Abstract
Aim: To explore the effects of viable allogeneic disc tissue supplementation in younger patients with discogenic chronic low back pain (CLBP). Patients & methods: VAST was a randomized placebo-controlled trial of disc allograft supplementation in 218 patients with discogenic CLBP. We conducted a post hoc analysis of change from baseline to 12 months in Oswestry Disability Index (ODI) and visual analog scale for pain intensity scores stratified by patient age. Results: Patients aged <42 years receiving allograft experienced greater improvement in ODI (p = 0.042) and a higher ODI response rate (≥10-, ≥15- and ≥20-point reductions in ODI) than those receiving saline (p = 0.001, p = 0.002 and p = 0.021, respectively). Conclusion: Young patients with discogenic CLBP may have significant functional improvement following nonsurgical disc allograft supplementation.
Lay abstract
The VAST trial evaluated a new treatment for patients with chronic back pain resulting from one or two degenerated spinal discs. The treatment consists of a single injection of disc tissue supplement. A total of 218 adults participated in the study; most received the active treatment, while a smaller number (39 patients) received an injection of saline. In this paper we explain what happened over the 12 months after the injections. Patients who were younger (<42 years old) experienced more functional benefits (i.e., ability to perform daily tasks) after active treatment compared with those who received the saline injection, as measured by disability score. In contrast, older patients (≥42 years old) experienced functional benefits with both active and saline treatments, with no differences between the groups. There were more side effects in both age groups in those who received the active treatment compared with those who received saline, but almost all of the side effects were temporary and not serious.
Clinical Trial Registration number: NCT03709901 (ClinicalTrials.gov)
Low back pain (LBP) is a leading cause of disability, with approximately half a billion cases worldwide [Citation1–3]. In the USA alone in 2019, there were 136,190 cases of back-related problems that led to missed days from work [Citation4]. As a consequence of its high prevalence among working adults, LBP carries a significant direct cost burden related to healthcare resource utilization [Citation5]. Additionally, the prominence of LBP among working-age people is such that it is the leading cause of work-loss days, and thus its economic impact via indirect (employer-paid work-loss) costs is perhaps even more significant [Citation3,Citation6,Citation7]. In the USA alone, the costs associated with treating LBP exceed $100 billion per year, with two-thirds of these costs related to lost productivity and wages [Citation8].
A leading cause of chronic LBP (CLBP) in adults is degenerative disc disease, and in one study, patients with discogenic CLBP had a significantly lower mean age (44 years) than patients with facet or sacroiliac joint pain (60 and 61 years, respectively) [Citation9]. CLBP also represents a leading indication for the prescription of opioid analgesics in clinical practice [Citation10,Citation11]. The current treatment paradigm for LBP typically starts with conservative nonsurgical measures (i.e., rest, anti-inflammatory medications, manual manipulation/chiropractic, steroid injections, electrical stimulation, back braces and heat or ice therapy) and, depending on severity, may progress to surgical management, such as discectomy, disc replacement or disc fusion [Citation12,Citation13]. At present the outcomes of interventions for CLBP of putative discogenic origin that directly target the degenerated disc(s) are often suboptimal. The reported success rates for surgical care of degenerative disc disease are reported to be as low as 41–54% [Citation14], with an early complication rate as high as 16% [Citation15]. Suboptimal outcomes across the spectrum of care, coupled with increased costs associated with surgery relative to conservative management, delineate a gap in the treatment algorithm for the management of degenerative disc disease.
