Abstract
Aim: In a single-center, double-blind, randomized controlled trial, we evaluated whether pupillometry-controlled use of sufentanil is better than free-choice administration of sufentanil by anesthesiologists. Patients methods: 61 patients undergoing daycare gynecological or abdominal surgery were enrolled. A pupillometry pain index score chart was introduced for administration guidance of sufentanil. Results: The first objective, patient well-being, did not show a significant difference with painkiller usage and health state index at day 1 postoperatively. Second, we experienced difficulty in interpretation of the pupillometry score. Third, opioid usage was higher in the intervention group (20.1 vs 14.8 mcg; p = 0.017). Conclusion: The use of pupillometry and pain index chart for bolus sufentanil with our protocol showed an unwanted higher sufentanil usage without a significant difference in patient wellbeing. (Ethics Committee EC17/28/319 of the University Hospital of Antwerp. Registration at clinicaltrials.govNCT03248908).
Plain language summary
Communication with patients under general anesthesia is impossible. A potential solution is to measure pain. One of seven commercially available options is to use a pupillometer. Automated painful stimuli are given and the dilation of the pupil is measured. We hoped to use this method to better control the dose of the opioid sufentanil. During daycare gynecological and abdominal procedures, we had a 25% higher sufentanil usage in the intervention group. We experienced difficulties in reaching the right pain score in both groups. The well-being of the patients, namely pain and painkiller usage at day 1 after surgery, did not show any significant difference. With our protocol, there is no benefit to controlling the dose of sufentanil by pupillometer measurement.
Pain assessment in noncommunicative patients is challenging. Under general anesthesia, communication is impossible due to unconsciousness. Adequate measurement of nociception may allow the anesthesiologist to perform individual titration of analgesics, avoiding over- or under-dosage. Correct nociceptive assessment, and therefore appropriate individually based treatment, may be an ideal scenario [Citation1,Citation2]. Nowadays there are seven devices for measuring perioperative pain; Ledowski reviewed the current commercial solutions in 2019, and the conclusion of that review was that the optimum solution for monitoring nociception is not yet known [Citation3]. The seven devices use between one and four parameters. Our study uses pupillometry, a one-parameter device. Infrared pupillometry has existed for decades [Citation4]. Larson et al. and Packiasabapathy et al. published reviews about pupillometry [Citation4,Citation5], but there are only a few studies about portable video pupillometry in anesthetized patients, of which most have used remifentanil [Citation4,Citation5]. To our knowledge, only two studies have used sufentanil [Citation6,Citation7]. Both studies used a continuous drip of sufentanil and did their study during cardiac surgery [Citation6,Citation7].
Patients & methods
Study design & data collection
This was a single-center, double-blind, randomized controlled trial carried out at the University Hospital of Antwerp, Belgium. Only daycare patients were included. The study was performed in accordance with the ethical standards of ICG-GCP (International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use) and the Declaration of Helsinki after study approval by the institutional review board and the Ethics Committee (EC17/28/319) of the University Hospital of Antwerp by G Ieven on 31 July 2017. Registration at clinicaltrials.gov (NCT03248908) was executed before study inclusion. This manuscript adheres to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines [Citation8].
We anticipated that a pupil reflex dilation evaluation, and in addition a pupillometry pain index (PPI) score with increasing tetanic stimulation, may be related to analgesic treatment in anesthetized patients. An initial pilot study was done with 38 patients (aged 24–74 years) enrolled [Citation9]. Patients were anesthetized with propofol continuous target-controlled infusion. One measurement was done before opioid administration, and a second measurement after opioid administration. After opioid administration, patients needed a higher stimulation intensity (45.26 vs 30.79 mA; p = 0.00001) to have a pupil dilation of >13%. Power analysis showed a need for n = 28 to measure a stimulation intensity difference between T0 and T1 measurement of 10 mA (α: 0.05, power 0.9) [Citation9].
