Abstract
Objective: This study presents real-world data from a cross-sectional follow-up survey of patients who previously received 60-day peripheral nerve stimulation (PNS) treatment for pain. Materials & methods: A survey including validated pain and other related outcome measures was distributed to patients who previously underwent implantation of temporary PNS leads for 60-day PNS treatment. Results: Among survey respondents who were at least 3 months from the start of treatment, most reported sustained clinically significant improvements in pain and/or quality of life, with the length of follow-up at the time of survey completion ranging from 3 to 30 months. Conclusion: These real-world data support recent prospective studies indicating that 60-day percutaneous PNS provides significant and sustained relief across a wide range of pain conditions.
Plain language summary
This study presents the findings from a survey that was sent to patients who previously received a 60-day peripheral nerve stimulation (PNS) treatment for their chronic pain. Patients were asked about their current pain levels, how their quality of life and physical function have changed since their PNS treatment, and whether they had changed their usage of pain medications. The survey showed that most patients who were at least 3 months from the start of the PNS treatment continued to have meaningful pain relief and/or improvement in their quality of life. This information is consistent with clinical studies that were previously published and supports that the 60-day PNS treatment can provide patients with long-term relief of chronic pain.
Chronic pain is a major global health issue with substantial socioeconomic impacts. The estimated prevalence of high-impact chronic pain is 4.8–8% in the USA [Citation1,Citation2]. Pain often leads to discouragement, anger, embitterment and general suffering with a strong negative correlation between pain severity and quality of life [Citation3–6]. In addition, long-term treatment of pain is a significant financial burden with costs estimated at $635 billion annually across the USA [Citation7].
Peripheral nerve stimulation (PNS) has been used successfully to treat a wide range of pain conditions, including intractable neuropathic pain, post-traumatic nerve pain, causalgia, chronic axial back pain, postoperative pain, joint pain and postherpetic neuralgia [Citation8–16]. However, PNS systems were historically limited by the need for permanent implantation of leads in close contact with nerves and suffered from high rates of lead migration and subsequent loss of long-term efficacy.
Subsequently, a percutaneous PNS system was developed and US FDA-cleared that provides temporary PNS treatment with fine-wire, open-coil leads designed to reduce infection risk, enabling implantation for up to 60 days without necessitating permanent implantation of hardware [Citation17,Citation18]. Prospective clinical trials with follow-up periods up to 12 months across multiple pain indications, including chronic back pain, shoulder pain and post-amputation pain, have demonstrated that most subjects experienced sustained pain relief following the end of up to 60 days of PNS treatment [Citation19–23]. The most commonly reported adverse event across these studies has been adhesive-related skin irritation. A review found fine-wire, coiled leads to have an estimated 60-day infection rate of 0.1% and 25-fold lower risk of infection compared with conventional cylindrical leads [Citation24], which is similar to the infection rate in published clinical trials to date using temporary percutaneous leads (one superficial infection at a lead exit site across 601 implanted leads, 0.17%) [Citation21–23,Citation25–35].
However, few real-world data have been published regarding the use of 60-day PNS outside of small retrospective case series and individual case reports [Citation36–39]. Real-world data have an important role in providing insight into treatment effectiveness and safety in routine clinical practice (i.e., outside of the environment of clinical research), and calls to increase the collection and sharing of real-world data to improve clinical practice and clinical guidelines have risen recently, including in the neuromodulation field [Citation40,Citation41]. Therefore, the present work summarizes the results of a cross-sectional, follow-up survey of patients who previously underwent implantation of 60-day PNS leads for the treatment of pain with the goals of beginning to validate previously published prospective findings and expanding insights into the use of 60-day PNS in the real world. This study incorporated validated measures of pain, quality of life and physical function to provide a multidimensional assessment of patient improvement after withdrawal of the temporarily implanted leads. This study presents the largest set of real-world data to date regarding the effectiveness and long-term impact of 60-day PNS treatment.
Methods
Study design & sample
The study was a cross-sectional follow-up survey of patients who previously underwent implantation of temporary PNS leads (SPRINT®; SPR Therapeutics, OH, USA) for up to 60 days for the treatment of pain. The survey was distributed via email to an existing real-world database populated with records from 2028 patients who received the FDA-cleared SPRINT PNS treatment between March 2018 and December 2020 and agreed to opt in to a follow-up survey. Due to the nature of this market research and the use of anonymized data listings, ethical committee approval was not required.
As a cross-sectional survey, patient-reported outcomes were queried at a single point in time. The survey was not intended to reflect longitudinal assessments of a consistent population over time, and instead were anticipated to provide initial insights into the potential rates of sustained improvements in pain and other outcomes in the real world following the end of treatment (EOT).
