2,Citation3]. Injury to nerves during surgery has been proposed as a major contributor to the development of CPSP, and the proportion of CPSP cases that are neuropathic in nature has been reported to range from a low of 6–9% for patients receiving total hip or knee arthroplasty to a high of 68–74% in patients undergoing breast surgery [Citation4]. Neuropathic pain is associated with more severe, persistent pain than non-neuropathic pain, has a greater impact on patients’ quality of life and increases the risk of depression [Citation3,Citation5,Citation6].
Chronic pain, including CPSP, is often treated in the first line with several different types of drugs, such as antiepileptics, epidural steroid injections, opioids, N-methyl-d-aspartate receptor antagonists, capsaicin, neurotoxins and local anesthetics. However, evidence for the effectiveness of these drugs is unclear [Citation6]; opioids, in particular, are unsuitable for chronic treatment. Neuromodulation has been used for CPSP in the second-line setting. Peripheral nerve stimulation has been used previously for neuropathic pain, including CPSP [Citation7], but this technology requires focal stimulation at the site of pain and nearby placement of a generator, which can be inconvenient or impractical for some patients. There have been some successes in treating CPSP with conventional, paresthesia-dependent spinal cord stimulation (SCS). A decades-old study of Italian subjects who had chronic neuropathic pain from conditions related to CPSP, including phantom limbs, reflex sympathetic dystrophy and failed back surgery, found high initial responder rates that declined significantly over time using conventional SCS [Citation8]. A more recent study in subjects limited to CPSP following herniorrhaphy found that all subjects reported >75% pain relief after 12 months of treatment [Citation9]. In these previous studies, however, subjects were not selected specifically for neuropathic pain, nor were they tested for neurological deficits or improvements in response to SCS treatment.
In the past several years, high-frequency SCS delivered at 10 kHz has been shown to provide superior pain relief to conventional SCS in subjects with chronic low back and leg pain [Citation10–13] as well as effectiveness in treating chronic peripheral polyneuropathy (PPN) [Citation14,Citation15]; high-frequency SCS offers the advantage of paresthesia-independent pain relief. This prospective study was undertaken in order to evaluate the efficacy and safety of 10-kHz SCS for treating subjects with CPSP. Subjects were also tested not only for pain relief, but also neurological functioning to determine whether 10-kHz SCS is a suitable option to help meet the unmet need for pain management in this population.
Patients & methods
Study design & subject selection criteria
The study plan, protocols, informed consent forms and all amendments were reviewed and approved by the Ethics Committee of the institute, and the study was compliant with all relevant recommendations of the 18th World Medical Assembly in Helsinki, Finland, for physicians involved in biomedical research. As the study was observational, public registration of this study was not required, and the study was submitted to the Privacy Commission of Belgium and registered in their database prior to enrollment (registration number: VT005076953). The first patient was enrolled on 12 April 2017, the last patient was enrolled on 28 September 2018 and the final subject visit was completed on 14 October 2019.
Subject eligibility was determined using the inclusion and exclusion criteria detailed in . Eligible subjects had a diagnosis of chronic, focal, neuropathic pain following surgery involving the trunk or limbs, with an average pain intensity in the primary affected region of ≥5 out of 10 cm on a visual analog scale (VAS) for a minimum of 3 months. Subjects with significant pain from failed back surgery were excluded, as well as any subjects with pain in areas that are not suitable for SCS treatment.
Table 1. Key inclusion and exclusion criteria.