After written consent, patients planned for elective abdominal or gynecological surgery were recruited for study inclusion from October 2017 until August 2021. Inclusion criteria were: elective abdominal of gynecological surgery, no locoregional anesthetics, age >18 years and American Society of Anesthesiologists Physical Status Classification System (ASA) category I, II or III [Citation10]. Exclusion criteria were: medical history of eye surgery, known bilateral eye disease, known nervus opticus or nervus oculomotorius deficit, active pheochromocytoma, active psychiatric disease, opioid usage >7 days preoperatively or active oncological treatment with chemotherapy. Also excluded were patients who used: medication that interferes with pupillary measurements, such as high-dose α-1 or β-blocker (no intake on the day of surgery); benzodiazepines on the day of surgery; topical atropine or phenylephrine; or scopolamine or dopamine antagonists. During anesthesia it was forbidden to give dehydrobenzperidol (DHBP), alizapride, phenylephrine or atropine, as an effect on the pupil diameter was examined in two studies beforehand [Citation11,Citation12]. Because of the high risk of postoperative nausea or vomiting of some patients, we tolerated the administration of DHBP or alizapride after the last PPI measurement.
We used a CE-approved NeuroLight Algiscan® (IDMed, Marseille, France) pupillometer using infrared video recording allowing quantitative pupil size assessment. The company provided a nonvalidated PPI score chart. The subjects underwent consecutive pupil measurements under general anesthesia.
For nociceptive stimulation, two Ag-AgCl electrodes were placed at the skin area innervated by the median nerve. Optimal skin contact with low electrode impedance was defined on the touch-screen display. Constant current stimulations were generated during pupil measurement, increasing the voltage automatically according to the resistance. Voltage was limited to a maximum of 300 V; therefore, for a current fixed at 60 mA, the maximum acceptable resistance was 5 kΩ.
The upper eyelid of the measured eye was opened during pupil assessment. A rubber cup placed to the orbit ensured optimal device position, pupil–camera distance and environmental darkness. There was never direct contact with the cornea. By convention, the left eye was assessed after confirmation of the absence of pupil syndrome disorder. The contralateral eye was closed, reducing the effect of the consensual light response. The PPI-modus was selected for dynamic pupil measurement via the touch-screen display. This inbuilt measurement protocol generated an automatic electric stimulation pattern. The operating principle is the application of a standardized noxious stimulation (10–60 mA in incremental steps of 10 mA, with a duration of 1 s and pulse width of 200 μs) in increasing intensity, until pupillary dilation of more than 13% ([maximal diameter − minimal diameter] / maximal diameter × 100). When the defined criteria were reached, stimulation automatically stopped and PPI score was determined (). When the pupil variation (VAR) was >20%, a +1 was added to the score. The measurable pupil size (diameter) ranged between 0.1 and 10 mm. Furthermore, baseline (as minimum diameter per measurement), pupil reflex dilation amplitude (PRDA), VAR, stimulation intensity (Int) and PPI score were recorded each measurement. Depending on necessary stimulation intensity, pupil measurement duration was between 2 and 16 s (Supplementary Video 1).
Table 1. Pupillometry pain index score.
The enrolled subjects were divided into two groups: group 1 was the sufentanil flowchart or intervention group and group 2 was the sufentanil control group. For the double-blind randomized controlled trial, randomization was performed using the site www.randomization.com. Permuted block randomization was used.
Before induction, demographic data were collected. Height and weight were registered, and ideal body weight (IBW) was calculated by height (cm) − 100 for men and height (cm) − 105 for women. If actual body weight was lower than IBW, then actual body weight was used. When actual body weight was higher than IBW, then corrected body weight (CBW) was used. CBW was calculated by IBW + 0.4 × (weight [kg] − IBW [kg]). Further ASA classification, oxygen saturation (SpO2) before administration of oxygen, blood pressure and heart rate (HR) were collected. Also, bispectral index monitoring (BIS) awake (NeuroSENSE Monitor©, NeuroWave Systems, Inc., Beachwood, OH, USA) was recorded [Citation13]. The use of any antihypertensive drug, including β-blockers, was checked.
