Abstract
Aim: To provide real-world evidence for the effectiveness and tolerability of lidocaine 700 mg medicated plaster (LMP) in localized peripheral neuropathic pain (l-PNP) treatment compared with first-line oral medications (OM). Patients & methods: This was a noninterventional, retrospective 6-month cohort study in patients refractory to at least one recommended OM, using anonymized medical care data from the German Pain eRegistry. Treatment groups were matched by propensity scoring, considering seven predefined confounding factors. The primary effectiveness end point was the absolute change in average pain intensity index from baseline at weeks 4, 12 and 24 of treatment and over the treatment period. Results: A total of 3081 datasets were retained per treatment group. LMP provided superior pain reductions and significantly greater improvements in pain-related impairments of daily living and quality of life with significantly better tolerability (p < 0.001 for all parameters) than OM. Conclusion: These real-world data confirm the effectiveness and good tolerability of LMP for l-PNP treatment under routine medical care.
Lay abstract
Conditions such as shingles, diabetes and surgery may lead to chronic localized neuropathic pain. This pain is often described as burning or stabbing and can limit functioning in daily activities and diminish quality of life. Several oral and topical medications are available for neuropathic pain treatment. The current study compared the effectiveness and tolerability of lidocaine 700 mg medicated plaster applied directly at the painful skin area with oral medications. Anonymized patient data collected in a German pain registry were selected based on predefined criteria (3081 patient data sets per treatment). Lidocaine plaster treatment resulted in superior pain relief, significantly fewer restrictions in daily life activities and better quality of life than the oral medications evaluated and was significantly better tolerated. This study showed that lidocaine 700 mg medicated plaster is effective and well tolerated in the treatment of chronic localized neuropathic pain in routine medical practice.
Chronic neuropathic pain originates in the peripheral or central nervous system and is now recognized as a separate entity in the ICD-11 [Citation1]. Peripheral neuropathic pain is caused by a lesion or disease of the peripheral somatosensory nervous system [Citation1]; the pain can be spontaneous (burning, throbbing, and shooting pain) and/or stimulus-evoked (allodynia and hyperalgesia) [Citation2]. It includes pain indications such as postherpetic neuralgia (PHN), diabetic polyneuropathy (pDPN), postsurgical neuropathy (PSNP) and others. In many of these indications, pain is localized (e.g., the area of maximum pain is often characterized by a specific, clearly circumscribed area [Citation3]). The prevalence of localized peripheral neuropathic pain (l-PNP) among chronic pain patients is high [Citation4] and the burden of pain is considerable with a profound impact on quality of life [Citation4–6].
As treatment recommendations vary across countries, the analgesic treatment patterns in primary care also differ [Citation4]. International guidelines generally recommend oral systemic medications such as tricyclic antidepressants (TCAs), the antiepileptics pregabalin and gabapentin, and the selective serotonin-norepinephrine reuptake inhibitors (SSNRIs) duloxetine and venlafaxine as first-line treatment [Citation7,Citation8]. The topical agents lidocaine 700 mg medicated plaster (LMP) and capsaicin 8% patch are recommended for second-line treatment; LMP is considered for first-line treatment in PHN [Citation7] or when there are tolerability concerns about the use of systemic medications – in particular, in the frail and elderly [Citation7,Citation8].
Topical treatments have also been suggested as first-line medications for the treatment of l-PNP because of their far better benefit/risk ratios compared with oral medications (OMs), the targeted treatment at the site of the pain and the low systemic exposure and thus a reduced risk of systemic side effects and drug–drug interactions [Citation9]. Two appraisals of the clinical evidence of the lidocaine plaster suggest LMP as first-line treatment of localized neuropathic pain either as a single agent or as part of a multimodal approach [Citation10,Citation11]. To date, LMP is approved in 54 countries worldwide for PHN and additionally in 15 of these countries for l-PNP treatment.
Randomized controlled trials (RCTs) constitute the best available standard of evidence of treatment efficacy [Citation12]; nevertheless, real-world data from electronic health records, health insurance claims databases, prescription data or patient registries provide additional evidence for benefit/risk evaluations of a treatment [Citation12,Citation13]. Real-world data obtained from the German Pain eRegistry (GPeR) were used to evaluate the benefit/risk profile of LMP for PHN in a recently published paper [Citation14] and confirmed the effectiveness and good tolerability of LMP in this condition. Based on treatment guidelines, LMP has, however, also been used in other l-PNP conditions in Germany. Therefore, GPeR data provide a unique opportunity to study the benefit/risk of LMP in a large population of l-PNP patients including analyses of data from a population of patients with l-PNP other than PHN. The present noninterventional retrospective cohort study analyzed real-world practice data from the GPeR with the aim to provide real-world evidence for the use of the lidocaine 700 mg medicated plaster in the treatment of l-PNP and in particular of l-PNP other than PHN.