Implant procedures
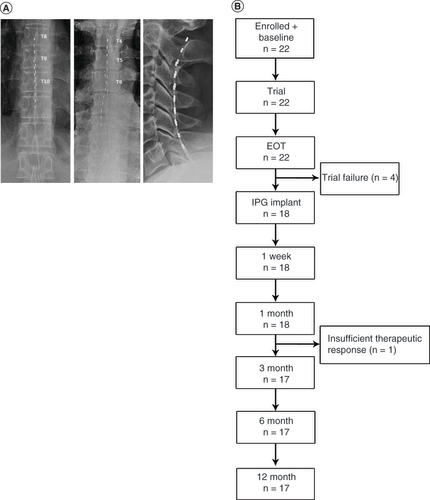
Investigators implanted octapolar, epidural leads in eligible subjects, depending on the location of their pain, with thoracic placement at T8/9–T11/12 for lower limb pain, cervical placement at C2–C6 for upper limb pain, and individualized placement (T4–T8) in cases of trunk pain (A). After implantation of the leads, subjects underwent trial stimulations for up to 2 weeks at a frequency of 10 kHz and a pulse width of 10 μs. Pulse amplitudes were individually adjusted to optimize pain relief without producing paresthesia in the subjects. As with previous studies of 10-kHz SCS, trial stimulations that resulted in at least 50% pain relief, as assessed by VAS scores, were deemed successful [Citation10,Citation16,Citation17], and those subjects were offered implantation with a permanent 10-kHz SCS system (Senza® System, Nevro Corp., CA, USA).
(A) Octopolar leads were placed based on the location of pain. Lead placements for subjects with lower limb pain are shown on the left, for pain of the trunk in the center and for upper limb pain on the right. (B) Flow chart showing subject progression through the study.
EOT: End of trial; IPG: Implantable pulse generator.
Clinical & safety outcomes assessments
The study did not have primary or secondary outcome assessments. Instead, it assessed both efficacy and safety outcomes as described below. Pain intensity was assessed at baseline and follow-up time points using patient-reported VAS scores (average pain intensity over 7 days) and the Douleur Neuropathique (DN-4) screening tool, which comprises four questions and is used to identify likely neuropathic pain [Citation18]. The pain experienced by subjects was also characterized using the short-form McGill Pain Questionnaire 2 (SF-MPQ-2), which assesses the contributions of four components of overall pain [Citation19], as well as the Pain Catastrophizing Scale (PCS) [Citation20] and the Pain Vigilance and Awareness Questionnaire (PVAQ) [Citation21]. Investigators also used the Pain Disability Index (PDI) [Citation22] to assess the degree of interference by pain on subjects’ normal functioning, and pain interference with subjects’ sleep was determined using the Pain and Sleep Assessment (PSQ-3) [Citation23].
Subjects’ general health was assessed using the 12-item Short Form Health Survey (SF-12), a generic measure of clinical outcomes which includes the Physical Component and Mental Component Summary Scale Scores [Citation24]; the Global Assessment of Functioning (GAF), an overall assessment of psychological, social and occupational functioning, was used to determine subjects’ overall mental health status [Citation25]. Finally, the subjects’ and investigators’ perceptions of the effectiveness of 10-kHz SCS was evaluated with the Patient Global Impression of Change and Clinician Global Impression of Change questionnaires [Citation26] and a survey of subjects’ satisfaction with the treatment.
Investigators recorded all adverse events (AEs) and tested neurological functioning at baseline and follow-up visits.
Data analysis
Outcomes were analyzed using descriptive statistics. Continuous variables are reported as means and standard deviations or medians and ranges, if appropriate, and categorical variables are reported as counts and percentages. After implantable pulse generator implantation, responder rates were calculated based on subjects whose VAS scores decreased by ≥50% from baseline levels, and remitter rates were determined by the number of subjects whose VAS scores were ≤2.5 cm [Citation10].
Results
Study subjects
A total of 22 subjects were enrolled in this study, underwent baseline assessments and received implanted leads for a trial stimulation (B). The study population included 14 men (64%), and the subjects had a median age of 52.5 years (range: 22–77). Subjects’ demographic information and baseline pain characteristics are summarized in . The most frequent region affected by CPSP among this cohort was the lower limb, which was affected in 16 subjects (73%), and pain was unilateral in 20 subjects (91%). The subjects had from one to six previous surgeries, and the onset of pain followed surgery in 21 subjects (95%). Median duration of pain prior to enrollment was 4 years (range: <1–22).
Table 2. Subject demographic information and baseline pain characteristics.
