Abstract
Aim: Comparison of tapentadol prolonged release (PR) with other oral WHO-III PR opioid analgesics (morphine, oxycodone ± naloxone, hydromorphone) in routine medical care of chronic low back pain. Patients & methods: Noninterventional, retrospective 12-week study using anonymized clinical practice data from the German Pain eRegistry. Six effectiveness, tolerability, and safety criteria were aggregated in a primary composite end point (treatment responder). Propensity scoring matched 2331 datasets per treatment cohort. Results: All six single criteria showed significantly better outcomes for tapentadol PR (all parameters p < 0.001). There were significantly more treatment responders under tapentadol PR (65.7 vs 14.2%; p < 0.001). Conclusion: Tapentadol PR showed significantly better effectiveness and tolerability in severe chronic low back pain unsuccessfully treated with WHO-I/II analgesics compared with the other oral WHO-III PR opioids investigated.
Lay abstract
Chronic low back pain is a common condition often resulting in impaired functioning in daily life and reduced quality of life and well-being of the patient. In case treatment with less potent pain medications is unsuccessful, opioid treatment might be required. Our study compared the effectiveness and tolerability of the prolonged release formulation of the atypical opioid tapentadol with other strong opioids commonly used for chronic pain treatment in Germany (morphine, hydromorphone, oxycodone ± naloxone). Anonymized patient data from German clinical practices collected in a pain registry were used (2331 comparable patients per treatment group). Patients receiving 12 weeks of tapentadol treatment experienced significantly greater pain relief, greater improvements in daily living activities, sleep, and quality of life compared with those receiving the other strong opioids investigated. Neuropathic pain components (pain features resembling nerve pain, often described as shooting, burning or stabbing pain) were reduced to a greater extent in the tapentadol treatment group. Tapentadol was also significantly better tolerated. This study showed that tapentadol is effective and well tolerated in chronic low back pain treatment in routine medical practice in patients still in considerable pain despite treatment with less potent pain medications.
Low back pain (LBP) is the leading cause of disability worldwide [Citation1] and is associated with a substantial individual and societal burden [Citation2]. Prevalence and burden of the condition increase with age [Citation2]. For the majority of patients, LBP does not have a specific cause [Citation2], and progression to a chronic state is common [Citation3]. Besides substantial restrictions in daily life activities due to pain and disability, chronic LBP (cLBP) is often associated with co-morbidities such as depression, panic and anxiety disorders, and sleep disturbances [Citation4], and quality of life can be markedly reduced [Citation5]. Frequently, both nociceptive and neuropathic pain mechanisms are involved (mixed pain); the latter component is more difficult to diagnose and challenging to treat [Citation6].
Treatment of cLBP is mostly symptomatic and aims to reduce pain, improve function and prevent worsening of the condition [Citation7]. A multimodal approach with pharmacological and nonpharmacological treatments is typically advised [Citation6]. A recent overview of clinical practice guidelines for the management in primary care found that most of the guidelines for cLBP recommended exercise therapy, psychosocial interventions, and if required, the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and antidepressants [Citation8]. Opioids should only be initiated if less potent analgesics and adjuvant treatments are unsuccessful [Citation9]. Patients should be carefully selected, abuse/misuse risk factors should be evaluated and regular monitoring/follow-up should be implemented. When prescribing opioids, the broad range of side effects of this medication class also needs to be considered, which might influence treatment adherence potentially resulting in insufficient analgesia or cessation of treatment [Citation10]. The GI tract, for example, is a significant site of opioid-induced side effects, summarized under the term ‘opioid-induced bowel dysfunction’ with opioid-induced constipation as the most common and debilitating symptom [Citation11,Citation12]; GI adverse events (AEs) are also a major reason for treatment discontinuation [Citation13].
The atypical opioid tapentadol combines the two mechanisms of action μ-opioid receptor agonism and noradrenaline reuptake inhibition in one molecule (dual mechanism of action). These mechanisms synergistically provide strong analgesia in a broad range of chronic nociceptive and neuropathic pain conditions; the medication is generally well tolerated [Citation14,Citation15]. In clinical trials, the prolonged release (PR) tapentadol formulation reduced pain intensity and improved health-related quality of life in the treatment of moderate or severe cLBP with or without a neuropathic component, with better gastrointestinal tolerability compared with oxycodone controlled release or oxycodone/naloxone PR [Citation16–20]. Network meta-analyses also demonstrated the tolerability advantages versus other classical strong opioids, for example, hydromorphone or morphine [Citation21]. Furthermore, long-term treatment was effective and well tolerated [Citation22–26].
Randomized controlled trials are the gold standard for evidence of treatment efficacy [Citation27], but this trial design has narrowly defined criteria to achieve homogenous trial populations and thus cannot necessarily be generalized to the entire patient population treated in a real-life practice setting. Medication use in controlled trial situations might also differ from the use during routine medical care. Real-world data collected from electronic health records, health insurance claims data bases, prescription data or patient registries include the diverse patient populations seen in routine clinical practice and can provide supportive evidence for benefit/risk evaluations from traditional clinical trials [Citation27,Citation28]. The German Pain eRegistry (GPeR), a national web-based pain registry developed on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin), for example collects all data deemed necessary for the comprehensive routine medical care of pain patients (demographic characteristics, medical history, previous treatment and all pain treatment-related data) and serves to facilitate information flow between patient and physician. Data are entered via the online documentation service iDocLive® (predominantly by patients) and are reviewed together with the treating physician at practice visits. Data from such a registry allow insight into the pain management of a broad spectrum of patients under routine care and therefore are a valuable addition serving to complement controlled trials.
The present noninterventional retrospective cohort study analyzed anonymized real-world clinical practice data collected in the GPeR in order to investigate the effectiveness and tolerability of tapentadol PR in comparison to other oral WHO-III PR opioid analgesics (morphine, oxycodone ± naloxone, hydromorphone) under routine medical care in cLBP patients unsuccessfully treated with WHO-I/II analgesics.
Patients & methods
This exploratory, retrospective, noninterventional, 12-week parallel cohort study compared the effectiveness and tolerability of tapentadol PR with oral WHO-III PR opioid analgesics (defined under ‘medication under evaluation’) in adult cLBP patients unsuccessfully treated with WHO-I/II analgesics and with pain severe enough to be in need of treatment with a strong opioid analgesic. The study used anonymized data collected in the GPeR; core parameters of the system are based on patient questionnaires for pain documentation, the German Pain Questionnaire and the German Pain Diary [Citation29] both recommended by the German Pain Association, the German Pain Society and the German Pain League. These instruments include a broad spectrum of validated questionnaires for the assessment of intensity, severity, phenomenology and chronification stage of pain, pain-related impairments of daily life, health-related quality of life, overall well-being, depression, anxiety and stress and also data on pain treatment and treatment-related AEs.