Survey procedures
Eligibility for inclusion required that the patient opted in to data collection at the time of treatment and provided contact information to enable follow-up survey distribution. Interested individuals completed the survey without the presence of their physician or a device representative. The first screen of the survey included a written consent agreement that acknowledged the use of de-identified survey data for research purposes and potential publication. If patients declined the consent agreement, the survey was ended and patients were excluded from further survey data collection or analysis. Survey data were collected from December 2020 to February 2021. Patients were compensated $15 for their time to complete the follow-up survey.
Patients were asked to respond to survey questions specifically regarding the region of pain for which they previously underwent PNS treatment. Survey questions were based on validated pain and other related outcomes measures (Supplementary data). The survey asked patients to report their worst and average pain in the previous week (Brief Pain Inventory – Short Form [BPI-SF] items #3 and #5) as well as their percent pain relief at the time of survey completion (BPI-SF #8) [Citation42]. Patient Global Impression of Change (PGIC) was assessed separately for quality of life and physical function on a seven-point scale (-3 to +3, or very much worse to very much improved). Additional questions collected information about changes in analgesic medication usage since starting the PNS treatment and utilization of other pain therapies.
60-day PNS treatment
Each patient included in the survey study previously underwent commercial implantation of the 60-day PNS system, and no additional interventions were administered as part of the present survey study. The system consists of coiled percutaneous leads that are typically implanted under ultrasound or fluoroscopic guidance and connected externally to a body-mounted pulse generator () [Citation23,Citation28,Citation43]. The stimulating leads are placed remote (e.g., 0.5–3 cm) from the target nerve to deliver a stimulation waveform (asymmetric charge-balanced biphasic pulse train, 1–30 mA, 10–200 μs) at 100 Hz with the goal of producing comfortable sensations covering the region of pain, or at 12 Hz with the goal of producing comfortable, cycling muscle activity secondary to the activation of the nerve [Citation44]. Patients are able to adjust the intensity of stimulation using a wireless remote to maintain comfort throughout the treatment period. Stimulation is delivered for up to 60 days, after which the percutaneous leads are removed by clinical staff by applying gentle traction to the external portion of the lead, and patients proceed to follow-up as directed by their physician.
Stimulation is delivered through percutaneous, coiled leads typically placed under ultrasound or fluoroscopic guidance.
Study outcomes & analysis
All patients who completed the consent agreement and follow-up survey questions were included in the analysis. No survey questions required an answer, so not all patients completed every question, and actual sample sizes are reported as appropriate. Follow-up survey data for each respondent were combined with existing database records including pain and PGIC data collected at baseline and at the end of the 60-day PNS treatment.
The primary outcome evaluated the proportion of patients who were responders to PNS at the end of the 60-day treatment and at the time of the follow-up survey. Studies in the field of pain management have historically focused on pain intensity as a primary outcome, whereas recent publications have begun to recognize the importance of multiple patient-centric domains in addition to pain intensity in the clinical study of chronic pain, including physical function, emotional function, and quality of life or global improvement [Citation45–48]. Pain intensity outcomes alone may underrepresent the overall benefit of pain therapies, suggesting that a wider range of key outcome measures should be considered when assessing the real-world effectiveness of pain interventions [Citation45,Citation49–51]. Therefore, in the present study, patients were considered treatment responders if they obtained ≥50% pain relief (based on patient report of the percentage of pain relief) and/or had at least minimal clinically significant improvement (i.e., at least Minimally Improved) on the PGIC for quality of life. Although physical function is considered a key outcome, it is not included as a criterion in the responder analysis because it was not regularly assessed at the end of PNS treatment.
Analysis focused on patients who were at least 3 months from the start of treatment (i.e., at least 1 month post-lead removal) at the time of survey completion, and patient outcomes were stratified by time since the start of treatment to evaluate whether improvements at EOT were sustained. Additional outcomes included analysis of the individual components of the primary composite outcome (i.e., the proportion of patients reporting ≥50% pain relief or improvement in quality of life as measured by PGIC), and the proportion of patients reporting reduction or cessation of opioid or anti-convulsant medications from before PNS treatment to the time of the follow-up survey. All data are summarized descriptively as mean (standard deviation [SD]) or as proportions of the total number of survey respondents for a given outcome measure.