In recent years, efforts to improve the treatment of painful degenerative disc disease have increasingly focused on regenerative medicine using various biological approaches, such as intradiscal injection of growth factors, juvenile chondrocytes and allogeneic mesenchymal stem cells [Citation12,Citation16–18]. The aim of delivering biologically active factors to a damaged disc is to prevent further degeneration and potentially encourage regeneration. Minimally invasive therapies that aim to closely mimic the composition and structure of native tissues may offer the most potential for safe and effective treatment of painful disc degeneration [Citation16]. A viable disc tissue allograft (VIA Disc®) has been developed to provide the benefits of allogeneic tissue supplementation that combines a matrix replete with viable cells to address tissue loss attributable to degeneration [Citation12,Citation19]. The VAST (Viable Allograft Supplemented Disc Regeneration in the Treatment of Patients with Low Back Pain With or Without Intervertebral Disc Herniation; NCT03709901) trial was a blinded, prospective, randomized controlled trial designed to assess the safety and efficacy of this allograft in adults with discogenic back pain [Citation19]. At 12 months, durable, clinically meaningful improvements in function (Oswestry Disability Index [ODI]) and pain (visual analog scale [VAS] scores) were reported versus baseline in the overall population. Post hoc analyses of sex and number of levels treated were consistent with the overall results, demonstrating that treatment effects were similar in men and women and regardless of whether patients were treated at one or two intervertebral disc levels [Citation19]. In contrast, comparison of treatment effects in study subjects at or above the median age with those younger than the median age suggests an association with treatment outcome () [Citation19]. In light of these observations, we undertook further post hoc analyses to explore the potential effects of age on treatment outcomes in patients undergoing allogeneic disc tissue supplementation.
ODI: Oswestry Disability Index; VAS: Visual analog scale.
Reproduced with permission, Pain Physician, ASIPP Publishing [Citation19].
![Figure 1. Subgroup analysis for age, sex and levels treated on response measured by visual analog scale and Oswestry Disability Index at 12 months.ODI: Oswestry Disability Index; VAS: Visual analog scale.Reproduced with permission, Pain Physician, ASIPP Publishing [Citation19].](/cms/asset/94979e75-9b52-40e8-991f-cf459e5dd3be/ipmt_a_12344487_f0001.jpg)
Patients & methods
VAST trial
The VAST trial has been reported previously, including a complete description of study methods [Citation12,Citation19]. Briefly, VAST was a multicenter, blinded, prospective, randomized controlled trial of 218 patients with one- or two-level degenerative disc disease (from L1 to S1) categorized as modified Pfirrmann grades 3–6 who met the following inclusion criteria: CLBP that had persisted for ≥6 months and that remained unresponsive to nonoperative treatment modalities; with moderate to severe disability (ODI ≥40%) and pain (score of ≥40 mm on VAS for pain intensity). Key exclusion criteria were spondylolisthesis, prior lumbar spine surgery, type III Modic endplate changes, facet joint arthrosis and spinal stenosis. Patients were randomized (3.5:1:1) to either viable disc allograft, saline or nonsurgical conservative management. Patients randomized to active allograft or saline received intradiscal injections into the center of the lumbar intervertebral disc with either the viable disc allograft or saline. The VIA Disc® treatment provides a minimum of 6 million cells with 75% viability combined with 100 mg of lyophilized nucleus pulposus allograft reconstituted with 1 cm3 of saline, comprising a total injection volume of approximately 1.75 cm3. Cell activity was measured in a viability assay at thaw by a live/dead assay. Further details regarding assessment of cell activity and preparation of the VIA Disc treatment have been described in previous reports [Citation12,Citation19]. After 3 months, all patients in the conservative care group crossed over to the allograft group (crossover group) and received viable disc allograft treatment. Patient condition before and after treatment was evaluated using standardized outcome measures, and disc space height, spinal alignment and degree of disc degeneration were assessed using plain radiographs and MRI scans. Patients were followed for 12 months after treatment. The co-primary end points were change from baseline in ODI and VAS average back pain at 12 months after treatment; secondary end points included improvement in ODI and VAS scores at 6 months and rates of adverse events (AEs) and serious AEs (SAEs) after treatment.
Post hoc age-stratified analysis
In the current post hoc analysis of data from the VAST trial, two subgroups comprising patients aged <42 and ≥42 years, respectively, were analyzed. The cutoff for defining these groups was the overall median age of 42 years [Citation19]. All data were summarized using descriptive statistics, including means, standard deviations, medians and ranges for continuous variables and frequency and percentages for categorical variables.