According to GPeR standard operating procedures, all data extracted for the purpose of a specific biometrical analysis have to be deleted after completion of the analyses specified in the respective statistical analysis plan.
Data selection
There was no formal sample size calculation for this analysis. All datasets of patients with newly initiated treatment with one of the medications under evaluation before 31 December 2019 were selected from the GPeR database, according to defined inclusion and exclusion criteria. Inclusion criteria were a cLBP diagnosis (ICD-10 M40-54; patients also marked their pain location on a body scheme) of at least 3 months between 1 January 2015 and 31 December 2019, newly initiated treatment with tapentadol PR or another oral PR WHO-III analgesic chosen by the treating physician based solely on individual patient needs, and data documentation over the 12-week observation period. Physicians assessed pain severity mainly using the parameters pain intensity, pain-related impairments and distress. Data of patients discontinuing treatment during the observation period were retained in the analysis and missing outcomes were imputed (details can be found under statistical analysis). Exclusion criteria were a current cancer diagnosis, previous spine surgery and a switch from another WHO-III opioid analgesic (i.e., patients on WHO-III opioid rotation). Treatment initiation was defined as no use of WHO-III analgesics in the 12 weeks prior to treatment; the date of first treatment dose was set as the starting date for the 12-week observation period.
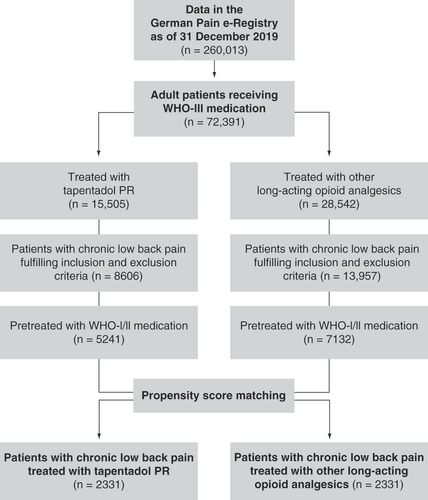
The data selection process is shown in . To obtain comparable study cohorts, a matched pair approach using a propensity score model was chosen. Treatment status was regressed on the observed baseline characteristics age, sex, bowel function index (BFI), average 24-h pain intensity index (PIX), duration of current pain symptoms, co-morbidities, and previous analgesic treatment using the nearest neighbor method without replacement (caliper 0.15). Propensity score matching resulted in datasets of 2331 patients for each treatment group.
Medications under evaluation & concomitant analgesics
Medications under evaluation were tapentadol PR (TAP PR cohort) versus the oral PR formulations of the opioids morphine, oxycodone, oxycodone/naloxone and hydromorphone, the classical oral WHO-III PR opioids prescribed in Germany (WHO-III PR cohort). In the following, the two cohorts are referred to as TAP PR and WHO-III PR. All treatment decisions such as selection of analgesic medication and concomitant analgesics, initial dosing and dose adjustments were at the discretion of the treating physician and were solely based on individual patient needs.
Outcome measures
Outcome measures included pain intensity, pain-related impairments of daily living, health-related quality of life, pain phenomenology, the occurrence of opioid-induced constipation, the occurrence of drug-related adverse events (DRAEs), treatment discontinuation due to DRAEs, use of concomitant analgesics and pain-related sick leave.
Pain intensity was rated on a 100-mm visual analogue scale (VAS) with 0 = no pain to 100 = worst imaginable pain. A PIX was calculated as the arithmetic mean of the lowest, average and highest 24-h pain intensities. Pain-related impairments in daily activities related to ‘home and family,’ ‘recreation,’ ‘social activities,’ ‘occupation,’ ‘self-care/personal maintenance,’ ‘sleep’ and ‘overall quality of life’ were reported using a modified version of the original pain disability index [Citation30] (mPDI) on a 100-mm VAS (0 = none to 100 = worst imaginable) to harmonize the different instrument scales. Health-related quality of life (from here on termed quality of life) was assessed with the eight physical and mental domains of the Short Form 12 (SF-12) questionnaire [Citation31] and summarized in a physical and a mental component score (PCS/MCS). Pain phenomenology and related pain type categorization were carried out using a patient-based version of the painDETECT questionnaire (PDQ), originally developed by Freynhagen et al. [Citation32]. This version includes the seven sensory symptom items (PDQ7) [Citation33]. Each question was rated from 0 = never to 5 = very strongly. A sum score of 0–10 indicates that a neuropathic pain component is unlikely (negative), of more than 10 to less than 18 that a neuropathic component might be present (unclear), and of ≥18 that a neuropathic component is likely present (positive). Opioid-induced bowel dysfunction was evaluated using the validated BFI with the three items ‘ease of defecation,’ ‘feeling of incomplete bowel evacuation’ and ‘personal judgment of constipation’ on a VAS from 0 = freedom from symptom to 100 = maximum difficulty or most severe symptom [Citation34,Citation35]. The BFI score is calculated as the mean of the three item scores. The defined reference range is 0–28.8 [Citation35]. Pain-related sick leave at the end of observation was compared with baseline using the respective parameter from the von Korff questionnaire (number of sick leave days within the last 3 months) [Citation36].
The primary end point was the proportion of treatment responders at the end of the 12-week treatment period and included six criteria aggregated into three dimensions. A patient was defined as a treatment responder, if the following criteria were fulfilled:
No relevant deterioration of bowel function of ≥the minimal clinically important difference (MCID) from baseline: worsening of ≥12 mm VAS [Citation34].
At least two of the following:
○ Reduction of pain intensity ≥ MCID from baseline measured by the PIX: decrease by ≥20 mm VAS [Citation37].
○ Reduction of pain-related impairments in daily life ≥ MCID from baseline: decrease by ≥20 mm VAS [Citation29].
○ Improvement in physical quality of life ≥ MCID from baseline based on SF-12: increase in PCS >3.77 [Citation38].
○ Improvement in mental quality of life ≥ MCID from baseline based on SF-12: increase in MCS >3.29 [Citation38].
No discontinuation of the prescribed study medication during the observation period due to DRAEs.
As in clinical trials, in routine clinical practice a treatment also has to be i) safe (i.e., no occurrence of DRAEs, which might lead to treatment discontinuation), ii) well tolerated (i.e., no BFI worsening) and iii) effective (i.e., lead to a clinically important improvement of pain intensity, pain-related functional disabilities and/or physical/mental quality of life). But in contrast to clinical trials, in daily clinical practice, it is rather irrelevant which of the three aspects lead to treatment failure. This study therefore combined the three dimensions in one composite primary end point.