Results
Study population
A total of 354 patients responded to the survey, and analysis focused on 252 of those patients who completed follow-up survey outcomes and were at least 3 months from the start of treatment. The analysis population (mean age 56.9 ± 17.0 years) included patients who underwent treatment for a variety of pain conditions, including chronic axial back pain, complex regional pain syndrome, neuralgia, osteoarthritis, chronic postoperative pain and meralgia paresthetica. The four most frequently treated pain regions were the low back, shoulder, knee and ankle/foot, and the remaining pain regions were grouped together in a fifth ‘other’ category (). The most frequent nerve targets were the medial branches of the dorsal ramus, suprascapular nerve and femoral nerve, with additional nerves targeted throughout the periphery (). Most patients (64%, 162/252) received stimulation from one lead, whereas the remaining 36% (90/252) underwent implantation of two leads (e.g., targeting pain bilaterally or two different nerves innervating the region of pain). Most patients (51%, 128/250) reported average baseline pain defined as severe (numerical rating scale [NRS] >6 [Citation52]) with a mean average pain of 6.4 (SD 1.7), and 94% (238/252) reported worst baseline pain rated as severe with a mean worst pain of 9.0 (SD 1.3; ).
Table 1. Survey respondent baseline and treatment characteristics.
60-day PNS treatment outcomes
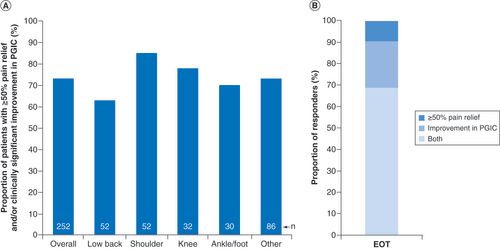
Although the primary goal of the cross-sectional survey was to assess long-term patient outcomes after the short-term 60-day PNS treatment, outcomes at EOT were summarized to determine which survey respondents were responders to PNS treatment as of the end of the 60-day treatment period. At the end of the 60-day PNS treatment, 73% of patients (185/252) reported substantial pain relief (≥50%) and/or improvement in PGIC, ranging from 63% in the low back pain subgroup to 85% in the shoulder pain subgroup (A). Among those who qualified as responders, more than two-thirds reported both ≥50% pain relief and improvement in quality of life, whereas the remainder qualified as responders based on either pain relief or quality of life (B).
(A) Proportion of patients who were responders during peripheral nerve stimulation treatment with substantial (≥50%) pain relief and/or improvement in PGIC (n = 252 total). Responder rates are shown overall and for the most common body regions treated. (B) Among those who were responders in (A) (185/252), the proportions are shown reporting both ≥50% pain relief and improvement in PGIC, ≥50% pain relief alone or improvement in PGIC alone.
EOT: End of treatment; PGIC: Patient Global Impression of Change.
Follow-up survey outcomes
As a cross-sectional survey, information was collected from patients at a single time point, and therefore the duration of follow-up at the time of the survey varied for each patient. Of the 252 patients at least 3 months from the start of treatment (i.e., at least 1 month following PNS lead removal), 75% (188/252) were within the first year (3–12 months from start of treatment), 21% (52/252) were between 1 and 2 years, and 4% (12/252) were more than 2 years from the start of treatment. Due to the commercial availability of the 60-day PNS system beginning in late 2017, this distribution of follow-up durations among survey respondents was anticipated and mirrors the natural growth in the patient population for the 60-day PNS treatment. At the time of the follow-up survey, 50% (125/252) of patients reported sustained substantial (≥50%) reductions in pain and/or improvement in quality of life compared with baseline, ranging from 41% in the knee subgroup to 56% in the shoulder subgroup.
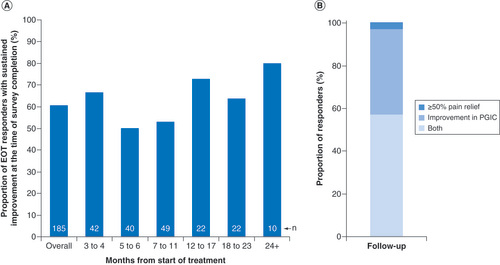
In addition to the overall responder rate at follow-up, the question of whether patients who initially reported improvement during the PNS treatment sustained those improvements long-term is better assessed by evaluating follow-up outcomes specifically among the 185 patients who were initially responders at the end of the 60-day PNS treatment period (EOT responders). Among the EOT responders, 61% (112/185) continued to report sustained improvement (at least 50% pain relief and/or improvement in quality of life) at the time of survey completion. The time of survey completion relative to the start of treatment varied by patient, so responses were stratified by months since the start of treatment at the time of follow-up survey completion (A), demonstrating that long-term responder rates were relatively stable through at least 24 months among the subsets of patients within each time strata. Among EOT responders who also had sustained improvements at the time of survey completion, most reported both ≥50% pain relief and improvement in quality of life, whereas the remainder qualified as responders based on either pain relief or quality of life (B).