Methods
This noninterventional retrospective cohort study compared l-PNP treatment with either LMP or recommended oral first-line treatments (according to the current German guideline for the treatment of neuropathic pain [Citation15]). Only patients refractory to at least one previous first-line OM (single drug or combination of drugs) were included in this assessment. The analysis used data of the GPeR, a German web-based pain treatment registry developed on behalf of the German Pain Association. According to GPeR standard procedures, data made available for healthcare research are anonymized, temporarily extracted from the database and deleted after the completion of the analyses specified in the research project.
GPeR data are originally prospectively collected for routine purposes via the online documentation service iDocLive®, a standardized program that facilitates the exchange of information between patient and pain physician with respect to individual pain treatment and patient care. Data can be entered by both patients and their physicians. The patient questionnaires used on iDocLive are recommended by the German Pain Association, the German Pain Society and the German Pain League with core parameters based on the German Pain Questionnaire and German Pain Diary [Citation16]. They include a variety of validated instruments for the assessment of intensity, severity, phenomenology and chronification stage of pain; pain-related impairments of daily life; quality of life; well-being; and depression, anxiety and stress; they also collect data on pain treatment and treatment-related adverse events (AEs).
Data selection
There was no formal sample size calculation for this analysis. All data sets of patients with newly initiated treatment with one of the study medications before 31 December 2018, were selected from the GPeR database as of 31 July 2019, according to defined inclusion and exclusion criteria. Included data sets were of adult outpatients with a medically confirmed l-PNP and a pain history of at least 3 months unsuccessfully treated with at least one first-line OM (single drug or a combination of drugs). Exclusion criteria included a diagnosis of cancer and/or cancer-related pain; chemotherapy-induced neuropathic pain; any type of evidence for HIV and/or HIV-related neuropathy; a diagnosis of low back pain, osteoarthritis, complex regional pain syndrome, myofascial pain and trigeminal autonomic cephalalgia; and any painful lesions of the cranial nerves not caused by l-PNP. Treatment initiation was defined as no study medication use in the 12 weeks before treatment; the date of the first dose was set as the starting date for the 6-month assessment period.
The data selection process has been previously described [Citation14]. Briefly, the database contained data sets of 243902 patients; 22,702 were adult noncancer patients fulfilling the preset criteria for entry into the study. LMP treatment was documented for 4014 patients, OM treatment for 18,688 patients. A matched pair approach using propensity score matching was chosen to obtain comparable study cohorts. The selection of the variables to generate the propensity score was based on patient characteristics anticipated based on available literature and treatment guidelines to have an influence on treatment selection such as age, sex, 24-h pain intensity index, pain severity [Citation17], chronification stage [Citation18], pain duration, comorbidities (ICD-10 code, first three digits), indications/diagnosis for treatment, and previous medication (ATC code, first three digits and/or medication group). For study-specific reasons, the reason for treatment switching (effectiveness, tolerability, drug-related AEs) was also included. The nearest neighbor method for these predefined confounding factors matched the data sets of 3081 patients in both groups.
Medication under evaluation & concomitant analgesic medication
Medications under evaluation were LMP versus a recommended first-line OM. All treatment decisions (e.g., selection of analgesic medication and concomitant analgesic medication, initial dosing, dose adjustments) were at the discretion of the treating physician and were based on the individual patient’s needs.
Outcome measures
Several instruments were used to assess treatment effectiveness. Pain intensity was rated on a 100-mm visual analog scale (VAS) with 0 = no pain to 100 = worst imaginable pain. A pain intensity index (PIX) was calculated as the arithmetic mean of the lowest, average and highest 24-h pain intensities. Pain-related impairments in daily activities related to ‘home and family,’ ‘recreation,’ ‘social activities,’ ‘occupation,’ ‘self-care/personal maintenance,’ ‘sleep’ and ‘overall quality of life’ were reported using a modified version of the pain disability index [Citation19] (mPDI) on a 100 mm VAS (0 = none to 100 = worst imaginable). Pain-related quality of life was measured by the quality-of-life impairment by pain inventory questionnaire (QLIP). This instrument rates well-being, sleep, pain, impairments and mood with a sum score ranging from 0 to 40 points. A sum score of ≤20 points indicates severe impairment. Overall quality of life was assessed with the eight physical and mental domains of the Short Form 12 version 2 (SF-12v2) questionnaire [Citation20] and summarized in a physical and a mental component score (PCS/MCS). Patients’ clinical pain phenotype was determined using the modified seven-item version of the validated PainDETECT questionnaire (PDQ7 [Citation21]). Patients reported their change in health status using the Patient Global Impression of Change (PGIC) scale ranging from 1 = very much improved to 7 = very much worse [Citation22]. Tolerability assessments were based on the occurrence of drug-related adverse events (DRAEs) and treatment discontinuation due to DRAEs. A DRAE was defined as an AE possibly, probably or definitely related to the study medication.