Further investigated outcomes were changes from baseline at the end of observation in pain-related sleep impairments assessed by the mPDI subscale 6 (MCID: decrease by ≥20 mm VAS), in pain phenomenology, in concomitant analgesics and in pain-related sick leave.
Statistical analysis
All analyses were conducted using PASW Statistics version 18 (Copyright IBM Corporation, NY, USA). Any data of patients with a record of at least one dose of the relevant treatment under evaluation and for whom at least one post-baseline measure had been documented were included.
Missing data were imputed using baseline observation carried forward (BOCF)/last observation carried forward (LOCF) approaches. A BOCF analysis was performed for datasets of patients discontinuing treatment due to a DRAE, death, or lack of efficacy for all analyzed variables except BFI where LOCF was used. LOCF was used for the latter as bowel function issues might have been the reason for discontinuation, which would have not been reflected in a BOCF approach. LOCF imputations were also carried out for any data missing at random or due to treatment discontinuation when pain treatment was no longer required. Sensitivity analyses included the different imputation methods pure BOCF, pure LOCF, mean observation carried forward, best observation carried forward and worst observation carried forward as well as no imputation data (as observed), and a 12-week completers analysis.
Mean data are presented with standard deviation or 95% CI. For statistical comparison of the two study cohorts, McNemar’s test, Pearson’s Chi-square test, paired sample t-test or Wilcoxon’s signed rank test were performed using a two-sided significance level of 0.05. As all comparisons were exploratory, significance levels were not adjusted for multiplicity.
AEs were encoded using the Medical Dictionary for Regulatory Activities (MedDRA version 22.0, 2019). A DRAE was defined as an AE assessed by the treating physician as possibly, probably or definitely related to the medication under evaluation.
Results
Patients
The registry contained data of 260,013 patients at cutoff date (31 December 2019); 15,505 and 28,542 patients had been treated with TAP PR and WHO-III PR, respectively (). Propensity score matching resulted in the selection of datasets of 2331 cLBP patients per treatment group; 275 patients in each group had received WHO-I and 2056 patients both WHO-I and WHO-II pretreatment. Patients in the WHO-III PR cohort were treated with oxycodone (40.8%), morphine (23.5%), hydromorphone (19.2%) or oxycodone/naloxone (16.5%). Baseline data for the matched cohorts are shown in . A third of all patients had suffered from cLBP for more than a year; patients had visited a median of five physicians, mainly general practitioners (tapentadol PR: 91.5%/WHO-III PR: 91.8%), for pain treatment.
Table 1. Baseline characteristics (n = 2331 in each group).
Analgesic treatment
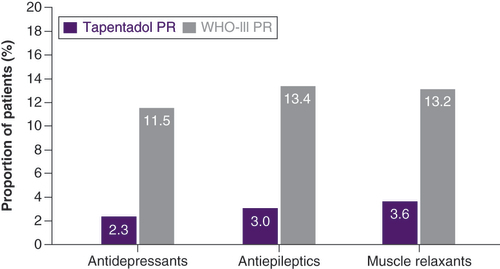
The majority of patients in both cohorts had received WHO-I and WHO-II analgesics before initiation of tapentadol PR or another oral WHO-III PR analgesic; 34.4% also took antidepressants and 21.6% received antiepileptics, two medication classes typically used for neuropathic pain symptoms (). The main reason for initiation of the opioid treatment under evaluation was insufficient effectiveness of previous medications (64.4% of patients in both groups). There were also no relevant differences between the groups for all additional reasons. These included reasons such as insufficient tolerability of previous treatment, insufficient balance between effectiveness and tolerability, drug interactions with concomitant medication, lack of compliance, and insufficient quality of life (reflecting the patient’s situation due to pain, pain-related impairments or side effects).
Table 2. Previous and concomitant analgesic medications (n = 2331 in each group).
Patients in the tapentadol PR cohort received a mean daily dose of 33.8 ± 10.8 mg morphine equivalent dose (MED) (95% CI: 33.3, 34.3) at treatment initiation, which increased over the 12-week observation period to a mean 100.5 ± 36 mg MED (98.8, 102.2; corresponding to a mean 251.3 mg/day tapentadol PR). For the WHO-III PR cohort, a mean daily dose of 28.2 ± 10.2 mg MED (27.7, 28.7) at treatment initiation and a mean 105 ± 38.2 mg MED (103.2, 106.8) at end of observation were documented. Mean treatment duration was 72.3 ± 22.4 days for tapentadol PR patients and 64.7 ± 26.3 days for the WHO-III PR cohort. The majority of patients (75.2% TAP PR and 61% WHO-III PR) received treatment over the entire 12-week evaluation period; 24.8 versus 39% of patients discontinued treatment before the end of observation for the following reasons: treatment with strong opioids no longer required (9.4 vs 3.7%), ineffectiveness of treatment (8.1 vs 12.3%) or occurrence of DRAEs (7.4 vs 22.9%). Of the patients who received 12 weeks of treatment (TAP PR n = 1752, WHO-III PR n = 1423), 50.7 versus 24.2% did not continue with the medication under evaluation because they reached their individual treatment target (21.5 vs 5.2%), owing to tolerability issues (3.1 vs 12.5%) or owing to other reasons (6.6 vs 2.6%). No reason was given by 19.6 versus 3.9% of the patients.
At treatment initiation (baseline), concomitant WHO-I and WHO-II medications were documented for 100 and 88.2% of all patients, respectively (). Administration of these medication classes had decreased in both treatment groups at end of observation but to a significantly higher extent in tapentadol PR patients compared with the WHO-III PR cohort (p < 0.001): WHO-I medication was still received by 47.3% of tapentadol patients and 71.1% of patients in the WHO-III PR cohort and WHO-II medication by 6.3 and 31.2% of patients, respectively. The use of antiepileptics (TAP PR 8.3% vs WHO-III PR 23%), antidepressants (11.8 vs 28.3%) and muscle relaxants (20.5 vs 32.2%) was also significantly reduced (p < 0.001) after 12-treatment weeks in the tapentadol PR cohort compared with the WHO-III PR cohort; use of antiepileptics and muscle relaxants even slightly increased in the WHO-III PR cohort (). Initiation of these adjuvant analgesics during the observation period was also less frequent in the tapentadol cohort ().
Main effectiveness parameters
Average 24-h pain intensity was reduced in both treatment groups over the observation period but to a significantly greater extent in tapentadol PR patients (p < 0.001; A). Greater improvements with tapentadol PR were also observed for the lowest and highest 24-h pain intensities and the 24-h PIX (p < 0.001; ). Significantly more tapentadol PR patients attained their individual pain treatment target (p < 0.001; 63.1 vs 49.8% in the WHO-III PR cohort).
*p < 0.001 in favor of tapentadol PR.