Trial stimulation phase
Trial stimulations with 10-kHz SCS were deemed successful in 18 of the 22 enrolled subjects (82%) based on pain relief of ≥50% relative to baseline values. The mean VAS score in permanently implanted subjects declined from 7.9 cm at baseline to 1.1 cm after the trial stimulation period, an 86% reduction. All 18 subjects who had successful trial stimulations were implanted with permanent 10-kHz SCS systems, but one subject withdrew before the 3-month follow-up visit due to insufficient therapeutic response (B).
Pain outcomes
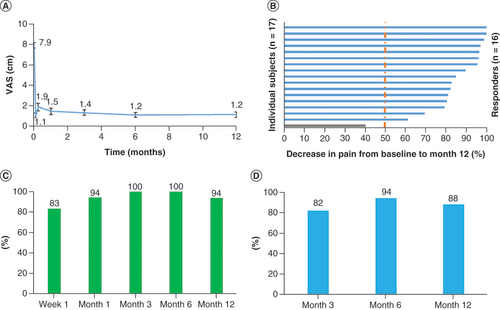
Mean pain scores for all subjects are shown in A. The pain reduction achieved with the trial stimulation was maintained at all assessments through the 12-month follow-up. The mean VAS score was 1.4 cm after 3 months of stimulation, a pain reduction of 82%, and 1.2 cm after 12 months, an 85% pain reduction. After 12 months of stimulation, 16 of 17 subjects (94%) continued to be responders, with ≥50% pain relief compared with baseline (B), and the responder rate was high at all intermediate time points, including 100% after 3 months and 6 months of 10-kHz SCS (C). Finally, the remitter rate (subjects with VAS ≤2.5 cm) was 82% after 3 months of stimulation, and this rate was durable over the course of the study, at 88% after 12 months of treatment with 10-kHz SCS (D).
(A) Mean VAS declined immediately upon initiation of trial stimulation and remained low through the end of the study at 12 months. (B) All but one of the subjects were responders, with ≥50% pain relief, after 12 months of stimulation. (C) Responder rates at 3-, 6- and 12-month assessments. (D) Remitter rates (VAS ≤2.5 cm) at 3, 6 and 12 months.
VAS: Visual analog scale.
Subject satisfaction & quality of life
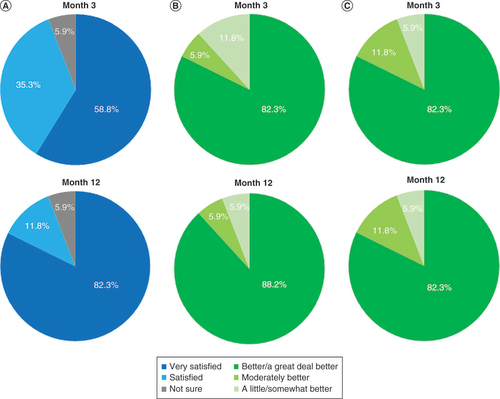
When surveyed regarding their level of satisfaction with 10-kHz SCS as a treatment for their pain due to CPSP, 59% of patients reported they were ‘very satisfied’ after 3 months of stimulation. This proportion rose to 82% after 12 months, as shown in , while the rest of the subjects chose either ‘satisfied’ or ‘not sure’. Subjects who reported that their general health status was ‘better/a great deal better’ on the Patient Global Impression of Change questionnaire increased from 82% after 3 months to 88% after 12 months of stimulation (), while investigators’ answers on the Clinician Global Impression of Change questionnaire were identical at both the 3- and 12-month follow-up visits, with 82% reporting their subject’s health status as ‘better/a great deal better’.
(A) Patient satisfaction shown after 3 and 12 months of stimulation. (B) Responses to the Patient Global Impression of Change questionnaire after 3 and 12 months of stimulation. (C) Responses to the Clinician Global Impression of Change questionnaire after patients had received 3 and 12 months of stimulation.