(A) Proportion of patients who were responders at EOT (EOT responders) and maintained substantial (≥50%) pain relief and/or improvement in PGIC at long-term follow-up (n = 185 total), stratified by months from the start of treatment at the time of survey completion. Sample size (n) for each follow-up duration is shown. (B) Among those who were responders in (A) (n = 112/185), the proportions are shown reporting both ≥50% pain relief and improvement in PGIC, ≥50% pain relief alone or improvement in PGIC alone.
EOT: End of treatment; PGIC: Patient Global Impression of Change.
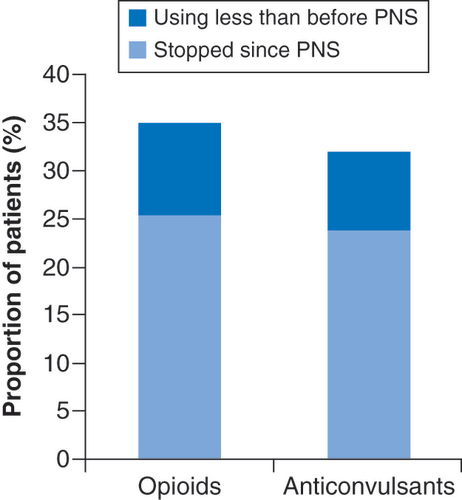
Additionally, 35% (44/126) of those who reported using opioids before beginning the 60-day PNS treatment reported reducing or ceasing usage at the time of survey completion (). Similarly, 32% (47/147) of patients reporting anticonvulsant (i.e., gabapentin, pregabalin) usage at baseline reported reducing or ceasing usage as of the time of survey completion (). Antidepressant use for pain management was less common at baseline, and 15% (15/97) reported ceasing or reducing usage compared with before PNS treatment.
PNS: Peripheral nerve stimulation.
Discussion
This study presents real-world evidence from a cross-sectional survey regarding the long-term effectiveness of 60-day percutaneous PNS for the treatment of chronic pain with the goal of providing insights into the use of 60-day PNS in the real world. Among the 252 survey respondents at least 3 months post start of treatment, most patients reported significant and sustained improvements in pain and/or quality of life at the time of survey completion, which ranged from 3 to 30 months from the start of treatment.
Temporary, percutaneous PNS has been studied in numerous prospective trials across multiple pain indications including chronic low back pain, shoulder pain, knee pain and post-amputation pain. These studies consistently found that most subjects reported substantial pain relief (≥50%) and resulting improvements in function, including in multiple randomized controlled trials and in multiple studies with follow-up durations up to 12 months. Applying a similar composite end point strategy to the subject-level data from previously published studies shows an aggregate rate in the literature of 72% (134/186) of subjects with substantial (≥50%) improvements in pain and/or function during treatment [Citation19–23,Citation25–27,Citation32,Citation35]. Treatment success rates were largely sustained over time in those published studies, with 72% (119/166) of subjects with data at 3 months and 58% (56/97) of subjects with data at 12 months continuing to report substantial improvement in pain and/or function [Citation19–23,Citation25–27,Citation35]. The present survey’s 73% success rate at the end of the 60-day PNS treatment () and 61% long-term success rate among EOT responders with at least 3 months of follow-up (including 58% of EOT responders with at least 12 months of follow-up; ) provide real-world evidence that most patients can achieve sustained long-term improvements even outside the more rigorous patient selection and procedural guidelines of prospective trials, and indicate that previously published prospective study outcomes can be replicated in broader clinical practice.
Consistent with trends in clinical pain research that recognize the importance of multidimensional assessments of patient responses to analgesic treatments, the present study used a primary composite outcome consisting of ≥50% pain relief and/or quality of life improvement. Improvement in quality of life as measured by the PGIC tended to correlate with clinically significant levels of pain relief, with most patients reporting both ≥50% pain relief and improvement in PGIC ( & ), and on average, patients reporting that they were ‘Minimally Improved’ in PGIC achieved clinically significant levels of pain relief. Even though pain relief and quality of life or functional improvements are not always correlated clinically, these data suggest that patients reporting at least minimally clinically significant improvement in PGIC also tended to report clinically significant levels of pain relief on average. Additionally, a subset of patients who were responders as of the end of the 60-day PNS treatment maintained improvement in quality of life with long-term levels of pain relief that ultimately fell below 50% but remained near the threshold for clinical significance (≥30%), supporting the notion that pain relief and other improvement (quality of life, function, etc.) can be decoupled in some patients. For example, these patterns could be indicative of a behavioral priority among some patients toward increasing function or reducing medication usage to the detriment of maximizing pain relief, but to the benefit of a patient’s overall global improvement [Citation45,Citation49]. These types of insights highlight the value of multifaceted assessments of patient responses to pain treatments and therapies and support the growing use of composite measures in clinical research, especially in real-world settings, that can elucidate patient improvement across multiple domains.