BL: Baseline; mPDI: Modified pain disability index; PR: Prolonged release; SF: Short form; VAS: Visual analogue scale; W: Week.
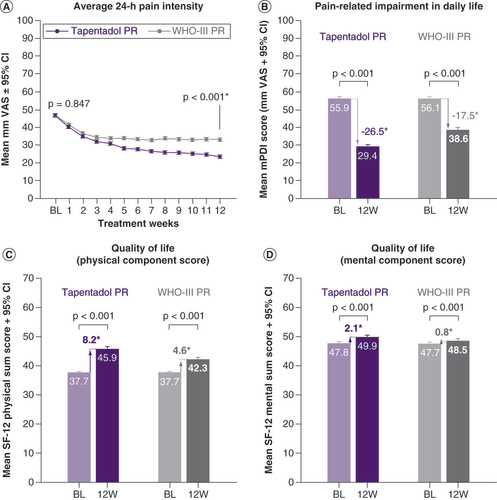
Table 3. Mean changes in pain intensity over the evaluation period (n = 2331 in each group).
Patients reported considerable impairments in daily life activities at baseline, which improved under both treatments over the observation period (p < 0.001 in favor of tapentadol PR; B). A relative improvement ≥50% versus baseline in the mPDI sum score was documented for significantly more tapentadol PR patients (52.6 vs 32.1%; p < 0.001). The proportion of patients showing improvements ≥MCID after 12 treatment weeks was significantly greater under TAP PR than under WHO-III PR treatment (58 vs 38.6%; p < 0.001).
Quality of life with respect to the physical components was markedly affected at baseline but had improved in both cohorts at end of observation with a significant treatment difference in favor of tapentadol PR (p < 0.001; C). Mental component score of quality of life only slightly changed over the 12-week treatment period but to a significantly greater extent in the tapentadol PR cohort (p < 0.001; D).
Further outcome parameters
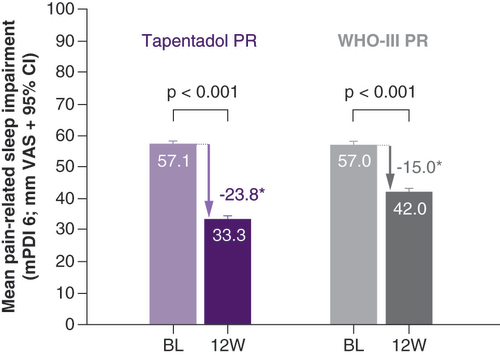
Patients experienced marked impairments in sleep at baseline, which improved in both treatment groups over the evaluation period (). Improvements were significantly higher under tapentadol treatment (p < 0.001). A total of 50.1% of tapentadol PR and 35.7% of WHO-III PR patients had an absolute reduction in sleep impairment of ≥MCID (p < 0.001 in favor of tapentadol PR).
*p < 0.001 in favor of tapentadol PR.
mPDI: Modified pain disability index; PR: Prolonged release; VAS: Visual analogue scale; W: Week.
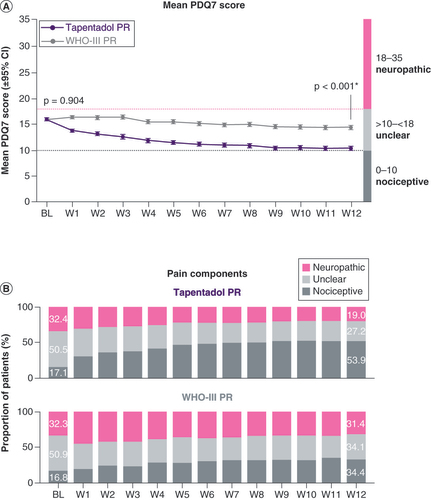
Mean PDQ7 scores had decreased from 15.8 ± 5.4 (95% CI: 15.5, 16.1) in the tapentadol PR group and 15.8 ± 5.3 (15.6, 16.0) in the WHO-III PR cohort at baseline to 10.2 ± 7.8 (9.8, 10.6) and 14.2 ± 8.2 (13.8, 14.6), respectively, at end of week 12 (p < 0.001 in favor of tapentadol PR; A). At baseline, the proportion of patients with predominantly nociceptive pain was low (17.1% of tapentadol PR patients, 16.8% of WHO-III PR patients). A neuropathic component was likely for 32.4 and 32.3% of the patients, respectively, and could not be excluded for a further 50.5 and 50.9%. Over the evaluation period, the proportion of tapentadol PR patients likely to have a neuropathic pain component decreased to 19%, whereas there were only slight changes in the WHO-III PR cohort (31.4%; B).
(A) Mean PDQ7 score. (B) Pain components.
*p < 0.001 in favor of tapentadol PR.
BL: Baseline; PDQ: PainDETECT questionnaire; PR: Prolonged release; W: Week.
Pain-related sick leave was reduced in both treatment groups from 32.5 ± 21.2 to 21.7 ± 20.1 days under tapentadol PR and from 32.5 ± 20.2 to 26.1 ± 20.4 days in the WHO-III PR cohort (range: 0–72 days for all data). The reduction was significantly higher under tapentadol PR treatment (p < 0.001).
Tolerability
A total of 35.6% of all patients reported BFI scores beyond the reference range (0–28.8) at baseline. During the 12-week tapentadol PR treatment, the mean BFI remained within the reference range, in other words, the majority of the patients did not show a relevant indication of OIC (). In contrast, bowel function scores markedly worsened in the WHO-III PR cohort. Overall, 76.4% of tapentadol PR patients had a BFI score within the (normal) reference range at end of week 12, whereas 73.7% of patients in the WHO-III PR cohort had scores above the reference range (p < 0.001). At baseline, 27.6 and 27.5% of patients were prescribed laxatives, respectively. This proportion declined to 11.9% in the tapentadol PR group over the 12-week evaluation period but increased to 66.8% in the WHO-III PR cohort (p < 0.001 in favor of tapentadol PR).
A BFI reference range of 0–28.8 indicates normal bowel function.
BFI: Bowel function index; BL: Baseline; PR: Prolonged release; VAS: Visual analogue scale; W: Week.
Reproduced with permission from the International Association for the Study of Pain [Citation40].
![Figure 6. Change in bowel function over the observation period.A BFI reference range of 0–28.8 indicates normal bowel function.BFI: Bowel function index; BL: Baseline; PR: Prolonged release; VAS: Visual analogue scale; W: Week.Reproduced with permission from the International Association for the Study of Pain [Citation40].](/cms/asset/603c1ecc-48fc-41f0-bc80-27a8c0d96ecd/ipmt_a_12344475_f0006.jpg)
DRAEs were significantly less frequent in the tapentadol group (13 vs 36.9% of patients in the WHO-III PR cohort, p < 0.001). In both groups, they consisted mainly of gastrointestinal disorders (4.7 vs 26.9%) and nervous system disorders (11.3 vs 13.7%). Fatigue and headache were the most common DRAEs in the tapentadol PR group, constipation and nausea in the WHO-III PR cohort (). Most DRAEs were mild or moderate in intensity, severe DRAEs were noted for 4.7% of tapentadol PR and 8% of WHO-III PR patients. Treatment discontinuation due to DRAEs occurred in 7.4% of patients under tapentadol PR and in 22.9% of patients in the WHO-III PR cohort.