Statistical analysis
Data sets of patients who received at least one dose of the relevant treatment under assessment and recorded at least one postbaseline/postdose measure were included in the analysis. When changes from baseline to end point were assessed, data were included only if there was both a baseline and corresponding post-baseline measure. For the primary effectiveness end point, missing data were imputed using the baseline observation carried forward (BOCF) approach for data sets from patients who discontinued treatment (due to an AE, death or lack of effectiveness). Otherwise, the last observation carried forward (LOCF) method was used for the primary and all secondary variables.
The primary effectiveness population was the group of patients with l-PNP other than PHN. The primary effectiveness end point was determined for this population and also for the overall population of patients with l-PNP. It was defined as the absolute change in the average PIX from baseline following 4, 12 and 24 weeks of treatment and over the treatment period and was assessed by mixed-model repeated measures (MMRM) analysis of covariance (ANCOVA) adjusted for potential confounding factors such as age, sex, pain severity, stage of chronification, history/duration of pain, comorbidity, comedication, a subtype of l-PNP condition, previous medication and baseline values. On the basis of the model, the difference in least squares (LS) mean and two-sided 95% CIs between the treatments following 4, 12 and 24 weeks of treatment was calculated across all three time points. It was defined a priori that LMP noninferiority to OM was confirmed in case the upper bound of the 95% CI did not exceed +10.0. In case noninferiority was confirmed and the MMRM covariance analysis indicated a significant difference between the treatment groups in favor of LMP, a supplemental superiority analysis was conducted. Superiority was rejected if either the 95% CI for the primary end point measure of both treatment groups overlapped, and/or the 95% CI of the LS mean difference for the primary effectiveness variable between both treatment groups included ‘0,’ and/or the upper bound of the 95% CI was higher than -10.0. LMP superiority was confirmed, if all of the aforementioned three effectiveness criteria were fulfilled and if the number of reported treatment discontinuations due to DRAEs was significantly less for LMP than those documented for the comparator group.
Secondary effectiveness end points were the change from baseline after 4, 12 and 24 treatment weeks in average 24-h PIX (relative change), in pain-related impairments in daily life, in clinical pain phenotype, in quality-of-life impairment by pain and in overall quality of life, response to treatment (defined as ≥30 and ≥50% reduction in PIX), patients’ impression of change in health status, patients with DRAEs, reasons for premature discontinuation, discontinuation of treatment due to DRAEs and change in concomitant medications during the observation period in various predefined populations. Treatment differences were compared by conducting MMRM ANCOVA analyses.
Student’s t-test, Pearson’s chi-square test and Fisher’s exact test were used for between-group comparisons of continuous and categorical variables. For within-group comparisons, paired-samples t-tests were performed. All statistical tests were carried out using a two-sided significance level of 0.05. There were no adjustments for multiplicity. The effect size of the comparisons for the primary variable was determined with Cohen’s d. AEs were encoded using the Medical Dictionary for Regulatory Activities (MedDRA version 22.0).
All analyses were conducted using PASW Statistics version 18.
Results
Following propensity score matching, the two resulting cohorts of 3081 patients were comparable for baseline data (). They included 1711 PHN, 732 pDPN and 531 PSNP patients, and 107 patients with other l-PNP indications (OTHNP) in each group. The majority (64%) was female, half were >60 years old (LMP patients 50.6%, OM patients 51.3%) and nearly all (96%) had comorbidities and received concomitant medications. Pain had been present for a mean of 1.6 years in both groups. In comparison, patients with l-PNP other than PHN were slightly older than those suffering from PHN (mean [SD] age: 62.7 [14.6] vs 59.2 [14.3] years, respectively; ).
Table 1. Baseline characteristics of all patients with localized neuropathic pain conditions (n = 3081 in each group).
Table 2. Baseline characteristics of all patients with localized neuropathic pain conditions treated with the lidocaine 700 mg medicated plaster stratified for patients with postherpetic neuralgia (PHN) and patients with diabetic polyneuropathy, postsurgical neuropathy or other peripheral localized neuropathic pain conditions (l-PNP other than PHN).
Analgesic treatment
The mean treatment duration in the LMP group was 144.2 ± 47.2 days (95% CI 142.5–145.9). Overall, 27.3% of the patients discontinued LMP treatment earlier than 24 weeks; the main reasons are listed in . Patients in the OM group received one of the following treatments for their l-PNP: antiepileptic medications (36%), SSNRIs (32.8%) or TCAs (31.2%; ). The most used medications were pregabalin (29.9%), duloxetine (24.7%) and amitriptyline (15.7%). The mean treatment duration was 102 ± 66.6 days (95% CI: 99.6–104.3). Treatment discontinuation before 24 weeks was documented for 54.9% of OM patients; the main reasons are shown in .