Pain perception
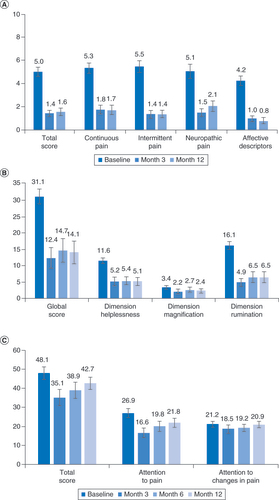
The results of the SF-MPQ-2 showed that overall pain decreased from 5.0 at baseline to 1.4 after 3 months of 10-kHz SCS and remained at 1.6 after 12 months, and that all four components of pain were decreased, including neuropathic pain (A). The subjects’ mean global score on the PCS likewise decreased from 31.1 at baseline to 12.4 after 3 months of stimulation and was 14.1 through 12 months of treatment, as shown in B. Scores for the PCS dimensions of helplessness and rumination decreased by 56 and 60%, respectively, after 12 months of treatment with 10-kHz SCS, while the mean score for the PCS magnification dimension was already low (3.4) at baseline in these subjects. The mean total PVAQ score decreased from 48.1 to 35.1 after 3 months of stimulation and was 42.7 after 12 months (C).
(A) Pain declined both overall and in all four components of the MPQ-2. (B) Mean scores on the pain catastrophizing scale. (C) Mean responses for the Pain Vigilance and Awareness Questionnaire.
MPQ-2: McGill Pain Questionnaire.
Functional capacity, sleep & assessment of neuropathy
The results for disability, functional capacity, sleep quality and the likelihood of neuropathy assessments are shown in . Mean scores from the GAF increased by 18 points after 3 months of treatment with 10-kHz SCS, a 30% rise, and this increase was maintained through the 12-month follow-up period (75.7 ± 3.6). Mean PDI scores decreased from 46.5 ± 3.5 to 26.7 ± 3.2 at the 3-month time point, reflecting a reduction in subjects’ overall disability, and this decrease in scores was, likewise, maintained through the end of the study (23.5 ± 3.9).
Table 3. Results of assessments for disability, functional capacity, sleep quality and the likelihood of neuropathy.
In the current study, subjects’ mean SF-12 physical component score increased by 10.9 points after 3 months of 10-kHz SCS (); after 12 months of stimulation, it was 40.4 ± 2.4, a rise of 12.9 points over the baseline score. The mean mental component score rose by 8.2 points after 3 months of treatment and was 4.2 points higher than baseline after 12 months (47.4 ± 2.4). Scores on all three questions of the PSQ-3 fell from means of 6.0–6.8 at baseline to 0.9–1.6 after 12 months of treatment with 10-kHz SCS, corresponding to improvements in falling asleep and maintaining sleep.
Finally, the DN-4 was administered at baseline and at the 3-month and 12-month assessments. Scores ≥4 identify subjects with likely neuropathic pain [Citation18]; the subjects’ baseline mean score was 6.4 ± 0.6 and fell by 4.3 points after 3 months. The mean DN-4 score was 3.8 ± 0.6 after 12 months of stimulation.
Safety
A total of ten AEs were reported in seven subjects (39%) during the course of this study and are detailed in . All AEs were resolved by the time of the 12-month follow-up, except for one case of medical device pain, which was ongoing. No subjects experienced any serious AEs or unanticipated AEs.
Table 4. Study-related adverse events.
Discussion
There is an unmet need for safe and effective treatment of CPSP, which is often neuropathic in nature. Recent work suggests 10-kHz SCS is safe and effective in chronic, neuropathic pain with other etiologies. This study was undertaken to test the safety and effectiveness of 10-kHz SCS in subjects who had focal, neuropathic CPSP, as shown by the mean baseline VAS score (7.9 cm) and DN-4 score (6.4). 10-kHz SCS is known to provide paresthesia-independent pain relief, likely due to the preferential activation of inhibitory interneurons in the spinal dorsal horn over dorsal column fibers, as demonstrated in rodent models [Citation27]. The results presented here compare favorably with and support previously published data showing 10-kHz SCS is safe and effective in subjects with CPSP [Citation28].