Table 4. Drug-related adverse events during the 12-week observation period (n = 2331 in each group).
Responder analysis
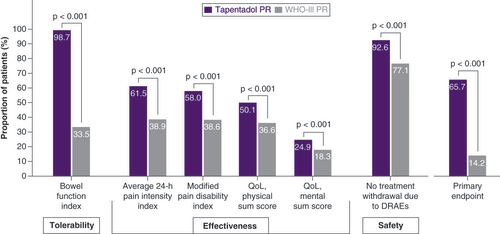
The responses according to the six single criteria for the responder analysis were significantly better for tapentadol PR than for WHO-III PR patients (p < 0.001 for all criteria; ). The primary study end point was attained by significantly more tapentadol PR patients (65.7 vs 14.2%; p < 0.001; ), resulting in an odds ratio for treatment response of 11.56 (95% CI: 10.0, 13.4) and a relative risk of 2.88 (95% CI: 2.59, 3.2; based on the proportion of responders in both cohorts). A total of 8.4% of patients in the tapentadol PR group and 0.5% of patients in the WHO-III PR cohort fulfilled all six criteria; no response to any of the six criteria was only observed in the WHO-III PR cohort (16.6%).
The primary end point included the three dimensions: tolerability, effectiveness and safety. A treatment responder had to fulfil the tolerability criterion and the safety criterion plus at least two criteria from the dimension effectiveness.
DRAE: Drug-related adverse event; PR: Prolonged release; QoL: Quality of life.
The broad range of sensitivity analyses employed showed that the conclusions were robust to different imputation methods (analyses available upon request).
Discussion
In routine clinical practice, the usefulness of a drug in the treatment of chronic pain relies on its safety, tolerability and effectiveness. Treatment with a potent analgesic is not worth continuing, if its side effects interfere significantly with patients’ well-being or force them to stop treatment, and if it does not show clinically relevant pain relief or improvement of pain-related functional disabilities with respect to daily life activities or quality of life. All the above points are reflected in the primary end point of our study, which only considered a patient a treatment responder if safety, tolerability, and effectiveness criteria were met.
In the present study, effective pain relief accompanied by improvements in daily living activities and sleep was observed in cLBP patients treated with tapentadol PR; use of concomitant analgesics could be reduced and quality of life of the patients improved. The reduction in pain-related impairments under tapentadol PR treatment also led to a decline in pain-related sick leave. Two thirds of the tapentadol PR patients were considered treatment responders. When these outcomes were compared with matched pairs of patients treated with classical oral WHO-III PR opioid analgesics, all six single criteria included in the composite primary end point showed a statistically significantly better outcome in favor of tapentadol PR. Furthermore, a more effective overall treatment response was observed: compared with 66% of responders in the tapentadol PR cohort, only 14% of patients in the WHO-III PR cohort fulfilled the relevant criteria.
cLBP was considered as mixed pain, in other words, involving both nociceptive and neuropathic pain mechanisms in the majority of the patients in our study. A neuropathic pain component could not be excluded/was likely for more than 80% of patients in both groups. This is in line with an epidemiological survey in Germany of approx. 8000 cLBP patients: a neuropathic pain component could not be excluded/was likely for 77% of patients suffering from severe pain [Citation32]. The survey found that patients with a neuropathic pain component had higher pain intensity ratings, more frequent and more severe co-morbidities such as depression, panic/anxiety disorders and sleep disorders, and more severe limitations in daily functioning [Citation32]. Management of pain with a neuropathic component is challenging, and clinically meaningful analgesia is often not achieved [Citation6]. Clinical trials did, however, demonstrate effectiveness of tapentadol PR in cLBP both with and without a neuropathic component [Citation17–19]. In the current analysis, the proportion of patients in whom a neuropathic pain component was detected or could not be excluded decreased in the tapentadol PR cohort over the observation period and was smaller (46%) than in the WHO-III PR cohort (66%). This is further supported by the observation that use of adjuvant analgesics for neuropathic pain such as antidepressants and antiepileptics was considerably reduced under the 12-week tapentadol treatment (antidepressants from 34.4 to 11.8% of patients and antiepileptics from 21.6 to 8.3%). In contrast, antidepressant use only slightly declined in the WHO-III PR cohort, the proportion of patients using antiepileptics and muscle relaxants slightly increased, and more than 10% of the patients were initiated with these medications during the observation period. The reason why treatment with the newly administered antidepressants and antiepileptics was not effective is not fully understood. One must, however, consider that the time of initiation of these medications was not investigated and that initiation most likely occurred after titration of the index medication. Considering the slow titration and long time to onset of action of these drugs, an effect might not have been observed in the observational period.
The overall higher use of additional analgesic medications, in particular analgesics for neuropathic pain, in the WHO-III PR cohort in combination with the lower pain relief and smaller improvements in pain-related functioning and sleep seem to indicate that the WHO-III PR opioids investigated do not address the neuropathic component of LBP as well as tapentadol PR in a real-world setting. This is supported by the findings of clinical trials [Citation18,Citation19] and is thought to be due to the dual mechanism of action of the tapentadol molecule, whereby the descending noradrenergic modulation activity contributes to its analgesic action on neuropathic pain components [Citation41,Citation42]. Considering that a neuropathic pain component cannot be excluded for the majority of cLBP patients, tapentadol PR might be a more suitable treatment than the WHO-III PR opioid analgesics investigated.
cLBP patients may suffer severe restrictions in daily living activities owing to pain and impairment in functioning, which might impact considerably on their well-being and quality of life. When questioned about their treatment targets, less than 5% of the patients in a chronic pain survey stated complete pain relief as their individual treatment goal [Citation43]. Improvement of functioning, the ability to perform activities of daily living, and individual autonomy were rated as far more important. This is not surprising as all these goals in conjunction with a reduction in pain and good tolerability of the pain medication allow independence and participation in social and work life. A dialogue between physician and patient regarding individual treatment targets might result in more individualized pain treatment, better outcomes, and a more realistic expectation of the patient with regard to pain relief. This might save from frustration and in turn lead to better treatment compliance.