This post hoc analysis stratified by age group was conducted in the same way as the primary analysis. Specifically, changes from baseline in ODI and VAS scores at 12 months were compared among the three treatment groups (allograft, saline, crossover) using the Kruskal–Wallis test to obtain an overall p-value, and the Dwass–Steel–Critchlow–Fligner method was used to assess all pairwise comparisons. Responder analyses were conducted using a responder definition of a change of ≥15 points on the ODI and a ≥50% reduction in VAS average back pain. Responder analyses were conducted using the Fisher exact test. All analyses were completed using a two-sided α = 0.05, without controlling for multiple testing.
Regression analysis
A regression analysis was also performed to assess the impact of age on the results. An analysis of covariance model was fitted to the change from baseline for ODI and VAS, with treatment group, age group and an interaction term (treatment group × age group) included as independent variables. The interaction term was used to assess treatment effect across the whole age range. Cofactors were not included in the analysis because no other variable besides age was correlated with outcome, including pain at baseline, Pfirrmann score, Modic change, number of levels involved or sex.
Results
Patients
Among younger patients (<42 years), 68 were treated with the allograft, 19 received saline and 17 began in the conservative care group before crossing over to allograft treatment. Within the older group (≥42 years), there were 72 allograft recipients, 20 who received saline and 22 in the conservative care group who crossed over to allograft treatment. Baseline demographic and disease characteristics were similar across the three treatment groups in both patient age groups (<42 or ≥42 years; ).
Table 1. Baseline demographic and disease characteristics according to age.
Post hoc age-stratified analysis
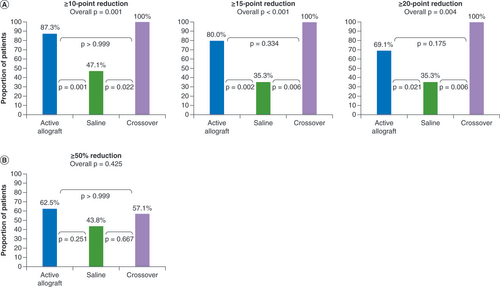
The post hoc analysis performed according to age showed that younger patients who received allograft (either initially or after crossing over from conservative care) had a more favorable functional outcome than those receiving saline (overall between-group p-value of 0.004; p-values of 0.042 and 0.025 for allograft vs saline and crossover vs saline, respectively, for ODI mean change from baseline at 12 months; ). Statistical differences in ODI reductions between treatments were not seen in older patients (overall p-value of 0.244). Responder analyses confirmed these findings: among younger patients, there was a significant between-group treatment effect (p < 0.001), with significantly greater proportions of patients receiving allograft (both those receiving allograft initially and those crossing over from conservative care) achieving a reduction of ≥15 points in ODI versus those receiving saline (p = 0.002 and 0.006, respectively; A). Additional responder analyses at cutoffs of ≥10- and ≥20-point reductions in ODI also showed significant improvements versus saline in younger patients receiving allograft initially (p = 0.001 and p = 0.021, respectively) and in those crossing over from conservative care (p = 0.022 and p = 0.006, respectively; A). No significant differences between treatments were seen for VAS changes () or VAS responder rates in the younger patients (B).
Table 2. Mean change in visual analog scale score and Oswestry Disability Index at 12 months according to age.
Data derived from a subpopulation analysis using the modified intent-to-treat population. p-values are derived from the Fisher exact test. Conservative care 12-month crossover visit is used. Month 3 value is used as baseline.
Note: active allograft, n = 55; placebo, n = 17; crossover, n = 7.
ODI: Oswestry Disability Index; VAS: Visual analog scale.
In patients aged ≥42 years, there were no statistically significant between-treatment differences in ODI or VAS score changes () and no significant differences in VAS responder rates. Furthermore, in patients <42 years of age receiving one level of treatment, there was a significant benefit of allograft treatment versus saline on the ODI, but this was not the case for those ≥42 years of age (). There was no association of severity of disc degeneration as quantified by Pfirrmann grade with either outcome in either age group.
Table 3. Responder analyses at 12 months in patients aged <42 years by number of levels treated.