Patients were anesthetized in a fully equipped operation room. No premedication was administered before surgery. On arrival in the operating theater, standard monitoring and safe surgery checklist were executed. A venous catheter was inserted in a cubital vein. Noninvasive blood pressure was recorded every 5 min. HR, ECG, SpO2, end-tidal carbon dioxide concentration and BIS were recorded continuously.
Induction was established, after preoxygenation, by administration of a propofol continuous target-controlled infusion (Marsh Model: injectomat TIVA Agilia, Fresenius Kabi, Germany) until the value of BIS was between 40 and 60 [Citation14]. The effect site concentration of propofol (CE-Prop) was noted. When necessary, administration of lidocaine and dexamethasone was allowed, as there is no known interference with pupil measurement [Citation11,Citation12]. Manually assisted ventilation with 100% oxygen began as soon as patients became apneic.
The observer performed the first (T0) measurement at the moment the patient had a BIS value between 40 and 60. When necessary, mask ventilation with minimal manipulation was conducted during measurement. Minimal manipulation does not have a significant nociceptive influence in comparison with the standardized nociceptive stimulation by the pupillometer. Of note, no opioid or curare were given before the T0 measurement. After the first measurement, the anesthesiologist gave the opioid as denoted by the group allocated at the randomization. When necessary, curare was also administered after the first measurement.
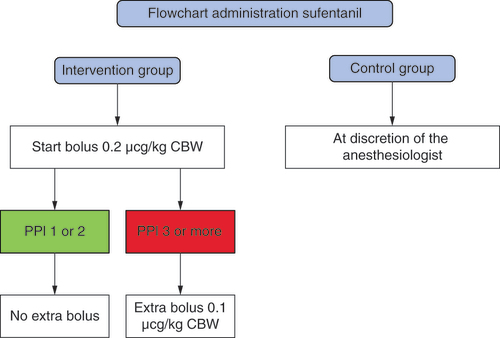
The first bolus of sufentanil in the intervention group was 0.2 mcg/kg CBW (). For the control group, sufentanil administration was at the discretion of the anesthesiologist. After a waiting time of 6 min, the T1 measurement was performed, then a new measurement was conducted every 10 min. When the PPI score was 1 or 2, no extra sufentanil was given in the intervention group. With a PPI score of 3 or more, a new 0.1 mcg/kg CBW bolus of sufentanil was given. The last measurement was taken at the start of wound closure or when there was no wound at the end of surgery. At each measurement we also collected the blood pressure, HR, movement and BIS.
CBW: Corrected body weight; PPI: Pupillometry pain index.
At the end of the surgery, the observer noted the time of stopping CE-Prop, surgery stop time, temperature, neuromuscular transmission monitoring by train-of-four test (TOF-watch®, Draeger, Heide, Wemmel, Belgium), SpO2 and BIS. The time of extubation was also noted and the anesthesiologist was asked whether acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), tramadol, morphine or local wound infiltration was given and whether anti-emetic dexamethasone or ondansetron was given. If necessary, we tolerated the administration of DHBP or alizapride, but only after the last measurement to prevent measurement influence.
The anesthesiologist had to fill in a blind form with the study group and the total dose of sufentanil. The form went in a closed envelope which was only opened after all the measurements. Via the post-anesthesia care unit staff we collected time of arrival and departure of the recovery period. The Aldrete score at arrival and departure, the need for anti-emetic, vomiting and/or nausea was noted. The need for supplemental oxygen and, if needed, the oxygen flow were noted. Patients were asked about pain and, if necessary, a note was made of which and how much rescue painkiller was given, followed by pain reassessment.