The phenomenon of sustained long-term improvement following short-term PNS treatment has generated recent discussion regarding the potential underlying mechanisms of action. Conventional PNS therapies have long been postulated to provide relief via the gate control theory by which activation of large-diameter sensory fibers transiently modulates the spinal processing of peripheral pain signals, a strategy that requires permanent implantation of hardware for continuous stimulation to provide durable relief [Citation44,Citation53–55]. In contrast to conventional PNS approaches, the 60-day PNS treatment studied here utilizes large monopolar electrodes temporarily implanted remotely from the nerve that deliver waveforms more selective for large-diameter fibers (i.e., remote selective targeting) [Citation44,Citation53,Citation56]. Sixty-day PNS, when delivered in the manner described here, is theorized to augment the traditional gating mechanism by producing physiological signals from the periphery that recondition the CNS to reverse maladaptive features of the centralized pain state, enabling patients to experience pain relief or subsequent improvements in function or quality of life long after EOT [Citation19,Citation20,Citation44]. The present survey included patients who received two variations of the short-term PNS treatment: 100-Hz stimulation intended to produce comfortable sensations in the distribution of the stimulated nerve(s) (e.g., femoral or sciatic nerve stimulation for knee pain or foot/ankle pain), and 12-Hz stimulation intended to activate efferent fibers resulting in secondary comfortable cycling muscle tension (e.g., medial branch stimulation for low back pain, axillary nerve stimulation for shoulder pain) [Citation44]. Similar success rates were observed among the low back, shoulder, knee, foot/ankle and other subgroups (), suggesting that both stimulation paradigms produced comfortable, robust, physiological signals focal to the region of pain that were sufficient to recondition the CNS to produce sustained long-term clinical improvement.
Reducing analgesic medication usage, especially opioid analgesics, has become a priority of physicians in recent years in response to the opioid epidemic and CDC recommendations prioritizing nonpharmacological therapies for pain [Citation57]. Reducing chronic analgesic usage can also help mitigate the substantial socioeconomic costs and burden on the healthcare system of opioid abuse and misuse [Citation58,Citation59]. At the time of the present survey, 35% of patients who reported using opioids at baseline and 32% of those using gabapentin or pregabalin at baseline had reduced or stopped the medications since the start of the PNS treatment. As a nonpharmacological intervention that does not require permanent implantation and may help reduce reliance on opioid and nonopioid analgesics, 60-day PNS can be an important and minimally invasive part of the treatment options available to physicians and patients for pain relief.
The survey had a response rate of 17.5%, which could introduce risks of nonresponse bias in the results. However, although self-selection and nonresponse bias can influence the interpretation of survey findings, studies of the impact of nonresponse rates on nonresponse bias have repeatedly found that nonresponse rate alone is a poor predictor of whether the data contains nonresponse bias [Citation60–62]. One method to help determine whether the survey respondent population was representative is to compare the survey respondents against known population means for the larger group of survey recipients. All survey recipients gave written agreement to provide data to the device manufacturer as part of their use of the device prior to their treatment (i.e., prior to knowing whether the treatment would provide pain relief). Of the 2028 patients to whom the follow-up survey was distributed, 1646 had percentage of pain relief and/or PGIC data available at EOT. Therefore, it is possible to compare the outcomes at the end of the 60-day treatment for the survey respondents with the outcomes for a larger population of survey recipients, even though follow-up data were not available for those who did not reply to the survey. A total of 74.3% (1223/1646) reported at least 50% pain relief and/or clinically significant improvement on the PGIC. In the primary survey analysis population (i.e., 252 of the 354 survey respondents who were at least 3 months post treatment at the time of survey completion), 73.4% (185/252) were similarly classified as responders at EOT. These data indicate that, at least as of the end of the 60-day PNS treatment, the survey respondent subgroup was representative of the larger population of survey recipients, suggesting that the nonresponse rate does not significantly bias the interpretation of the survey results.
Study limitations
A primary limitation of the present study is that follow-up data were collected as part of a cross-sectional survey and do not include longitudinal assessments of patients at multiple follow-up timepoints post treatment. These data therefore represent a single time point after the completion of treatment for each patient, and future longitudinal analyses would provide added insight. Furthermore, because of the natural growth of the patient user population over time since the commercial availability of the PNS system began in 2017, a much greater proportion of survey responses came from patients <12 months from the start of treatment compared with longer follow-up durations (e.g., 2+ years). The present study did not control or collect detailed information about other interventions used since the end of the 60-day PNS treatment that could affect pain, nor specific dosing information to determine the clinical significance of changes in medication usage. Follow-up survey responses could therefore be influenced by the use of other pain interventions since the time of the PNS treatment. Nonetheless, patient reports of reduced opioid and anticonvulsant medication usage do support clinical findings that pain relief from the 60-day PNS treatment may enable patients reduce or eliminate oral medication dosage and/or frequency [Citation20,Citation28].