Regression analyses
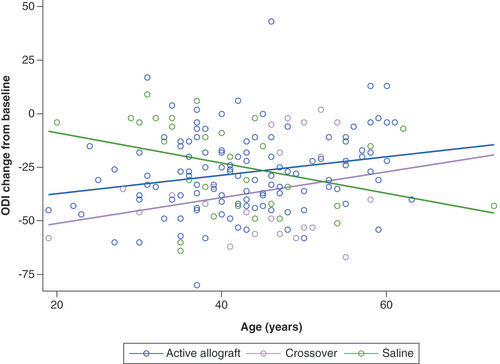
While the responder analyses described above showed between-group differences, they cannot establish age as a predictor of response; therefore a regression analysis using an analysis of covariance model was used to assess the impact of age on the results. This analysis showed that change from baseline in the ODI demonstrated a strong interaction between treatment and age (p = 0.0071; R2 = 0.099; ). There was no significant interaction between treatment and age in VAS scores (p = 0.3501), and there were no significant benefits of allograft treatment on VAS score in younger versus older patients (p = 0.567).
Data derived from a subpopulation analysis using the modified intent-to-treat population. Conservative care 12-month crossover visit is used with Month 3 value as baseline. The interaction plot illustrates the differences in change from baseline between patients receiving allograft and those who received saline. The plot shows that younger patients (<42 years) showed a benefit that was not observed in older patients (≥42 years) (p = 0.0071; R2 = 0.099). Note: modified intent-to-treat population: active allograft, n = 119; placebo, n = 30; crossover, n = 24.
ODI: Oswestry Disability Index.
Adverse events
AEs were reported in 30.9, 10.5 and 17.6% of younger patients in the allograft, saline and crossover groups, respectively (overall p = 0.155), compared with 28.8, 15.8 and 9.1% of older patients in these groups (overall p = 0.136; ). The most common AE across all treatment groups and patient ages was back pain. Ten younger patients (14.7%) and six older patients (8.2%) in the active allograft group had treatment- or procedure-related AEs, versus two (11.8%) and one (4.5%), respectively, in the crossover group; no saline-treated patients had treatment- or procedure-related AEs [Citation19]. Procedure-related back pain was reported as an AE by eight patients (5.7%) and one patient (2.9%) in the allograft and crossover groups, respectively. The maximum duration of procedure-associated back pain was 3 weeks; in most cases, pain resolved after a few days.
Table 4. Adverse events by age.
One younger patient in the allograft group experienced four SAEs (administration site pain, osteomyelitis, pneumonia and back pain). The diagnosis of pneumonia was determined to be unrelated to either treatment or procedure but may have led to seeding at the site of the injection. The back pain and diagnosis of osteomyelitis were both deemed to be unrelated to allograft treatment, but possibly related to procedure.
Four older patients in the allograft group reported a total of seven SAEs, comprising myocardial infarction (n = 1), bacteremia (n = 1), osteomyelitis (n = 1), back pain (n = 2), spinal osteoarthritis (n = 1) and transient ischemic attack (n = 1). The myocardial infarction and the transient ischemic attack were not related to the treatment and procedure, and the patients recovered. The bacteremia and osteomyelitis were both noted as related to procedure and the back pain was possibly related to the procedure.
One older patient in the crossover group who was treated at a single level developed intervertebral disc degeneration at another level during the study period.
Discussion
We report the results of post hoc analyses performed on data from the VAST trial, stratified according to patient age (<42 and ≥42 years). These analyses demonstrated that younger patients in both the allograft group and the conservative care group who crossed over to allograft had significantly greater improvements in ODI scores at 12 months than the saline group. Given that disc degeneration has long been shown to be positively correlated with aging [Citation20], we can speculate that the regenerative capacity may have been greater in the younger population, and therefore it would not be surprising that an intervention in this group might yield a better response.