Table 3. Main reasons for discontinuation from treatment.
Table 4. Oral recommended first-line medications documented for the treatment of localized neuropathic pain conditions in the OM group after switching from previous medication (n = 3081).
The mean normalized daily dose (percentage of the maximum recommended dose) was 50.5 ± 26.3% (95% CI: 49.6–51.5) in the LMP group and 71.9 ± 31% (95% CI: 70.8–73.0) for OM patients (p < 0.001).
All patients in both treatment groups received concomitant analgesic medications at baseline (). After 6 months of treatment with the study medication, significantly more LMP patients than OM patients had discontinued concomitant analgesic medications (43.1% vs 10.5%; p < 0.001). shows the changes over the observation period for different analgesic medication classes. The proportion of patients who had stopped taking background medication was significantly higher in the LMP group with a relative risk of 4.12 (95% CI: 3.69–4.60; p < 0.001) for overall concomitant analgesic medications and of 1.49 (95% CI: 1.42–1.57; p < 0.001) for rescue medication.
Table 5. Change in concomitant analgesic medication at end of observation.
Pain intensity
At baseline, the mean PIX in the overall l-PNP population was 64.5 ± 14.5 mm for both treatment groups. In the primary effectiveness population (patients with l-PNP other than PHN), the mean (standard error [SE]) change in absolute scores for average 24-h PIX over the 24-week treatment period was -30.49 mm (0.32) and -17.39 mm (0.32) in LMP and OM patients, respectively (). At all three time points, the scores were substantially improved from baseline in both treatment cohorts (data not shown). The LS mean difference (SE) between the cohorts was -13.10 mm (0.45) with a 95% CI of -13.99 to -12.21 (p < 0.001). Because the upper bound of the 95% CI did not exceed +10.0 mm, noninferiority of LMP treatment to OM treatment was confirmed. In addition, the predefined superiority criteria relating to CIs were fulfilled, and discontinuations due to DRAEs were significantly higher in the OM than in the LMP group. Thus, the superiority of LMP over OM could be demonstrated in patients with l-PNP conditions other than PHN.
Table 6. Absolute mean change from baseline (standard error) in average 24-h pain intensity index (averaged over 4, 12 and 24 weeks after treatment initiation).
Similar results were obtained in the overall population. Reductions in the absolute change in the average PIX from baseline were significantly greater for LMP than OM patients overall () and at the three timepoints 4, 12 and 24 weeks of treatment () with a strong effect size (Cohen’s d >1.0 at all timepoints). After 24 weeks, the mean relative change from baseline in PIX was 52.5% for LMP patients and 29.4% for OM patients (p < 0.001, Cohen’s d = 0.878). After 24 weeks, more than 90% of the patients treated with LMP showed a 30% treatment response and more than 50% showed a 50% treatment response, whereas significantly lower responder rates were reported in the OM group ().
Table 7. Absolute mean change from baseline (standard deviation) in average 24-h pain intensity index over the observation period in the overall localized peripheral neuropathic pain population.
Table 8. Proportion of patients in the overall localized peripheral neuropathic pain population with a treatment response (≥30% and ≥50% reduction in the average 24-h pain intensity index) over the observation period.
Further effectiveness outcomes
Analyses of pain-related disability, quality of life and pain phenotype in the overall l-PNP patient population consistently revealed a significant difference in favor of LMP compared with alternative OMs (p < 0.001; ).
Table 9. Mean change from baseline (standard error) in further effectiveness parameters in the overall localized peripheral neuropathic pain population (averaged over 4, 12 and 24 weeks after treatment initiation).
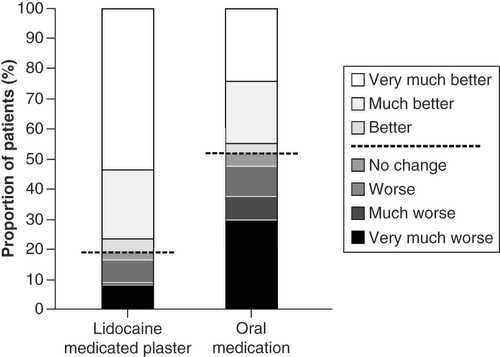
Change in health status was also rated significantly more favorable by LMP patients than OM patients (p < 0.001; ) with 76.2% versus 44.5% reporting ‘much better’ and ‘very much better’ improvements.