At home the patients were asked to fill in an online questionnaire for 5 days. The use of painkillers was asked about and calculated by the Medication Quantification Scale [Citation15]. A numeric rating scale (NRS) was used to assess various parameters: for pain, 0 is no pain and 10 is maximal pain; for activity, 0 is no activity and 10 is very active; and for sleep, an NRS score of 0 equals ‘did not sleep’ and 10 means ‘did sleep very well’. Patients used an online evaluation diary to provide their NRS scores. The questions “Did you have nausea in the last 24 hours?’”and “Did you vomit in the last 24 hours?’” were also asked. Also the EQ-5D-5L questionnaire was used (the calculation used the UK score as there is no Belgian score). Patients were asked to place a dot on the European Quality of Life Visual Analog Scale (EQ-VAS) scale about their feeling of health whereby 0 equals the worst health imaginable and 100 is the best health imaginable.
One of the authors obtained informed consent from the patient; the same author also performed all the perioperative measurements. The syringe was hidden from the observer, so it was not possible to make an estimation of the used amount of opioid. Also the participants, because they were under anesthesia, were blinded. The staff of the recovery ward was blinded because the anesthesiologist did not tell them to which group the patient had been allocated.
The study examined the usability of bolus sufentanil in combination with pupillometry and PPI score. The primary outcome parameter was postoperative pain intensity, measured as pain intensity score by NRS and the amount of painkiller usage. Our hypothesis was that patients with a good titrated sufentanil administration perioperatively should have less pain and less need of pain medication. Secondary outcome parameters were pupil reflex dilation characteristics such as Int, baseline pupil diameter, PRDA and PPI score. Tertiary outcome parameter was total opioid usage during surgery. Additional outcome parameters were recovery time, post-operative nausea and vomiting (PONV) and health state index using the EQ-5D-5L questionnaire.
Statistical analysis
Results were expressed as mean and standard deviation for continuous variables, as median and interquartile range for ordinal variables and as numbers and percentages for categorical variables. Normality of continuous variables per group was tested with Kolmogorov–Smirnov and Shapiro–Wilk tests. For normal distributions and independent samples, the t-test was used; in case of non-normality or ordinal variables, the Mann–Whitney U test was used. For categorical variables, the χ-square test or Fisher’s exact test was used as appropriate. In the study were also repeated measures for which two-way analysis of variance (ANOVA) was used; sphericity was assumed in both measurements. Statistical significance was set at a p-value of ≤ 0.05 for all tests. Statistical analysis was performed using SPSS Statistics v. 28.0.0.0 (IBM Corp., NY, USA).
Initially, we wanted to analyze the use of pain medication, pain score and health state index at day 5. Unfortunately, because of an 80% rate of uncompleted online questionnaires, we decided to only statistically analyze postoperative day 1.
Results
Demographic data
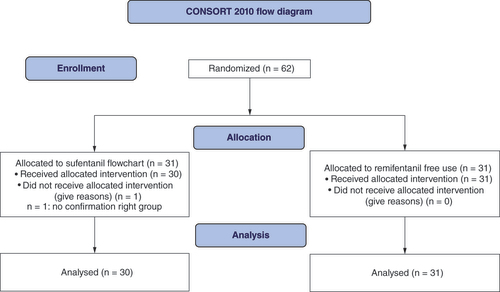
In total 62 patients participated in the study. By randomization, 31 patients were enrolled in the ‘sufentanil flowchart’ group or intervention group, and 31 in the ‘sufentanil control’ group. Among the sufentanil flowchart group, one patient was not confirmed as being in the group and their results were not analyzed (see CONSORT flow diagram, ). Baseline demographic data are presented in Supplementary Table 1. There were only 16.7 and 6.5% males, respectively, in the intervention and control groups due to the amount of gynecological patients with (76.7 and 83.9%). The BIS was calculated as (BIS right + BIS left) divided by 2 and designated BIS mean (BISm). There was no significant difference in BISm.
The baseline measurement at T0 was after induction with propofol, but before administration of sufentanil or curare. Results of the T0 measurement are presented in Supplementary Table 2. There was no significant difference in baseline pupil diameter, PRDA, VAR, Int or PPI. Respectively intervention versus control group, the mean baseline was 4.1 and 4.2 mm, and the mean PRDA was 1.58 and 0.94 mm; thus the variation was 23.9 and 22.8% at a median intensity of 35 and 30 mA. Both median PPI scores were 8. There was no statistically significant difference in systolic blood pressure, HR or BISm. The BISm reached the target value of 40–60 in 63.3% of the intervention group and 71% of the control group, which was not a statistically significant difference.