The minimum clinically important difference for ODI score change in patients undergoing lumbar spine surgery has been shown to be 12.8 points [Citation21]; however, a slightly more stringent responder definition of ≥15 points in ODI was used in the VAST responder analyses. Younger patients in the allograft and crossover groups had results superior to saline in the ODI responder analyses at this cutoff point (80 and 100% vs 35.3%, respectively). Significantly greater proportions of younger patients in the allograft and crossover groups also achieved ≥10- and ≥20-point reductions. Statistically significant differences between treatment groups on ODI mean change from baseline and ODI responder analyses were not seen in the older patients. However, there were clinically meaningful improvements from baseline in function and pain for all groups. Our post hoc regression analysis demonstrated a strong interaction between treatment and age (p = 0.0071) for change from baseline in ODI score, which was consistent with the benefits of allograft treatment on ODI being observed in younger but not in older patients, when compared with saline.
In an earlier study of 276 adults with degenerative disc disease who underwent one-level lumbar arthroplasty, postoperative outcomes at 2 years found no significant differences between two age groups (≤45 vs >45 years) in either ODI or VAS score changes [Citation22]. As that study used a different treatment modality than the VAST trial, its results cannot be directly compared with the age-stratified analyses reported here. One explanation for the difference might be that while both treatments seek motion preservation, a supplemental allograft that retains viable components of healthy disc tissue might be expected to support disc healing, whereas surgical approaches guided to retain motion at that spinal segment eliminate the natural disc with a prosthetic replacement. A more detailed discussion of procedural and other aspects of cell therapies used for the treatment of intervertebral disc degeneration is provided in an excellent review by Meisel et al. [Citation23].
The age effect observed in the VAST patient population may be explained by the differing etiologies of pain observed in older versus younger patients: back pain in younger patients is often discogenic in origin, whereas pain from other sources, such as facetogenic pain, is more common in older patients [Citation9]. The VAST trial was designed to assess patients with discogenic back pain who were treated with disc tissue allograft supplementation.
Several implications for clinical practice and key stakeholders, such as payers and employers, should be noted. First, the significant improvement in ODI in younger patients after receiving allograft in the VAST trial suggests that earlier intervention in younger patients, when CLBP is less severe or at an earlier stage of development, may be more effective and may allow a more rapid return to normal functioning. A second and related point, and one that holds particular significance for employers and payers, is that ODI may predict ability to return to work in patients with CLBP [Citation3,Citation24–28]. Among patients with CLBP undergoing lumbar spine surgery, higher baseline ODI scores have been found to reduce the odds of returning to work 12 months post-surgery [Citation26], while significantly lower postoperative ODI scores have been associated with earlier return to work (within ≤60 vs >60 days) [Citation27]. ODI has also been identified as one of the variables predicting recovery of work capability at 18 months in patients completing a multidisciplinary biopsychosocial rehabilitation program [Citation3]. Third, in two studies that explored the factors influencing work status in patients with CLBP, higher ODI scores were found to be predictors of prolonged work absenteeism [Citation24] and underemployment (a work status defined as unemployed, employed with modified work duties, or disability) [Citation25]. These findings suggest that patients who achieve ODI reductions through treatment of their CLBP may be able to return to the workforce more quickly than those whose ODI scores do not improve after intervention.
Younger and older patients in the VAST trial showed a similar AE profile, with no significant or noteworthy differences between groups. As expected, musculoskeletal back pain was the most common AE across all patient ages. There was a low rate of treatment- and/or procedure-related SAEs in both age groups. Osteomyelitis or discitis is an SAE that is known to occur after intradiscal injections, such as discography, and has been reported to occur in 0.2–4.92% of patients [Citation26,Citation27]. The rate of osteomyelitis observed in this study falls within the expected range. This safety profile, which provides an extension of previously reported safety data [Citation19], suggests that viable disc tissue allograft is safe to use according to its intended purpose, regardless of patient age.
The study was not powered to demonstrate statistical significance in the age subgroups. However, the improvements observed for ODI in patients <42 years old were statistically significant. The study lacked thorough data on healthcare resource utilization and return to work, and this aspect was further limited by small sample size when parsed to younger and older patient groups. In the future, it would be interesting to look at healthcare resource utilization and assess correlation with clinical outcomes. Beyond understanding the impacts on healthcare costs and utilization, it would be useful to assess longer-term clinical benefit, which will come from the 24- and 36-month assessments planned from VAST, and to evaluate real-world data.