Although the survey response rate (17.5%) is within the range commonly observed for email surveys [Citation63,Citation64], there is nonetheless potential for self-selection bias among survey respondents. In addition, because in-person interviews were unfeasible given the size and national distribution of the real-world database, follow-up outcomes were self-reported by patients. The survey was also distributed by the device manufacturer, although efforts were made to reduce self-report bias including no device representatives or clinical staff being present during survey completion and the survey consisting of validated outcome measures to reduce bias in the survey instrument itself. Regardless, the consistency of the survey outcomes with published prospective data supports the validity of the effectiveness of the 60-day PNS treatment in a real-world setting. Additional studies, including controlled studies, that address these limitations could provide further real-world corroboration of the previously published prospective clinical trials.
Conclusion
This study presents the largest body of real-world evidence published to date supporting the effectiveness of 60-day PNS treatment. The cross-sectional follow-up survey found that at the time of survey completion, which ranged from 3 to 30 months from the start of treatment, most patients reported clinically significant improvements in pain and/or quality of life, and many patients also reported improvements in physical function and reduced opioid and nonopioid analgesic usage. These real-world data validate the findings of published prospective studies that have demonstrated significant and sustained improvements following short-term PNS and support the benefits of 60-day PNS for the treatment of a wide range of pain conditions in broader clinical practice.
Recent studies across multiple pain indications have suggested that percutaneous peripheral nerve stimulation (PNS) treatment via implanted lead(s) for up to 60 days can produce significant, sustained improvements without permanent implantation.
This study presents real-world data from a cross-sectional follow-up survey of patients who previously received 60-day PNS treatment for pain.
Among survey respondents who were at least 3 months from the start of treatment, most reported sustained clinically significant improvements in pain and/or quality of life, with the length of follow-up at the time of survey completion ranging from 3 to 30 months.
Many patients also reported clinically significant improvements in physical function and reduced opioid and nonopioid analgesic medication usage.
These real-world data support recent prospective studies indicating that 60-day percutaneous PNS provides significant and sustained relief across a wide range of pain conditions.
Author contributions
ND Crosby and JW Boggs designed and conducted the data analysis. MJ Pingree, MFB Hurdle, DA Spinner, A Valimahomed, ND Crosby and JW Boggs contributed to interpretation of the data and writing and review of the manuscript. All authors approved the final version of the manuscript.
Ethical conduct of research
The authors state that SPR Therapeutics obtained patient consent for all survey participation. Due to the nature of this market research and the use of anonymized data listings, ethical committee approval was not required. Research conducted was consistent with the principles for the protection of research participants outlined in the IA Code of Standards and Ethics for Market Research and Data Analytics.
Supplemental document 1
Download MS Word (19.4 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pmt-2022-00052022-0019
Financial & competing interests disclosure
This study was supported by SPR Therapeutics. MJ Pingree, MFB Hurdle, DA Spinner and A Valimahomed are consultants to the World Academy of Pain Medicine United (WAPMU), which receives support from SPR Therapeutics. DA Spinner and A Valimahomed are consultants to SPR Therapeutics. ND Crosby and JW Boggs are employees of and hold stock options in SPR Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Pitcher MH , Von KorffM , BushnellMC , PorterL. Prevalence and profile of high-impact chronic pain in the United States. J. Pain20(2), 146–160 (2019).
- Dahlhamer J , LucasJ , ZelayaCet al. Prevalence of chronic pain and high-impact chronic pain among adults – United States, 2016. MMWR Morb. Mortal. Wkly. Rep.67(36), 1001–1006 (2018).
- Gore M , BrandenburgNA , DukesE , HoffmanDL , TaiKS , StaceyB. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manage.30(4), 374–385 (2005).
- Gormsen L , RosenbergR , BachFW , JensenTS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur. J. Pain14(2), 127.e121–127.e128 (2010).
- Haythornthwaite JA , Benrud-LarsonLM. Psychological aspects of neuropathic pain. Clin. J. Pain16(Suppl. 2), 101–105 (2000).
- Ciaramitaro P , MondelliM , LogulloFet al. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst.15(2), 120–127 (2010).
- Henschke N , KamperSJ , MaherSG. The epidemiology and economic consequences of pain. Mayo Clin. Proc.90(1), 139–147 (2015).
- Hassenbusch SJ , Stanton-HicksM , SchoppaD , WalshJG , CovingtonEC. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J. Neurosurg.84, 415–423 (1996).
- Novak CB , MackinnonSE. Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast. Reconstr. Surg.105(6), 1967–1972 (2000).