Perioperative measurements
The first perioperative measurement was conducted 6 min after administration of sufentanil and designated the T1 measurement; results are presented in Supplementary Table 3. Between the two groups, there was no significant difference in baseline pupil diameter, PRDA, VAR, Int or PPI. At T1 there was a significant difference in systolic blood pressure with 94 mmHg for the intervention group and 102 mmHg for the control group (p = 0.02). HR and BIS did not show a significant difference. The wanted PPI score of 1 or 2 was reached in five cases (16.7%) in the flowchart group and five cases (16.1%) in the control group (p = 0.955). The second perioperative measurement was conducted 10 min after T1 and designated the T2 measurement; results are presented in Supplementary Table 4. Also in T2 there was no significant difference between baseline pupil diameter, PRDA, VAR, Int or PPI. The systolic blood pressure did not show a significant difference at this measurement, and HR and BIS were not significantly different. The T2 measurement reached the wanted PPI score of 1 or 2 in eight (28.6%) of 28 cases of the intervention group and nine (29.0%) of 31 cases of the control group (p = 0.969).
The last measurement was at the beginning of closing the operative wound(s) and was designated the end measurement (TEnd); results are presented in . Also at this measurement there was no significant difference at baseline, PRDA, VAR, Int or PPI; systolic blood pressure, HR and BIS also did not show any significant differences. In nine of the 29 cases (31%), the end PPI was 1 or 2 in the flowchart group and in eight of 31 cases (26%) in the free use group (p = 0.653).
Table 2. End measurements.
compares the baseline pupil diameters and the PPI scores at the different times with confidence intervals. The baseline had a reduction of 50.9 and 51.0% between T0 and T1. The T1, T2 and TEnd baseline measurements for both groups remained in the same confidence intervals. For the sufentanil flowchart pupil baseline from T0 until TEnd was two-way ANOVA F = 256 with p < 0.001. When comparing the pupil baseline of both groups at the same time F = 0.345 with a non-significant p = 0.793. The PPI reduction in both groups was slower (14.5 and 9.7% between T0 and T1). The confidence interval was only statistically significant between T0 and T2 + TEnd in the intervention group and between T0 and TEnd in the control group. For PPI intervention group from T0 until TEnd was two-way ANOVA F = 18.1 with p < 0.001. When comparing the PPI score of both groups at the same time F = 1.0 with a non-significant p = 0.392.
Table 3. Two-way analysis of variance pupil baseline diameter and pupillometry pain index.
Supplementary Table 5 compares the two groups intraoperatively. The time between starting and stopping propofol had median values of 36 min for the intervention group and 37 min for the control group (p = 0.499). The time between stopping propofol and extubation had median values of 12 min for the intervention group and 13.5 min for the control group (p = 0.927). Two pieces of data in the flowchart group were missing. Wake-up conditions were not significantly different for temperature, SpO2 or BIS. The train-of-four count was 4 for all curarized patients. For one patient in the flowchart group it was only 55%, but for that patient the wake-up times were not noted. Perioperatively, all but one patient of the free use group received acetaminophen; that patient had an allergy to acetaminophen. Respectively for the intervention and control group, 87 and 81% received an NSAID (p = 0.731); 20 and 10% received tramadol (p = 0.301). None of the patients received morphine. 30% of the intervention and 36% of the control group received local wound infiltration (p = 0.786). Respectively for the intervention and control group, dexamethasone was respectively given to 63 and 68% of the patients (p = 0.791), and ondansetron to 13 and 23% (p = 0.508). DHBP or alizapride was given in 13% of both groups, only after the last measurement. As mentioned before, this is a deviation from the study protocol, but because there were no further pupillary measurements it did not influence the results. In the intervention group, the mean dose of sufentanil was 20.1 μg, while in the control group the mean dose was 14.8 μg, which is a significant difference (p = 0.017).