Tolerability
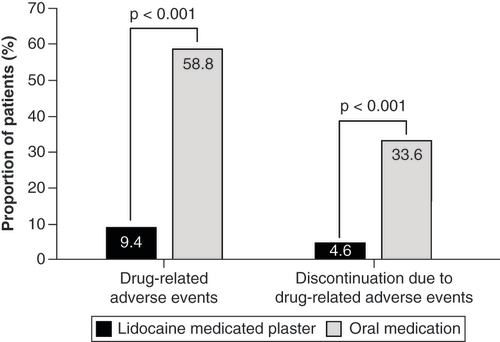
The proportion of patients with DRAEs was significantly lower under LMP treatment (9.4 vs 58.8% for OM patients; p < 0.001; ). In the LMP group, DRAEs were mainly application site reactions and other skin-related reactions (). OM patients reported mainly nervous system disorders (25.2%), psychiatric disorders (23.3%) and gastrointestinal disorders (17.5%; ). The most common DRAEs were somnolence (15.3% of patients) and dizziness (5.6%).
Table 10. All drug-related adverse events documented in patients receiving lidocaine 700 mg medicated plaster during the observation period (n = 3081).
Table 11. Main drug-related adverse events documented in patients receiving oral first-line medication during the observation period (n = 3081).
Treatment discontinuation due to DRAEs occurred significantly less often in the LMP group (4.6% vs 33.6% for OM patients; p < 0.001; & ).
Discussion
Localized neuropathic pain is a prevalent condition associated with a profound impact on quality of life and high burden of care [Citation4–6]. Based on its product characteristics and its clinical profile, LMP has been proposed as a first-line treatment for l-PNP [Citation9–11]. It has been registered in many countries for the treatment of PHN and in some for l-PNP. The aim of this study was to investigate if LMP is as effective in l-PNP conditions other than PHN such as PSNP and pDPN as in the already established PHN indication. Moreover, it also intended to compare its effectiveness, tolerability, effect on pain-related disability, quality of life and pain phenotype in the l-PNP population with oral first-line treatments. Additionally, an evaluation at weeks 4, 12 and 24 after treatment initiation (i.e., much longer than the usual 12 weeks covered in clinical trials) allowed for an evaluation of treatment effect development over time.
LMP application over a treatment period of 24 weeks under routine medical care resulted in pronounced reductions in pain intensity (by 52%) and in reduced impairments of daily activities, and better health status in a multimorbid l-PNP patient cohort with a high pain burden already refractory to at least one first-line OM. There was no evidence of a decrease in efficacy over time with LMP; on the contrary, efficacy was maintained, and quality of life improved also when concomitant analgesic medications were discontinued (observed in over 40% of LMP patients). The plaster was well tolerated. The overall incidence of LMP-related adverse events was low and consisted mainly of application site reactions and other skin-related tolerability issues.
LMP treatment provided significantly better pain relief and greater improvements in daily functioning and quality of life than OMs. Testing of the primary effectiveness end point in the l-PNP population other than PHN confirmed non-inferiority and additionally superiority of LMP over OMs. Similar results were obtained when comparing LMP and OM in the total population of patients with l-PNP. Furthermore, for the entire population of l-PNP patients, its tolerability profile was significantly better: systemic side effects were not reported in the LMP group in contrast to the 59% of OM patients with mainly nervous system, psychiatric and gastrointestinal drug-related AEs being reported. The treatment discontinuation rate due to drug-related events was also significantly lower (4.6 vs 33.6%).
The effectiveness of LMP was previously observed in a number of neuropathic or mixed pain conditions in clinical trials and clinical practice [Citation23] including refractory neuropathic pain [Citation24,Citation25]. In addition to a reduction in pain intensity, a significant decrease of the painful area was shown in patients with localized pain conditions [Citation26–28]. Previous network meta-analyses of RCTs have shown at least comparable treatment efficacy of the plaster compared with OMs [Citation29,Citation30]. The patient-reported outcomes reported here indicate a better treatment response to LMP than to OMs in the routine daily care of chronic l-PNP patients. Our data also confirm the good short- and long-term tolerability profile of the plaster [Citation31] and the generally better AE profile compared with OMs [Citation30,Citation32].
Several recent publications have focused on the efficacy of LMP in postsurgical neuropathic pain [Citation33–35]. All recent PSNP trials confirmed that LMP is well tolerated and showed a clinically relevant effect on pain, although not reaching statistical significance in one of the three trials [Citation35]. Moreover, LMP was shown to significantly alleviate pain and sleep disturbances in the treatment of chronic postthoracotomy neuropathic pain [Citation33] and to be effective in relieving localized neuropathic pain after knee surgery [Citation34].