Another advantage of the dual mechanism of action of the tapentadol molecule is the favorable gastrointestinal tolerability profile it confers. The synergistic contribution of the noradrenaline reuptake inhibition mechanism to the analgesic effect allows for a reduced μ-opioid load [Citation44], which is reflected in significantly lower incidences of the opioid-typical side effects nausea, vomiting, and constipation as shown in randomized controlled trials [Citation15]. In the present study, the mean BFI was within reference range before initiation of treatment with a strong opioid analgesic, in other words, the majority of patients did not show abnormal stool behavior. Treatment in the WHO-III PR cohort resulted in a gradual worsening of the mean BFI score over the evaluation period. After 12 weeks of treatment, 74% of patients in the WHO-III PR cohort were above the reference range of a normal bowel function due to opioid-induced bowel dysfunction. This was accompanied by a marked increase in the number of patients receiving prescriptions for laxatives (67% of patients at end of observation). In contrast, only 24% of tapentadol PR patients were above the BFI reference range after 12 treatment weeks and laxative prescriptions were low (12% of patients). DRAEs were generally less frequent in the tapentadol PR cohort (13 vs 36.9%) and treatment withdrawal due to DRAEs occurred considerably less often compared with the WHO-III PR cohort (7 vs 23%). However, DRAE numbers need to be evaluated with care. In the experience of the authors, DRAEs occurring in the real-world setting are not always as thoroughly documented as adverse events in interventional clinical trials, which might also be true for documentation in the pain registry. Overall, the good gastrointestinal tolerability of tapentadol PR observed in routine clinical practice supports the findings from clinical tapentadol studies [Citation15]. A more favorable safety and tolerability profile of tapentadol compared with oxycodone was also concluded by a Cochrane review [Citation45]. Furthermore, a conventional meta-analysis of 32 studies found a significantly lower incidence of AEs under tapentadol treatment compared with oxycodone, tramadol, oxycodone/naloxone, fentanyl and morphine [Citation21]. The same publication presents a network meta-analysis of 25 studies, which also revealed the lowest constipation incidence for tapentadol compared with oxycodone/naloxone, fentanyl, tramadol, morphine, oxycodone, buprenorphine, oxymorphone and hydromorphone [Citation21].
Initiation of opioid treatment is usually associated by both physicians and patients with the expectation that this treatment is likely to be continued. In other words, longer-term opioid treatment is often anticipated. According to guidelines, strong opioids should only be used as a short-term solution when less potent analgesics and adjuvant treatments have failed to provide pain relief and improvements in functioning. However, if given at the right time, effective and well-tolerated treatments might have an important impact on the biological processes underlying pain chronification. In the present study, 9.4% of tapentadol PR patients were able to stop tapentadol treatment during the observation period because treatment was no longer required. In addition, 21.5% of tapentadol PR patients completing the 12 weeks of treatment did not continue because they had reached their individual treatment target. This is, in our opinion, likely the most interesting finding of this real-world study. In conjunction with the finding that neuropathic pain symptoms could no longer be detected in a high number of patients, we conclude from this observation that neuropathic pain symptoms present in cLBP seemed to be addressed more effectively under treatment with tapentadol PR than under the other WHO-III PR analgesics investigated.
Patients were likely able to stop tapentadol PR treatment because pain was reduced to a threshold where opioid treatment was no longer required. Whether this was the case because the chronification process was stopped or for other reasons cannot be answered on the basis of the present data. It would have been interesting to follow-up those patients who stopped tapentadol treatment because it was no longer required. Further studies investigating this patient group might lead to interesting insights concerning the questions raised.
Another important point to consider prior to opioid initiation is the constipation status of the patient. Our data showed gradual worsening of the mean BFI over the course of treatment in the WHO-III PR cohort, whereas mean BFI data remained within the reference range under tapentadol PR. Patients already suffering from constipation symptoms or presenting with a high BFI might benefit from either improvement in bowel function before initiation of a strong opioid, or might alternatively be started on tapentadol PR. The German Pain Association recommends tapentadol in its practice guideline for opioid-induced constipation as a ‘viable alternative to conventional opioid therapy,’ in particular, in the presence of a neuropathic pain component or constipation unrelated to opioids [Citation46].
When evaluating real-world data as supporting evidence for the treatment with opioid analgesics, the question often arises if the treatment was in line with the indication of the opioid labels (which mainly state pain intensity). In interventional clinical trials, baseline pain severity is usually defined by pain intensity after washout of all analgesic pretreatments. This is impossible in a real-life setting where patients cannot be denied a treatment in order to evaluate the full extent of the pain without treatment. Furthermore, when choosing the appropriate treatment in real life, the physicians will also consider pain-related impairments of daily living and the pain-related distress of the patient in their assessment. This approach is reflected in the new ICD-11 coding, which proposes these three dimensions as extension codes to the diagnoses [Citation47]. In the present study, physicians had considered pain severity (intensity, impairments and distress) of the patients appropriate for the treatment with a WHO-III PR opioid.
This study has all the limitations inherent to an analysis of retrospective observational data. Propensity score matching was carried out to eliminate the main confounding factors and to reduce selection bias. There may be, however, other undetermined or undocumented factors which might have confounded outcomes. As these are real-world data, there has been no ‘washout’ of pretreatment analgesics; pain intensity at baseline can thus not be judged without bias. The registry data only show the prescribed dosages/regimen but do not give information about actual compliance. Differences in mean dosages within the WHO-III PR cohort are also not known and concomitant medications other than analgesics and laxatives were not reported. There might have been other than the reported reasons for physicians to choose a certain treatment, which might have biased treatment outcomes. There could also have been unclear reasons for prescribing further concomitant medication, which might have affected changes in pain phenomenology. It is also not known if physicians prescribing a WHO-III PR analgesic might have assessed changes in bowel function more often than when prescribing tapentadol PR or might have prescribed more laxatives as a precaution. Confirmation/verification of data to detect errors in measurement or misclassification was also not possible, as only anonymized data were available to comply with guidelines for the protection of data privacy. We were, for example, thus unable to determine a cause for the much higher rate of discontinuations without any documented reason in the tapentadol PR cohort (14.7 vs 2.4% for WHO-III PR). It should also be noted that for simplicity reasons the term MCID was used no matter if the underlying score was related to difference or change when evaluating treatment responders. Where possible, the individual-level change score was used (BFI, PCS/MCS, pain intensity).