Conclusion
Post hoc analyses of VAST trial data stratified according to patient age showed that younger patients (<42 years) with CLBP achieved statistically significant improvements (vs saline) in ODI score following allogeneic disc tissue supplementation. A reduction in ODI in younger patients would be predicted to be associated with a quicker return to work, suggesting that as a workforce health issue, there may be value to intervening earlier to address CLBP in younger, active patients in high-risk jobs with high recurrence of back pain.
At present, the outcomes of interventions for chronic low back pain (CLBP) of putative discogenic origin that directly target the degenerated disc(s) are often suboptimal.
The VAST trial was a blinded, prospective, randomized controlled trial to assess the safety and efficacy of disc tissue supplementation for the treatment of degenerated intervertebral discs in adults with CLBP.
At 12 months, clinically meaningful improvements in function (Oswestry Disability Index [ODI]) and pain (visual analog scale scores) were reported versus baseline in the overall population.
The analyses of age, sex and number of levels treated were consistent with the overall results and not driven by one subgroup. However, it was noted that younger patients had a more favorable outcome compared with older patients.
This post hoc analysis explored the potential effects of age on treatment outcomes.
Younger patients (aged <42 years) receiving allograft experienced greater improvement in ODI score (p = 0.042) and a higher ODI response rate (≥10-, ≥15- and ≥20-point reductions in ODI) than patients receiving saline (p = 0.001, p = 0.002 and p = 0.021, respectively).
Older patients showed similar improvements from baseline with both allograft and saline treatments, but no between-treatment differences in outcome.
These results suggest that earlier intervention in younger patients may be more effective and may allow for a more rapid return to normal functioning.
Conclusion: younger patients with discogenic CLBP may have significant functional improvement following nonsurgical disc allograft supplementation.
Author contributions
All authors contributed to the preparation of this manuscript, including its conception and critical review, and have approved the final draft and accept full responsibility for its content.
Ethical conduct of research
In the VAST trial, subjects were enrolled and data gathered under jurisdiction and oversight from the Sterling Institutional Review Board (GA, USA). The study was conducted in accordance with Good Clinical Practice guidelines and applicable regulatory requirements.
Acknowledgments
The authors are grateful to Megan Williams (Avania) and Martin Roessner (Parexel International) for contributions to statistical analysis.
Financial & competing interests disclosure
Vivex Biologics, Inc. sponsored this study. C Hunter provides consultancy for Saluda and Genecentrix; is in receipt of research funding from Abbott, Biotronik, Boston Scientific, DiscGenics, Mesoblast, Saluda, SKK, TissueGene and Vivex; is a member of the advisory boards of Abbott, Mainstay, Nalu, Spine BioPharma, PainTEQ, Vertiflex and Vivex; and is an equity shareholder of Axonics, Mainstay, Nalu, PainTEQ, Spine BioPharma and Vivex. R Guyer is a consultant for Vivex. M Froimson is a consultant for Vivex. M DePalma is a consultant for Vivex, Mesoblast and Avanos and has received research grants from Mesoblast, Vivex, DiscGenics, Avanos and SPR Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Professional medical writing and editorial assistance were provided by Parexel International, funded by Vivex Biologics, Inc., and Vivex employees.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: NCT03709901. The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- GBD Disease Injury Incidence Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392(10159), 1789–1858 (2018).
- Hurwitz EL , RandhawaK , YuH , CôtéP , HaldemanS. The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. Eur. Spine J.27(Suppl. 6), 796–801 (2018).
- Ibrahim ME , WeberK , CourvoisierDS , GenevayS. Recovering the capability to work among patients with chronic low back pain after a four-week, multidisciplinary biopsychosocial rehabilitation program: 18-month follow-up study. BMC Musculoskelet. Disord.20(1), 439 (2019).
- Bureau of Labor Statistics, US Department of Labor . Databases, tables & calculators by subject. https://data.bls.gov/timeseries/CSU00X32XXXX6P100
- Kim LH , VailD , AzadTDet al. Expenditures and health care utilization among adults with newly diagnosed low back and lower extremity pain. JAMA Netw. Open2(5), e193676 (2019).