Considering the difficulty of conducting placebo-controlled RCTs with LMP given its mechanisms of action and the feasibility of such studies (access to patients may be reduced when the medication is commercially available), alternative data such as real-world data can assist in evaluating the benefit/risk of the treatment compared with other available treatments. The additional advantage of such an approach is that observational studies generally include a more heterogeneous population that better reflects the patient population to be treated compared with clinical trial data from RCTs with their narrowly defined trial criteria resulting in homogenous but very specific trial populations.
The German Pain eRegistry facilitates the communication between patient and physician in routine medical care and focuses on outcomes established to improve patient care. In contrast to RCTs, the large database stores long-term data, whereas clinical trials are usually restricted to a predefined short-term period. All treatment decisions were solely made by the treating physicians (i.e., not influenced by the prospect of the patient’s participation in a research study).
Analyses using data extracted from the GPeR have all limitations inherent to observational retrospective studies. To strengthen the validity of the results, a study protocol and a statistical analysis plan were developed a priori and a matched pair approach with propensity score matching was employed to avoid comparison bias. This resulted in a large study population with 3081 data sets of patients per treatment group. The questionnaires used in the registry are standardized tools and include a broad range of validated instruments as required for quality-assured and standardized documentation of all treatment-relevant data for the routine care of chronic pain patients in Germany. It should, however, be noted that although the chosen statistical method of propensity score matching eliminated the main confounding factors, it does not exclude the possible presence of other confounders. Data on actual compliance in taking the prescribed medications under evaluation and information on why concomitant analgesics were prescribed are not captured by the standard documentation in iDocLive. Prescription of a specific treatment for l-PNP was based on individual patient needs and the response to previous therapies, which may also have an influence on the outcome. It is also not known if physicians prescribing OMs might have paid closer attention to side effects. In addition, an information bias based on documentation errors and misclassifications cannot be ruled out because only anonymized data were available due to data protection regulations.
These real-world findings add to the body of evidence accumulated in clinical trials demonstrating the good effectiveness and excellent short- and long-term tolerability of LMP compared with systemic medications. In routine daily care, LMP was effective in the overall l-PNP patient population and in the subgroup of patients with l-PNP other than PHN as reported in this study but also in PHN as reported previously [Citation14]. The reduced risk of systemic side effects and low potential for drug–drug interactions and the easy application at the site of the pain might increase patients’ treatment compliance and thus provide better treatment outcomes. This is particularly important for multimorbid elderly patients with an already high medication intake and is consistent with conclusions from other authors that ‘a therapy that promises a rapid onset of action without the risk of systemic side effects and sexual dysfunction is preferred by patients even if the application is time-consuming and associated with a higher risk of local side effects’ [Citation36]. The lidocaine 700 mg medicated plaster should therefore be considered a valuable alternative to oral medications in the treatment of l-PNP.
Conclusion
The analysis of real-world data extracted from the German Pain eRegistry confirmed the effectiveness of the lidocaine 700 mg medicated plaster in improving pain intensity, pain-related impairment and quality of life in the treatment of peripheral localized neuropathic pain. These improvements resulted in a marked reduction of concomitant pain medications under routine medical care without loss of efficacy. Tolerability was good, leading to few treatment discontinuations due to DRAEs. Moreover, this analysis showed significantly better outcomes with LMP treatment on all measured parameters compared with first-line oral medications in patients who failed on a first-line OM.
Localized peripheral neuropathic pain (l-PNP) is prevalent among chronic pain patients and associated with a profound impact on quality of life and high burden of care.
The topical lidocaine 700 mg medicated plaster (LMP) provides targeted treatment at the site of the pain, supporting its use in the treatment of l-PNP. LMP has demonstrated good short- and long-term efficacy with an excellent safety profile in the treatment of postherpetic neuralgia.
The present noninterventional retrospective cohort study investigated whether LMP is as effective in other l-PNP conditions such as diabetic polyneuropathy or postsurgical neuropathy and how its effectiveness and tolerability compare with oral first-line medications (OM) under routine medical care.
Real-world practice data were extracted from the German Pain eRegistry. Propensity score matching resulted in 3081 data sets per treatment group.
More than 60% of patients had experienced pain for more than a year and reported a high burden of pain and reduced quality of life.
Six months of LMP treatment provided significantly greater pain reductions and improvements in pain-related impairments of daily living and quality of life than OM treatment (p < 0.001 for all parameters). Testing of the primary effectiveness end point confirmed noninferiority and, additionally, superiority of LMP over OMs.
The proportion of patients with drug-related adverse events (DRAEs) was significantly lower under LMP treatment (9.4 vs 58.8% for OM patients; p < 0.001) and consisted mainly of application site reactions. The treatment discontinuation rate due to DRAEs was also significantly lower (4.6 vs 33.6% for OM patients; p < 0.001).