However, the data collected in this electronic data registry give insight into the routine medical pain management in the diverse patient population seen in routine clinical practice and can thus complement clinical trial data of narrowly defined trial populations. Unique strengths of the GPeR from a methodological point of view include the topographical representativity of the dataset, the diversity of the patient population, the freedom of physicians to choose treatment, standardized data aggregation, the avoidance of specific inclusion and exclusion criteria for participation, participation of mainly board-certified pain specialists and the large number of available patient datasets. These factors are essential prerequisites to optimize the unfolding of collective intelligence usually hidden in big datasets [Citation48]. Analyses of so-called real-world data not only provide insights into the reality of medical interventions in the environment in which patients are treated but also complementary approaches for efficacy, safety and tolerability analyses under everyday conditions. Therefore, the present analyses and results do certainly not fulfill the current requirements of the authorities for the comparative assessment of medicinal products with regard to the evidence level I studies to be provided. However, they offer the great advantage of the standardized use of patient-relevant end points and – in contrast to secondary data of health insurance companies or other sources – the possibility of direct correlations of prescription and effects. In this regard, it should also be noted that the questionnaires used for documentation in iDocLive® are standardized validated instruments that were developed to fulfill requirements by the German statutory health insurance funds for quality assured and standardized documentation of all treatment-relevant data for the routine care of chronic pain patients in Germany. However, it should be noted that these questionnaires had to be adapted for digitalization to permit larger collections of patient data, whereas the respective MCID scores refer to the original analog data.
Conclusion
In routine clinical practice, tapentadol PR was associated with significantly better effectiveness and gastrointestinal tolerability than the other oral WHO-III PR medications evaluated. It also addressed the neuropathic pain component more effectively. Tapentadol PR can therefore be considered a suitable treatment option for cLBP independent of the underlying pain mechanism.
Chronic low back pain (cLBP) is a debilitating condition associated with substantial restrictions in daily life activities, well-being and quality of life. Frequently, both nociceptive and neuropathic pain mechanisms are involved (mixed pain).
Clinical trials have shown the good efficacy and tolerability of the prolonged release formulation (PR) of the atypical opioid analgesic tapentadol HCl in the treatment of moderate or severe cLBP with or without a neuropathic component.
In order to get an insight into the use of tapentadol PR in cLBP management under routine medical care, anonymized real-world clinical practice data were extracted from the German Pain eRegistry and analyzed in a noninterventional retrospective cohort study.
The study compared effectiveness and tolerability of tapentadol PR with other oral WHO-III PR opioid analgesics (morphine, oxycodone ± naloxone and hydromorphone; WHO-III PR cohort) over 12 weeks in cLBP patients unsuccessfully treated with WHO-I/II analgesics and with pain severe enough to require strong opioid analgesics. Propensity score matching provided 2331 matching datasets.
After 12 treatment weeks, tapentadol PR patients showed significantly better outcomes than patients in the WHO-III PR cohort regarding pain reduction, improvements in daily living activities, sleep and quality of life.
In the majority of patients (>80%), a neuropathic pain component could not be excluded at baseline. In contrast to WHO-III PR patients, a stronger reduction in neuropathic pain symptoms was observed in the tapentadol PR cohort at end of observation.
At baseline, mean stool behavior was normal in both cohorts; after 12 treatment weeks, more than 75% of tapentadol PR patients were within the norm range of the bowel function index, whereas nearly 75% in the WHO-III PR cohort reported values above the norm range (p < 0.001).
Drug-related adverse events and treatment discontinuation due to drug-related adverse events occurred significantly less often under tapentadol PR treatment (p < 0.001).
As effectiveness, tolerability, and safety of a treatment are equally important in treatment selection in routine clinical practice, a composite primary study end point (treatment responder) was chosen including all three treatment aspects. The end point was attained by significantly more tapentadol PR patients (65.7 vs 14.2%; p < 0.001).
In routine clinical practice, tapentadol PR was associated with significantly better effectiveness and gastrointestinal tolerability than the other oral WHO-III PR medications evaluated. It also addressed the neuropathic pain component more effectively. Tapentadol PR can therefore be considered a suitable treatment option for cLBP independent of the underlying pain mechanism.
Author contributions
MA Überall, B Heckes, C Lefeber and M Heine contributed to the conception and design. MA Überall performed the data extraction and data analysis. All the authors were responsible for the data interpretation. All the authors critically revised for important intellectual content, approved the final manuscript version and agreed to the submission.
Ethical conduct of research
The study is registered in the European Union electronic Registry of Post-Authorization Studies (EUPAS 38332) through the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) coordinated by the European Medicines Agency (EMA) and was conducted in accordance with the Declaration of Helsinki and relevant national and regulatory requirements; approval was granted by the independent ethics committee of the German Pain Association and the German Pain Alliance. All the patients provided written informed consent prior to participation in the registry. All analyses were carried out using only anonymized data to comply with German guidelines on protection of data privacy and with the European Union General Data Protection Regulation.
Financial & competing interests disclosure
The German Pain Association received payment from Grünenthal GmbH, Germany, for the use of the data. MA Überall is a physician, pain specialist, medical director of the Institute of Neurological Sciences and CEO of O. Meany-MDPM GmbH, which was responsible for data extraction and biometrical analyses. MA Überall received payment for conference representation of the data. MA Überall has received financial support and/or expenses in form of research funds, consultancy fees and/or renumerations for lecture activities from: Allergan, Almirall, Amicus Therapeutics, Aristo Pharma, Bionorica, Esanum, Glaxo Smith Kline, Grünenthal, Hapa Medical, Hexal, IMC, Kyowa-Kirin, Labatec, Mucos, Mundipharma, Nestle, Pfizer, Recordati, Servier, SGP-Pharma, Shionogi, Spectrum Therapeutics, Strathmann, Teva, and Tilray. GHH Müller-Schwefe is a physician and pain/palliative care specialist and has received no financial support and/or expenses. All other authors are employees of Grünenthal GmbH, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing and editorial assistance was provided by E Grosselindemann and B Brett and was paid for by Grünenthal GmbH, Germany.
Additional information
Funding
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1789–1858 (2018).
- Hartvigsen J , HancockMJ , KongstedAet al. Low back pain 1. What low back pain is and why we need to pay attention. Lancet391, 2356–2367 (2018).
- Itz CJ , GeurtsJW , van KleefM , NelemansP. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur. J. Pain.17, 5–15 (2013).
- Freynhagen R , BaronR. The evaluation of neuropathic components in low back pain. Curr. Pain Headache Rep.13, 185–190 (2009).
- Morlion B . Chronic low back pain: pharmacological, interventional and surgical strategies. Nat. Rev. Neurol.9, 462–473 (2013).
- Baron R , BinderA , AttalN , CasaleR , DickensonAH , TreedeRD. Neuropathic low back pain in clinical practice. Eur. J. Pain.20, 861–873 (2016).
- Foster NE , AnemaJR , CherkinDet al. Low back pain 2. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet391, 2368–2383 (2018).
- Oliveira CB , MaherCG , PintoRZet al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur. Spine J.27, 2791–2803 (2018).
- O’Brien T , ChristrupLL , DrewesAMet al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain.21, 3–19 (2017).