Postoperative outcome
The median times to recovery in the flowchart and control groups were, respectively, 52 and 40 min (p = 0.025), a significant difference in favor of the control group (). The Aldrete score at arrival was 8 in both groups. At departure there were in the first group two cases with scores less than 10; in the second group there was one score of 9. None of the patients in either group suffered from PONV and none needed an anti-emetic; 16% in the intervention group versus 20% in the control group needed supplemental oxygen (p = 0.741). Respectively, two and three cases needed extra opioids, namely dipidolor, at recovery.
Table 4. Post-anesthesia care unit.
Follow-up
At day 1 the medication use was comparable between the two groups; the results are presented in . The median NRS was 2 for the flowchart group and 1 for the control group (p = 0.249). The level of activity median of both groups was 5 (p = 0.804). The level of sleep quality medians were, respectively 7 and 6, (p = 0.429). In the intervention group, 23% of the patients had nausea while none of the control group had nausea (p = 0.223); 15% of the intervention group and none of the control group reported vomiting (p = 0.480). The Health State Index of the flowchart group was 0.656 and that of the control group 0.702 (p = 0.464). The EQ-VAS scores at D1 were 56 in the intervention group and 70 in the control group (p = 0.152).
Table 5. Online questionnaire: day 1 results.
In range versus out of range
A comparison was made between a PPI score of 1 or 2 and a PPI score of ≥3 in the intervention and the control group separately (Supplementary Table 6). When we compared the amount of sufentanil used, there was no statistical significance associated with a score of 1 or 2 or higher in the two groups. Also the time taken for the operation, measured by the duration of propofol infusion, did not show any significance. When we looked further to the postoperative period, there was also no statistical significance in health state index or EQ-VAS score.
Discussion
Our primary outcome parameter was postoperative pain intensity. Patients in the study group had an NRS median of 2, while the control group had a NRS median of 1. There was no significant statistical difference and no clinical relevance. According to our opinion, it is good to have low pain scores; at our center we try to have a pain score less than 3. The pain medication use in both groups was also comparable. Unfortunately, there was a high drop-out rate with patients not completing or only partially completing the online questionnaire at day 1.
The second outcome parameter, namely pupil reflex dilation, was comparable in both groups. The PPI scores in the intervention group were significantly different between T0 and T2 and TEnd, while in the control group the significance was only between T0 and TEnd. The baseline pupil diameter was significantly lowered after T0 in both groups. The two-way ANOVA showed a significant difference for PPI (as for pupil baseline diameter) in time, but no significant difference between the study groups. The amount of desirable PPI scores (1 or 2) at the different times was too low to be acceptable, in our opinion, particularly as there was no significant difference in opioid dosage or well-being found between the patients with a score of 1/2 and those with scores of ≥3. However, the pupil baseline diameter lowered significantly at the T1 measurement in both groups, so it is possible that the baseline could be used in the next studies.
In terms of the tertiary outcome, we noticed the use of sufentanil was significantly lower in the control group. We can explain this due to the higher dosage of sufentanil used by the flowchart group in comparison with what we do daily at the hospital. With 12:00 min for the intervention group and 13:30 min for the control group, the wake-up times were comparable between the two groups. Thus the higher amount of sufentanil use does not cause longer wake-up times. The relatively long wake-up times are probably due to the continuous propofol administration.
The recovery ward times were significantly lower in the control group (40 vs 52 min). The minimum recovery ward care takes 30 min at our center; although the care priority is a comfortable patient, the use of time and resources cannot be neglected. None of the patients suffered from PONV at the recovery ward. At day 1 postoperatively, the health state index and the EQ-VAS score were comparable in the two groups but in favor of the control group. At day 1 nausea and vomiting were noted in the flowchart group, but not in the control group; this may be due to the higher amount of opioids administered.
There was no report of a serious adverse event during the whole study follow-up period.