A higher proportion of patients on LMP were able to discontinue concomitant analgesic medication without loss of efficacy.
In routine daily care, chronic l-PNP patients unsuccessfully treated with first-line oral medications had a significantly better response to newly initiated LMP treatment with a significantly better tolerability profile compared with OM treatment.
Author contributions
Conception, design and data interpretation: all authors. Data extraction and analysis: MA Überall. All authors critically revised for important intellectual content, approved the final manuscript version and agreed to the submission.
Ethical conduct of research
The study is registered in the European Union electronic Registry of Post-Authorization Studies (EUPAS 32826) through the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) coordinated by the European Medicines Agency and was conducted in accordance with the Declaration of Helsinki and relevant national and regulatory requirements. The concept and use of the German Pain e-Registry have been reviewed and approved by the steering committees of the German Pain Association and the German Pain League. All patients provided written informed consent prior to participation in the registry. All analyses were carried out using only anonymized data to comply with German guidelines on protection of data privacy and with the European Union General Data Protection Regulation.
Acknowledgments
The authors thank GHH Müller-Schwefe for his critical assessment and interpretation of the data.
Financial & competing interests disclosure
MA Überall is a physician, pain specialist, medical director of the Institute of Neurological Sciences, and CEO of O.Meany-MDPM GmbH, which was responsible for data extraction and biometrical analyses. Data extraction and biometrical analyses were paid for by Grünenthal GmbH, Germany. MA Überall has received financial support and/or expenses in form of research funds, consultancy fees and/or renumerations for lecture activities from Allergan, Almirall, Amicus Therapeutics, Aristo Pharma, Bionorica, Esanum, Glaxo Smith Kline, Grünenthal, Hapa Medical, Hexal, IMC, Kyowa-Kirin, Labatec, Mucos, Mundipharma, Nestle, Pfizer, Recordati, Servier, SGP-Pharma, Shionogi, Spectrum Therapeutics, Strathmann, Teva and Tilray. M Eerdekens, I Bösl, E Hollanders and I Sabatschus are employees of Grünenthal GmbH, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing and editorial assistance was provided by Elke Grosselindemann and Birgit Brett and was paid for by Grünenthal GmbH, Germany.
Additional information
Funding
References
- Scholz J , FinnerupNB , AttalNet al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain160, 53–59 (2019).
- Casale R , MattiaC. Building a diagnostic algorithm on localized neuropathic pain (LNP) and targeted topical treatment: focus on 5% lidocaine-medicated plaster. Ther. Clin. Risk Manage.10, 259–268 (2014).
- Mick G , BaronR , FinnerupNBet al. What is localized neuropathic pain? A first proposal to characterise and define a widely used term. Pain Manage.2, 71–77 (2012).
- Mick G , SerpellM , BaronRet al. Localised neuropathic pain in the primary care setting: a cross-sectional study of prevalence, clinical characteristics, treatment patterns, quality of life and sleep performance. Curr. Med. Res. Opin.37, 293–302 (2021).
- Van Acker K , BouhassiraD , DeBacquer Det al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab.35, 206–213 (2009).
- Serpell M , GaterA , CarrollSet al. Burden of post-herpetic neuralgia in a sample of UK residents aged 50 years or older: findings from the zoster quality of life (ZQOL) study. Health Qual. Life Outcomes.12, 92 (2014).
- Attal N , CruccuG , BaronRet al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol.17, 1113–e1188 (2010).
- Finnerup NB , AttalN , HaroutounianSet al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol.14, 162–173 (2015).
- Allegri M , BaronR , HansGet al. A pharmacological treatment algorithm for localized neuropathic pain. Curr. Med. Res. Opin.32, 377–384 (2016).
- de León-Casasola OA , MayoralV. The topical 5% lidocaine medicated plaster in localized neuropathic pain: a reappraisal of the clinical evidence. J. Pain Res.9, 67–79 (2016).
- Baron R , AllegriM , Correa-IllanesGet al. The 5% lidocaine-medicated plaster: its inclusion in international treatment guidelines for treating localized neuropathic pain, and clinical evidence supporting its use. Pain Ther.5, 149–169 (2016).
- Cave A , KurzX , ArlettP. Real-world data for regulatory decision making: challenges and possible solutions for Europe. Clin. Pharm. Ther.106, 36–39 (2019).
- Bolislis WR , FayM , KühlerTC. Use of real-world data for new drug applications and line extensions. Clin. Ther.42, 926–938 (2020).
- Überall MA , EerdekensM , BöslIet al. Lidocaine 700mg medicated plaster for postherpetic neuralgia: real-world data from the German Pain e-Registry. Pain Manage. (2021) ( Epub ahead of print).