In all, seven subjects (39%) experienced at least one study-related AE during the study, but there were no serious AEs. The AEs were procedure- and device-related, including pain, swelling and infection at the site of implantation, device pain and lead dislodgement. All but one AE were resolved by the end of the study, and there were no unexpected AEs, supporting the safety of 10-kHz SCS in this patient population.
The mean pain scores in the current study decreased by 82% after 3 months of stimulation, and these decreases were durable, with pain relief essentially unchanged after 12 months at 85%. These results were nearly identical to the 82% pain relief observed by Gupta et al. in 25 subjects with CPSP after 12 months of treatment with 10-kHz SCS [Citation28], and better than the 73% pain relief reported in a study of 10-kHz SCS in subjects with PPN. The responder rates after 12 months of stimulation in the current study were high (94%) and compared favorably with those previously published for 10-kHz SCS in subjects with CPSP (88%) [Citation28] and PPN (69%) [Citation14].
Pain outcomes assessed with the SF-MPQ-2 showed that baseline pain in this population was elevated in all four components of this questionnaire – namely, continuous pain, intermittent pain, neuropathic pain and affective descriptors of pain – and that all four domains benefited from treatment with 10-kHz SCS, as shown in A. Overall pain scores declined by 68% to 1.6 after 12 months in the current study, which was comparable to findings reported by Gupta et al. (79% decrease in overall pain) [Citation28].
The pain relief reported by the study subjects was accompanied by improvements in assessments of their general health and functioning. Both GAF and PDI scores showed rapid improvements following the initiation of 10-kHz SCS, and these improvements were maintained through 12 months of follow-up. The 27% reduction in GAF scores and 23-point improvement in PDI that we observed in the subjects also compares favorably with reductions previously reported in values for subjects with CPSP [Citation28]. Sleep quality, as assessed by the PSQ-3, showed durable improvement in all three questions, further reinforcing evidence of improved functioning and quality of life among these subjects.
In addition to relieving pain, 10-kHz SCS was effective at improving several outcomes related to mental health. The subjects’ baseline mean score on the PCS was 31.1; patients with PCS scores exceeding 30 have been shown to be at increased risk of being unemployed, of thinking of themselves as unable to work and of having moderate depression [Citation20]. However, after 12 months of stimulation, the mean PCS score was reduced by 17 points, and these reductions were principally due to improvements in the Helplessness and Rumination dimensions (B). Moreover, these reductions were similar to those reported previously for 10-kHz SCS treatment of CPSP [Citation28] and greater than the 7.6-point reduction in PCS reported in subjects with chronic intractable pain of the trunk and limbs using burst SCS, which has been noted for its effect on the emotional and affective aspects of pain [Citation29].
The baseline mean PVAQ score in the current subject sample (48.1) was similar to the value (47.5) reported in patients with chronic back pain [Citation21]; the PVAQ declined by 27% after 3 months of treatment with 10-kHz SCS, primarily due to a decline in the ‘Attention to Pain’ component (C). Although this decrease was less durable than other effects of high-frequency stimulation, the attention to pain score was still reduced by 19% after 12 months, a greater reduction in this component than the 7.6% reported with burst SCS in subjects with back pain [Citation30]. The SF-12, a generic health assessment tool, in contrast, showed little improvement in the mental component, despite a durable improvement in the physical component among these subjects.
Limitations
The primary limitations of this single-site, single-arm study are the possibility of bias in recruiting subjects and the lack of a control group. The lack of control subjects eliminates the possibility of making in-study comparisons between treatment groups. However, the chronic nature of the subjects’ pain provided some confidence that a spontaneous recovery was unlikely and that the observed improvements in outcomes were most likely due to the effects of the intervention. In addition, the consistency between our observations and the historical results cited above provides an additional measure of confidence in the current data.
Conclusion
There are few satisfactory treatment options for neuropathic CPSP. These results indicate that 10-kHz SCS may be a safe and effective nonpharmacological treatment option in patients with this condition. A high proportion of the current subjects responded to 10-kHz SCS with deep reductions in pain that was durable and not associated with any neurological deficits or unexpected AEs.