To our knowledge, our study is the first one that used pupillometry in combination with bolus sufentanil. Up to now, two articles describing pupillometry when using sufentanil have been published, but both were done in cardiac surgery using continuous sufentanil [Citation6,Citation7]. Berthoud et al. started with sufentanil effect-site target concentration at 0.3 ng/ml [Citation6]. With a PPI score of ≤3, they lowered the infusion by 0.1 ng/ml; with a score of 4–6 there was no change; and at ≥7 the infusion was raised by 0.1 ng/ml. The sufentanil dosage for the control group was at the discretion of the anesthesiologist. Berthoud et al. found a lower use of sufentanil in the intervention group, but no decrease of cumulative morphine use postoperatively nor less chronic pain at 3 months [Citation6]. The study of Bartholmes et al. also outlined a decrease of sufentanil use but did not explain the sufentanil adjustments [Citation7]. They reported a decrease of noradrenaline use and postoperative pain after 24 h [Citation7].
One of the limitations of our study was the investigation of only daycare patients. We had permission to include gynecological and abdominal elective surgery patients, so most patients were female and most operations were rather short. More evidence is needed for operations of more than 2 h or major operations like thoracic surgery.
Another limitation of our trial is the high drop-out rate for the online questionnaire; unfortunately this made it more difficult to reach statistically significant differences. Participation was always voluntary.
The anti-emetic management was not standardized. Questions can be raised about the lower NRS score postoperatively after dexamethasone. At the start of the study there was no meta-analysis to state the lower NRS score or lower morphine usage. Mitchell et al. published a meta-analysis in 2022 in which at 24 h postoperatively a -0.38 (-0.52 to -0.24) lower NRS score (p <0.05) was noted [Citation16]. In our study, 43% of the intervention group and 59% of the control group (p = 0.224) received dexamethasone. This was not a significant difference.
In our opinion, the two groups were comparable. We included patients from October 2017 until August 2021. The long time of inclusion was due to another study running in our center and because of COVID-19.
Conclusion
This study examined the usefulness of pupillometry in combination with sufentanil. There was no significant improvement of health state index at day 1 postoperatively. The control group had an NRS score of 1 at day 1 postoperatively, while the intervention group had an NRS score of 2.
In most patients, both intervention and control group, we did not reach the desired pain score of 1 or 2. The baseline pupil diameter dropped more rapidly, but we did not have a protocol using the baseline pupil diameter by itself.
There was less sufentanil use in the control group compared with the intervention group, and there was also a significantly shorter stay in the recovery ward for the control group. In our opinion, there is no reason to believe that this was due to a different type of surgery, duration of surgery or patient type. The lesser use of sufentanil has a consequence of faster recovery time. There was no difference in postoperative outcome, health state or pain score of the patients.
Our first conclusion is that there was no significant improvement of health or pain at day 1 postoperatively. The second conclusion is that our model of PPI score in combination with sufentanil does not guarantee a PPI score of 1 or 2.
In our study we can not recommend the additional usage of PPI score in combination with sufentanil during abdominal or gynecological daycare elective surgery. To our knowledge, no other published study has used pupillometry in combination with bolus sufentanil; further study is needed. A new protocol could be made in addition with the pupil baseline diameter and/or other sufentanil dosage.
Future perspective
Although anesthesiologists try to measure as much as possible, it is surprisingly difficult to measure the effects of opioids. We still believe that nociceptive pain measuring can create better healthcare, but to use pupillometry in combination with bolus sufentanil will be difficult to our opinion. However, it looks promising during cardiac surgery. Personal experience of Nociception Level- index monitorin looks also promising to us [Citation17].
Nociception is a possible way to titrate opioids in unconscious patients.
Seven devices are available to measure nociception during anesthesia.
A pupillometer measures pupil pain reflex during increasing stimulation.
To our knowledge, this is the first study using bolus sufentanil in combination with pupillometer and pupillometry pain index score.
The wanted pupillometry pain index score of 1 or 2 was reached only in 31% of the intervention group patients and 26% of the control group patients.
There was no effect on well-being between the study and the control group.
The study group received a higher amount of sufentanil.