- Ivanova JI , BirnbaumHG , SchillerM , KantorE , JohnstoneBM , SwindleRW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J.11(7), 622–632 (2011).
- Stewart WF , RicciJA , CheeE , MorgansteinD , LiptonR. Lost productive time and cost due to common pain conditions in the US workforce. JAMA290(18), 2443–2454 (2003).
- Katz JN . Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J. Bone Joint Surg. Am.88(Suppl. 2), 21–24 (2006).
- DePalma MJ , KetchumJM , SaulloT. What is the source of chronic low back pain and does age play a role?Pain Med.12(2), 224–233 (2011).
- Gudin J , KaufmanAG , DattaS. Are opioids needed to treat chronic low back pain? A review of treatment options and analgesics in development. J. Pain Res.13, 1007–1022 (2020).
- Young JC , JonssonFunk M , DasguptaN. Medical use of long-term extended-release opioid analgesics in commercially insured adults in the United States. Pain Med.21(4), 724–735 (2020).
- Beall DP , WilsonGL , BishopR , TallyW. VAST clinical trial: safely supplementing tissue lost to degenerative disc disease. Int. J. Spine Surg.14(2), 239–253 (2020).
- Yoshihara H , YoneokaD. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J.15(2), 265–271 (2015).
- Wei J , SongY , SunL , LvC. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Int. Orthop.37(7), 1315–1325 (2013).
- Ibrahim T , TleyjehIM , GabbarO. Surgical versus non-surgical treatment of chronic low back pain: a meta-analysis of randomised trials. Int. Orthop.32(1), 107–113 (2008).
- Buckley CT , HoylandJA , FujiiK , PanditA , IatridisJC , GradS. Critical aspects and challenges for intervertebral disc repair and regeneration-harnessing advances in tissue engineering. JOR Spine1(3), e1029 (2018).
- Hsieh PC , BuserZ , SkellyACet al. Allogenic stem cells in spinal fusion: a systematic review. Global Spine J.9(Suppl. 1), S22–S38 (2019).
- Orozco L , SolerR , MoreraC , AlbercaM , SanchezA , Garcia-SanchoJ. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation92(7), 822–828 (2011).
- Beall DP , DavisTT , DePalmaMet al. Viable disc tissue allograft supplementation; one- and two-level treatment of degenerated intervertebral discs in patients with chronic discogenic low back pain: one year results of the VAST randomized controlled trial. Pain Physician24(6), 465–477 (2021).
- Buckwalter JA . Aging and degeneration of the human intervertebral disc. Spine20(11), 1307–1314 (1995).
- Copay AG , GlassmanSD , SubachBR , BervenS , SchulerTC , CarreonLY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J.8(6), 968–974 (2008).
- Guyer RD , GeislerFH , BlumenthalSL , McAfeePC , MullinBB. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J. Neurosurg. Spine8(2), 101–107 (2008).
- Meisel HJ , AgarwalN , HsiehPCet al. Cell therapy for treatment of intervertebral disc degeneration: a systematic review. Global Spine J.9(Suppl. 1), S39–S52 (2019).
- Du Bois M , SzpalskiM , DonceelP. Patients at risk for long-term sick leave because of low back pain. Spine J.9(5), 350–359 (2009).
- Harris SA , RampersaudYR. The importance of identifying and modifying unemployment predictor variables in the evolution of a novel model of care for low back pain in the general population. Spine J.16(1), 16–22 (2016).
- Khan I , BydonM , ArcherKRet al. Impact of occupational characteristics on return to work for employed patients after elective lumbar spine surgery. Spine J.19(12), 1969–1976 (2019).
- Liow MHL , GohGS , YeoWet al. Time taken to return to work does not influence outcomes of minimally invasive transforaminal lumbar interbody fusion: a 5-year follow-up study. Spine44(7), 503–509 (2019).
- McGirt MJ , SivaganesanA , AsherAL , DevinCJ. Prediction model for outcome after low-back surgery: individualized likelihood of complication, hospital readmission, return to work, and 12-month improvement in functional disability. Neurosurg. Focus39(6), E13 (2015).