- Eisenberg E , WaisbrodH , GerbershagenHU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin. J. Pain20(3), 143–146 (2004).
- Mobbs RJ , NairS , BlumP. Peripheral nerve stimulation for the treatment of chronic pain. J. Clin. Neurosci.14(3), 216–221 (2007).
- Mirone G , NataleM , RotondoM. Peripheral median nerve stimulation for the treatment of iatrogenic complex regional pain syndrome (CRPS) type II after carpal tunnel surgery. J. Clin. Neurosci.16(6), 825–827 (2009).
- Deer T , PopeJ , BenyaminRet al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation19(1), 91–100 (2016).
- Hegarty D , GoroszeniukT. Peripheral nerve stimulation of the thoracic paravertebral plexus for chronic neuropathic pain. Pain Physician14(3), 295–300 (2011).
- Goroszeniuk T . The effect of peripheral neuromodulation on pain from the sacroiliac joint: a retrospective cohort study. Neuromodulation22(5), 661–666 (2019).
- Yakovlev AE , PetersonAT. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation10(4), 373–375 (2007).
- Allen S . New advances in neurostimulation for chronic pain. IEEE Pulse12(1), 12–15 (2021).
- Wilson RD , KimCH. Percutaneous and implanted peripheral nerve stimulation for the management of pain: current evidence and future directions. Curr. Phys. Med. Rehabil. Rep.8(1), 1–7 (2020).
- Gilmore CA , IlfeldBM , RosenowJMet al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain Med.45(1), 44–51 (2020).
- Gilmore CA , KapuralL , McGeeMJ , BoggsJW. Percutaneous peripheral nerve stimulation for chronic low back pain: prospective case series with 1 year of sustained relief following short-term implant. Pain Pract.20(3), 310–320 (2020).
- Wilson RD , GunzlerDD , BennettME , ChaeJ. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am. J. Phys. Med. Rehabil.93(1), 17–28 (2014).
- Wilson RD , HarrisMA , GunzlerDD , BennettME , ChaeJ. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation17(8), 771–776 (2014).
- Gilmore CA , DesaiMJ , HopkinsTJet al. Treatment of chronic axial back pain with 60-day percutaneous medial branch PNS: primary endpoint results from a prospective, multicenter study. Pain Pract.21, 877–889 (2021).
- Ilfeld BM , GabrielRA , SaulinoMFet al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract.17(6), 753–762 (2017).
- Chae J , WilsonRD , BennettME , LechmanTE , StagerKW. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract.13(1), 59–67 (2013).
- Chae J , YuDT , WalkerMEet al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am. J. Phys. Med. Rehabil.84(11), 832–842 (2005).
- Deer TR , GilmoreCA , DesaiMJet al. Percutaneous peripheral nerve stimulation of the medial branch nerves for the treatment of chronic axial back pain in patients after radiofrequency ablation. Pain Med.22(3), 548–560 (2021).
- Gilmore C , IlfeldB , RosenowJet al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain. Med.44(6), 637–645 (2019).
- Gilmore CA , KapuralL , McGeeMJ , BoggsJW. Percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation22(5), 615–620 (2019).
- Ilfeld BM , BallST , GabrielRAet al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation22(5), 653–660 (2019).
- Ilfeld BM , GilmoreCA , GrantSAet al. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J. Orthop. Surg. Res.12(1), 4 (2017).
- Rauck RL , CohenSP , GilmoreCAet al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation17(2), 188–197 (2014).
- Renzenbrink GJ , IJzermanMJ. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin. Rehabil.18(4), 359–365 (2004).
- Wilson RD , BennettME , NguyenVQCet al. Fully implantable peripheral nerve stimulation for hemiplegic shoulder pain: a multi-site case series with two-year follow-up. Neuromodulation21(3), 290–295 (2018).
- Yu DT , ChaeJ , WalkerME , FangZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch. Phys. Med. Rehabil.82(1), 20–25 (2001).
- Mainkar O , SolloCA , ChenG , LeglerA , GulatiA. Pilot study in temporary peripheral nerve stimulation in oncologic pain. Neuromodulation23(6), 819–826 (2020).
- Mainkar O , SinghH , GargyaA , LeeJ , ValimahomedA , GulatiA. Ultrasound-guided peripheral nerve stimulation of cervical, thoracic, and lumbar spinal nerves for dermatomal pain: a case series. Neuromodulation24(6), 1059–1066 (2021).
- Finneran JJT , FurnishT , CurranBP , IlfeldBM. Percutaneous peripheral nerve stimulation of the brachial plexus for intractable phantom pain of the upper extremity: a case report. A A Pract.14(14), e01353 (2020).