Further study is needed; a new protocol could be made in addition with the pupil baseline diameter and/or other sufentanil dosage.
Author contributions
D Van Vlaenderen, G Hans, S Vera and D Wildemeersch made all substantial contributions to the conception or design of the work, or to or the acquisition, analysis or interpretation of data for the work; and to drafting the work or revising it critically for important intellectual content. All authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Supplemental document 1
Download MP4 Video (13.4 MB)Supplemental document 2
Download MS Word (15.7 KB)Supplemental document 3
Download MS Word (15.4 KB)Supplemental document 4
Download MS Word (15.9 KB)Supplemental document 5
Download MS Word (16 KB)Supplemental document 6
Download MS Word (16.6 KB)Supplemental document 7
Download MS Word (15.5 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pmt-2022-0027
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data. Individual, de-identified participant data that underlie the results reported in this article (text, tables, figures and appendices) are available from the corresponding author following publication, including the clinical study report and study protocol.
References
- Cowen R , StasiowskaMK , LaycockH , BantelC. Assessing pain objectively: the use of physiological markers. Anaesthesia70(7), 828–847 (2015).
- Jiao Y , HeB , TongX , XiaR , ZhangC , ShiX. Intraoperative monitoring of nociception for opioid administration: a meta-analysis of randomized controlled trials. Minerva Anes.85(5), 522–530 (2019).
- Ledowski T . Objective monitoring of nociception: a review of current commercial solutions. Br. J. Anaesth.123(2), 312–321 (2019).
- Larson MD , BehrendsM. Portable infrared pupillometry: a review. Anesth. Analg.120(6), 1242–1253 (2015).
- Packiasabapathy S , RangasamyV , SadhasivamS. Pupillometry in perioperative medicine: a narrative review. Can. J. Anaesth.68(4), 566–578 (2021).
- Berthoud V , NguyenM , AppriouAet al. Pupillometry pain index decreases intraoperative sufentanyl administration in cardiac surgery: a prospective randomized study. Sci. Rep.10(1), 21056 (2020).
- Bartholmes F , MalewiczNM , EbelM , ZahnPK , Meyer-FriessemCH. Pupillometric monitoring of nociception in cardiac anesthesia. Dtsch. Arztebl. Int.117(49), 833–840 (2020).
- Schulz K , AltmanD , MoherD. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 340, c332 (2010).
- Wildemeersch D , BaetenM , PeetersN , SaldienV , VercauterenM , HansG. Pupillary dilation reflex and pupillary pain index evaluation during general anaesthesia: a pilot study. Rom. J. Anaesth. Intensive Care25(1), 19–23 (2018).
- Abouleish A , LeibM , CohenN. ASA Provides Examples to Each ASA Physical Status Class. ASA Newsletter. 79, 38–49 (2015).
- Kelbsch C , StrasserT , ChenYet al. Standards in pupillography. Front. Neurol.10, 129 (2019).
- Larson MD . The effect of antiemetics on pupillary reflex dilation during epidural/general anesthesia. Anesth. Analg.97(6), 1652–1656 (2003).
- Bibian S , DumontGA , ZikovT. Dynamic behavior of BIS, M-entropy and neuroSENSE brain function monitors. J. Clin. Monit. Comput.25(1), 81–87 (2021).
- Marsh B , WhiteM , MortonN , KennyGN. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaesth.67(1), 41–48 (1991).
- Harden R , WeinlandS , RembleTet al. Medication Quantification Scale Version III: update in medication classes and revised detriment weights by survey of American Pain Society Physicians. J Pain.6(6), 364–371 (2005).
- Mitchell C , CheukSJ , O’DonnellCM , BampoeS , WalkerD. What is the impact of dexamethasone on postoperative pain in adults undergoing general anaesthesia for elective abdominal surgery: a systematic review and meta-analysis. Perioper. Med.24(1), 11–13 (2022).
- Meijer F , HoningM , RoorTet al. Reduced postoperative pain using nociception level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. Br. J. Anaesth.125(6), 1070–1078 (2020).