Future perspective
The results from this study could encourage further research on treating chronic post-surgical neuropathic pain using 10 kHz SCS therapy and document longer term real world efficacy and safety. As more data is available and efficacy and safety of the therapy is established, 10 kHz SCS could be considered as an option in the earlier stages of pain management.
Chronic postsurgical pain (CPSP) is a common complication of surgery. The current study was conducted to address the unmet need in the treatment of CPSP and evaluate the efficacy and safety of 10-kHz SCS for CPSP.
The study had a prospective, single-arm design, and subjects with a diagnosis of CPSP and an average pain intensity of ≥5 cm on a 10-cm visual analog scale were enrolled.
Subjects who had pain relief of ≥50% (response) during trial stimulation (2 weeks) with epidural leads were permanently implanted with a 10-kHz SCS system and were assessed at intervals of 3 months, 6 months and 1 year.
A total of 22 subjects were enrolled and trialled with the 10-kHz SCS system; 18 had at least 50% pain relief and received permanent implants.
At 12-month assessment, 94% of subjects achieved ≥50% pain relief (response) and 88% achieved visual analog scale scores ≤2.5 cm (remission).
Pain decreased in all four components of the McGill Pain Questionnaire, including affective descriptors and measures of subjects’ disability and mental state; the Pain Catastrophizing Scale, the Pain Disability Index and the Global Assessment of Functioning improved at 12-month assessment.
The safety of 10-kHz SCS was as expected, and no unanticipated adverse events were reported during the study.
In conclusion, 10-kHz SCS may be a safe and effective treatment option for CPSP patients.
Author contributions
B Billet was responsible for conception and design planning, conduct, data analysis and interpretation. K Hanssens and O De Coster were involved in conducting the study, reporting, acquisition of data and interpretation of data. V Minne, A Santos and A Rotte were involved in acquisition of data, interpretation, preparing the draft outline and working with the external medical writer in drafting the manuscript. All authors have reviewed and approved the final manuscript.
Ethical conduct of research
The study plan, protocols, informed consent forms and all amendments were reviewed and approved by the AZ Delta Ziekenhuis Roeselare-Menen Ethics Committee, and the study was compliant with all relevant recommendations of the 18th World Medical Assembly in Helsinki, Finland, for physicians involved in biomedical research. Participants of the study signed informed consent forms prior to enrollment. Participants of the study signed an informed consent form to publish deidentified data.
Financial & competing interests disclosure
The study was funded by Nevro Corp. B Billet is a consultant for Nevro Corp. V Minne, A Santos and A Rotte are employees of Nevro Corp. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors wish to thank E MacLaren, Galen Medical Writing, LLC for drafting the manuscript and M Bhandaru for assistance in preparation of illustrations. Nevro Corp. provided funding for the writing assistance.
Data sharing statement
Clinical trial registration number: VT005076953. All the relevant data is included in the publication. Individual data cannot be shared due to privacy concerns.
Additional information
Funding
References
- Treede RD , RiefW , BarkeAet al. A classification of chronic pain for ICD-11. Pain156(6), 1003–1007 (2015).
- Kehlet H , JensenTS , WoolfCJ. Persistent postsurgical pain: risk factors and prevention. Lancet367(9522), 1618–1625 (2006).
- Macrae WA . Chronic post-surgical pain: 10 years on. Br. J. Anaesth.101(1), 77–86 (2008).
- Haroutiunian S , NikolajsenL , FinnerupNB , JensenTS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain154(1), 95–102 (2013).
- Jensen MP , ChodroffMJ , DworkinRH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology68(15), 1178–1182 (2007).
- Wylde V , DennisJ , BeswickADet al. Systematic review of management of chronic pain after surgery. Br. J. Surg.104(10), 1293–1306 (2017).
- Slavin KV . Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics5(1), 100–106 (2008).
- Broggi G , ServelloD , DonesI , CarboneG. Italian multicentric study on pain treatment with epidural spinal cord stimulation. Stereotact. Funct. Neurosurg.62(1–4), 273–278 (1994).