- Langford B , MauckWD. Peripheral nerve stimulation: a new treatment for meralgia paresthetica. Pain Med.22(1), 213–216 (2021).
- Chakravarthy Krishnan . Reframing the role of neuromodulation therapy in the chronic pain treatment paradigm. Pain Physician21, 507–513 (2018).
- Graham S , McDonaldL , WasiakR , LeesM , RamagopalanS. Time to really share real-world data?F1000Res.7, 1054 (2018).
- Cleeland CS , RyanKM. Pain assessment: global use of the brief pain inventory. Ann. Acad. Med. Singapore23(2), 129–138 (1994).
- Gabriel RA , IlfeldBM. Acute postoperative pain management with percutaneous peripheral nerve stimulation: the SPRINT neuromodulation system. Expert Rev. Med. Devices18(2), 145–150 (2021).
- Deer TR , EldabeS , FalowskiSMet al. Peripherally induced reconditioning of the central nervous system: a proposed mechanistic theory for sustained relief of chronic pain with percutaneous peripheral nerve stimulation. J. Pain Res.14, 721–736 (2021).
- Pilitsis JG , FaheyM , CustozzoA , ChakravarthyK , CapobiancoR. Composite score is a better reflection of patient response to chronic pain therapy compared with pain intensity alone. Neuromodulation24(1), 68–75 (2021).
- Gewandter JS , McDermottMP , EvansSet al. Composite outcomes for pain clinical trials: considerations for design and interpretation. Pain162(7), 1899–1905 (2021).
- Dworkin RH , TurkDC , FarrarJTet al. Core outcome measures for chronic pain clinical trials: impact recommendations. Pain113(1-2), 9–19 (2005).
- Offenbaecher M , KohlsN , EwertTet al. Pain is not the major determinant of quality of life in fibromyalgia: results from a retrospective “real world” data analysis of fibromyalgia patients. Rheumatol. Int.41(11), 1995–2006 (2021).
- Patel KV , AllenR , BurkeLet al. Evaluation of composite responder outcomes of pain intensity and physical function in neuropathic pain clinical trials: an action individual patient data analysis. Pain159(11), 2245 (2018).
- Turk DC , DworkinRH , McDermottMPet al. Analyzing multiple endpoints in clinical trials of pain treatments: impact recommendations. Pain139(3), 485–493 (2008).
- Bhardwaj P , YadavRK. Measuring pain in clinical trials: pain scales, endpoints, and challenges. Int. J. Clin. Exp. Physiol.2(3), 151–156 (2015).
- Woo A , LechnerB , FuTet al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann. Palliat. Med.4(4), 176–183 (2015).
- Strand NH , D’SouzaR , WieCet al. Mechanism of action of peripheral nerve stimulation. Curr. Pain Headache Rep.25(7), 47 (2021).
- Deer TR , NaiduR , StrandNet al. A review of the bioelectronic implications of stimulation of the peripheral nervous system for chronic pain conditions. Bioelectron. Med.6, 9 (2020).
- Chung JM , LeeKH , HoriY , EndoK , WillisWD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain19(3), 277–293 (1984).
- Ilfeld BM , GrantSA. Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: could neurostimulation replace continuous peripheral nerve blocks?Reg. Anesth. Pain Med.41(6), 720–722 (2016).
- Wenger S , DrottJ , FillipoRet al. Reducing opioid use for patients with chronic pain: an evidence-based perspective. Phys. Ther.98(5), 424–433 (2018).
- Kern DM , ZhouS , ChavoshiSet al. Treatment patterns, healthcare utilization, and costs of chronic opioid treatment for non-cancer pain in the United States. Am. J. Manag. Care21(3), e222–e234 (2015).
- Zhao X , ShahD , GandhiKet al. The association of pain interference and opioid use with healthcare utilization and costs, and wage loss among adults with osteoarthritis in the United States. J. Med. Econ.22(11), 1192–1201 (2019).
- Keeter S , MillerC , KohutA , GrovesRM , PresserS. Consequences of reducing nonresponse in a national telephone survey. Public Opin. Quart.64(2), 125–148 (2000).
- Groves RM . Nonresponse rates and nonresponse bias in household surveys. Public Opin. Quart.70(5), 646–675 (2006).
- Groves RM , PeytchevaE. The impact of nonresponse rates on nonresponse bias: a meta-analysis. Public Opin. Quart.72(2), 167–189 (2008).
- Shih TH , FanX. Comparing response rates in e-mail and paper surveys: a meta-analysis. Educ. Res. Rev.4(1), 26–40 (2009).
- Daikeler J , BošnjakM , LozarManfreda K. Web versus other survey modes: an updated and extended meta-analysis comparing response rates. J. Surv. Stat. Methodol.8(3), 513–539 (2019).