- German Society of Neurology ; SchlerethTet al.Diagnosis and noninterventional treatment of neuropathic pain, S2k guideline (in German), 2019. In: Guidelines for Diagnosis and Treatment in Neurology.www.awmf.org/uploads/tx_szleitlinien/030-114l_S2k_Diagnose-nicht-interventionelle-Therapie-neuropathischer-Schmerzen_2020-04_1.pdf
- German Pain Society . Manual for the German pain questionnaire (in German). www.schmerzgesellschaft.de/fileadmin/pdf/DSF_Handbuch_2020.pdf
- von Korff M , OrmelJ , KeefeFJ , DworkinSF. Grading the severity of chronic pain. Pain50, 133–149 (1992).
- Schmitt N , GerbershagenHU. The Mainz Staging System (MPSS) for chronic pain. Pain41(Suppl. 5), S484 (1990).
- Tait RC , ChibnallJT , KrauseS. The pain disability index: psychometric properties. Pain40, 171–182 (1990).
- Hayes CJ , BhandariNR , KatheN , PayakachatN. Reliability and validity of the medical outcomes study short form-12 version 2 (SF-12v2) in adults with non-cancer pain. Healthcare (Basel)5, 22 (2017).
- Cappelleri JC , BienenEJ , KoduruV , SadoskyA. Measurement properties of painDETECT by average pain severity. Clinicoecon. Outcomes Res.6, 497–504 (2014).
- Guy W . ECDEU Assessment Manual for Psychopharmacology (DHEW Publication no. ADM 76-338).US Government Printing Office, DC, USA (1976).
- Mick G , Correa-IllanesG. Topical pain management with the 5% lidocaine medicated plaster – a review. Curr. Med. Res. Opin.28, 937–951 (2012).
- Delorme C , NavezML , LegoutVet al. Treatment of neuropathic pain with 5% lidocaine-medicated plaster: five years of clinical experience. Pain Res. Manage.16, 259–263 (2011).
- Martini A , DelBalzo G , SchweigerVet al. Efficacy of lidocaine 5% medicated plaster (VERSATIS®) in patients with localized neuropathic pain poorly responsive to pharmacological therapy. Minerva Medica109, 344–351 (2018).
- Correa-Illanes G , CalderónW , RoaRet al. Treatment of localized post-traumatic neuropathic pain in scars with 5% lidocaine medicated plaster. Local Regional Anesth.3, 77–83 (2010).
- Casale R , MatteoMD , MinellaCEet al. Reduction of painful area as new possible therapeutic target in post-herpetic neuropathic pain treated with 5% lidocaine medicated plaster: a case series. J. Pain Res.7, 353–357 (2014).
- Amato F , DuseG , ConsolettiLet al. Efficacy and safety of 5% lidocaine-medicated plasters in localized pain with neuropathic and/or inflammatory characteristics: an observational, real-world study. Eur. Rev. Med. Pharmacol. Sci.21, 4228–4235 (2017).
- Wolff RF , BalaMM , WestwoodM , KesselsAG , KleijnenJ. 5% lidocaine-medicated plaster vs other relevant interventions and placebo for post-herpetic neuralgia (PHN): a systematic review. Acta Neurol. Scand.123, 295–309 (2011).
- Buksnys T , ArmstrongN , WorthyGet al. Systematic review and network meta-analysis of the efficacy and safety of lidocaine 700 mg medicated plaster vs. pregabalin. Curr. Med. Res. Opin.36, 101–115 (2020).
- Navez ML , MonellaC , BöslIet al. 5% lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther.4, 1–15 (2015).
- Katz P , PegoraroV , LiedgensH. Characteristics, resource utilization and safety profile of patients prescribed with neuropathic pain treatments: a real-world evidence study on general practices in Europe – the role of the lidocaine 5% medicated plaster. Curr. Med. Res. Opin.33, 1481–1489 (2017).
- Sansone P , PassavantiMB , FiorelliAet al. Efficacy of the topical 5% lidocaine medicated plaster in the treatment of chronic post-thoracotomy neuropathic pain. Pain Manage.7, 189–196 (2017).
- Pickering G , VouteM , MacianNet al. Effectiveness and safety of 5% lidocaine-medicated plaster on localized neuropathic pain after knee surgery: a randomized, double-blind controlled trial. Pain160, 1186–1195 (2019).
- Palladini M , BoeslI , KoenigSet al. Lidocaine medicated plaster, an additional potential treatment option for localized post-surgical neuropathic pain: efficacy and safety results of a randomized, placebo-controlled trial. Curr. Med. Res. Opin.35, 757–766 (2019).
- Schubert T , KernKU , SchneiderS , BaronR. Oral or topical pain therapy – how would patients decide? A discrete choice experiment in patients with peripheral neuropathic pain. Pain Pract.21, 536–546 (2021).