- Yakovlev AE , AlTamimi M , BarolatGet al. Spinal cord stimulation as alternative treatment for chronic post-herniorrhaphy pain. Neuromodulation13(4), 288–290 (2010).
- Kapural L , YuC , DoustMWet al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology123(4), 851–860 (2015).
- Baranidharan G , EdgarD , BrethertonBet al. Efficacy and safety of 10kHz spinal cord stimulation for the treatment of chronic pain: a systematic review and narrative synthesis of real-world retrospective studies. Biomedicines9(2), 180 (2021).
- Francio VT , PolstonKF , MurphyMT , HagedornJ , SayedD. Management of chronic and neuropathic pain with 10kHz spinal cord stimulation technology: summary of findings from preclinical and clinical studies. Biomedicines9(6), 644 (2021).
- Lee KY , LeeD , KaganZB , WangD , BradleyK. Differential modulation of dorsal horn neurons by various spinal cord stimulation strategies. Biomedicines9(5), 568 (2021).
- Galan V , ScowcroftJ , ChangPet al. 10-kHz spinal cord stimulation treatment for painful diabetic neuropathy: results from post-hoc analysis of the SENZA-PPN study. Pain Manag.10(5), 291–300 (2020).
- Gupta M , KnezevicNN , Abd-ElsayedA , RayM , PatelK , ChowdhuryB. Treatment of painful diabetic neuropathy –a narrative review of pharmacological and interventional approaches. Biomedicines9(5), 573 (2021).
- Al-Kaisy A , Van BuytenJP , SmetI , PalmisaniS , PangD , SmithT. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med.15(3), 347–354 (2014).
- Stauss T , ElMajdoub F , SayedDet al. A multicenter real-world review of 10-kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann. Clin. Transl. Neurol.6(3), 496–507 (2019).
- Bouhassira D , AttalN , AlchaarHet al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain114(1–2), 29–36 (2005).
- Dworkin RH , TurkDC , RevickiDAet al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain144(1–2), 35–42 (2009).
- Sullivan MJ . The Pain Catastrophizing Scale (2009). https://sullivan-painresearch.mcgill.ca/pdf/pcs/PCSManual_English.pdf
- Mccracken LM . ‘Attention’ to pain in persons with chronic pain: a behavioral approach. Behav. Ther.28(2), 271–284 (1997).
- Chibnall JT , TaitRC. The Pain Disability Index: factor structure and normative data. Arch. Phys. Med. Rehabil.75(10), 1082–1086 (1994).
- Ayearst L , HarsanyiZ , MichalkoKJ. The Pain and Sleep Questionnaire three-item index (PSQ-3): a reliable and valid measure of the impact of pain on sleep in chronic nonmalignant pain of various etiologies. Pain Res. Manag.17(4), 281–290 (2012).
- Jenkinson C , LayteR , JenkinsonDet al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health Med. 19(2), 179–186 (1997).
- Aas IH . Global Assessment of Functioning (GAF): properties and frontier of current knowledge. Ann. Gen. Psychiatry9, 20 (2010).
- Farrar JT , YoungJPJr , LamoreauxL , WerthJL , PooleRM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain94(2), 149–158 (2001).
- Lee KY , BaeC , LeeDet al. Low-intensity, kilohertz-frequency spinal cord stimulation differently affects excitatory and inhibitory neurons in the rodent superficial dorsal horn. Neuroscience428, 132–139 (2020).
- Gupta M , ScowcroftJ , KlosterDet al. 10-kHz SCS for chronic postsurgical pain: results from a 12-month prospective, multicenter study. Pain Pract.20(8), 908–918 (2020).
- Courtney P , EspinetA , MitchellBet al. Improved pain relief with burst spinal cord stimulation for two weeks in patients using tonic stimulation: results from a small clinical study. Neuromodulation18(5), 361–366 (2015).
- De Ridder D , PlazierM , KamerlingN , MenovskyT , VannesteS. Burst spinal cord stimulation for limb and back pain. World Neurosurg.80(5), 642–649e641 (2013).