- Benyamin R , TrescotAM , DattaSet al. Opioid complications and side effects. Pain Physician.11, S105–S120 (2008).
- Panchal SJ , Müller-SchwefeP , WurzelmannJI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int. J. Clin. Pract.61, 1181–1187 (2007).
- Farmer AD , DrewesAM , ChiarioniGet al. Pathophysiology and management of opioid-induced constipation: European Expert Consensus statement. United European Gastroenterol. J.7, 7–20 (2019).
- Lee AA , HaslerWL. Opioids and GI motility – friend or foe?Curr. Treat. Options Gastroenterol.14, 478–494 (2016).
- Tzschentke TM , ChristophT , KögelBY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs.28, 319–329 (2014).
- Baron R , EberhartL , KernKUet al. Tapentadol prolonged release for chronic pain: a review of clinical trials and 5 years of routine clinical practice data. Pain Pract.17, 678–700 (2017).
- Buynak R , ShapiroDY , OkamotoAet al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study. Expert Opin. Pharmacother.11, 1787–1804 (2010).
- Steigerwald I , MüllerM , DaviesAet al. Effectiveness and safety of tapentadol prolonged release for severe, chronic low back pain with or without a neuropathic pain component: results of an open-label, Phase 3b study. Curr. Med. Res. Opin.28, 911–936 (2012).
- Gálvez R , SchäferM , HansG , FalkeD , SteigerwaldI. Tapentadol prolonged release versus strong opioids for severe, chronic low back pain: results of an open-label, Phase 3b study. Adv. Ther.30, 229–259 (2013).
- Baron R , LikarR , Martin-MolaEet al. Effectiveness of tapentadol prolonged release (PR) compared with oxycodone/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, Phase 3b/4 study. Pain Pract.16, 580–599 (2016).
- Baron R , JansenJP , BinderAet al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, Phase 3b/4 trial. Pain Pract.16, 600–619 (2016).
- Meng Z , YuJ , AcuffMet al. Tolerability of opioid analgesia for chronic pain: a network meta-analysis. Sci. Rep.7, 1995 (2017).
- Wild JE , GrondS , KuperwasserBet al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract.10, 416–427 (2010).
- Strick V . Management of severe chronic pain with tapentadol prolonged release - long-term data from pain specialists. Curr. Med. Res. Opin.30, 2085–2092 (2014).
- Buynak R , RappaportSA , RodKet al. Long-term safety and efficacy of tapentadol extended release following up to 2 years of treatment in patients with moderate to severe, chronic pain: results of an open-label extension trial. Clin. Ther.37, 2420–2438 (2015).
- Finco G , MuraP , MusuMet al. Long-term, prolonged-release oral tapentadol for the treatment of refractory chronic low back pain: a single-center, observational study. Minerva Medica.109, 259–265 (2018).
- Gálvez Mateos R , SamperBernal D , TorresMorera LM , FerriCM , EsquiviasEscobar A. Long-term effectiveness and tolerability of pain treatment with tapentadol prolonged release. Pain Physician.24, E75–E85 (2021).
- Cave A , KurzX , ArlettP. Real-world data for regulatory decision making: challenges and possible solutions for Europe. Clin. Pharm. Ther.106, 36–39 (2019).
- Bolislis WR , FayM , KühlerTC. Use of real-world data for new drug applications and line extensions. Clin. Ther.42, 926–938 (2020).
- German Pain Association . Manual for the German Pain Questionnaire (in German). https://www.schmerzgesellschaft.de/fileadmin/pdf/DSF_Handbuch_2020.pdf
- Tait RC , ChibnallJT , KrauseS. The pain disability index: psychometric properties. Pain40, 171–182 (1990).
- Ware J Jr , KosinskiM , KellerSD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care34, 220–233 (1996).
- Freynhagen R , BaronR , GockelU , TölleTR. PainDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin.22, 1911–1920 (2006).
- Cappelleri JC , BienenEJ , KoduruV , SadoskyA. Measurement properties of painDETECT by average pain severity. Clinicoecon. Outcomes Res.6, 497–504 (2014).
- Rentz AM , YuR , Müller-LissnerS , LeyendeckerP. Validation of the bowel function index to detect clinically meaningful changes in opioid-induced constipation. J. Med. Econ.12, 371–383 (2009).
- Ueberall MA , Müller-LissnerS , Buschmann-KrammC , BosseB. The bowel function index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J. Int. Med. Res.39, 41–50 (2011).
- von Korff M , OrmelJ , KeefeFJ , DworkinSF. Grading the severity of chronic pain. Pain50, 133–149 (1992).
- Ostelo RWJG , de VetHCW. Clinically important outcomes in low back pain. Best Pract. Res. Clin. Rheumatol.19, 593–607 (2005).
- Díaz-Arribas MJ , Fernández-SerranoM , RoyuelaAet al. Minimally clinically important difference in quality of life for patients with low back pain. Spine42, 1908–1916 (2017).
- Schmitt N , GerbershagenHU. The Mainz Staging System (MPSS) for chronic pain. Pain41(Suppl. 5), S484 (1990).
- Überall MA . Poster presentation at the IASP 2021 Virtual World Congress on Pain: opioid-induced constipation in low back pain routine care with tapentadol.. The International Association for the Study of Pain.
- Schröder W , DeVry J , TzschentkeTM , JahnelU , ChristophT. Differential contribution of opioid and nonadrenergic mechanisms of tapentadol in rat models of nociceptive and neuropathic pain. Eur. J. Pain14, 814–821 (2010).
- Gonçalves L , FriendLV , DickensonAH. The influence of µ-opioid and noradrenaline reuptake inhibition in the modulation of pain responsive neurones in the central amygdala by tapentadol in rats with neuropathy. Eur. J. Pharmacol.749, 151–160 (2015).
- Müller-Schwefe GHH , ÜberallMA. Pain and quality of life (in German). Gesundh Ökon. Qual. Manag.16, S20–S22 (2011).
- Raffa RB , EllingC , TzschentkeTM. Does ‘strong analgesic’ equal ‘strong opioid’? Tapentadol and the concept of ‘μ-load’. Adv. Ther.35, 1471–1484 (2018).
- Santos J , AlarcãoJ , FareleiraF , Vaz-CarneiroA , CostaJ. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 20155, CD009923 (2015).
- German Pain Association . Practice guideline opioid-induced constipation (in German) (2019). https://www.dgs-praxisleitlinien.de/index.php/leitlinien/oic
- Treede RD , RiefW , BarkeAet al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain160, 19–27 (2019).
- Krenz P , WulfsbergJP , BruhnsF-L. Unfold Collective Intelligence! - opening up new potentials of value creation (in German). ZWF Zeitschrift für Wirtschaftlichen Fabrikbetrieb107(3), 152–157 (2012).