Abstract
Prescription-strength (8%) capsaicin topical system is a US FDA-approved treatment for painful diabetic peripheral neuropathy of the feet. A 30 min application of the capsaicin 8% topical system can provide sustained (up to 3 months) local pain relief by desensitizing and reducing TRPV1-expressing cutaneous fibers. Capsaicin is not absorbed systemically; despite associated application-site discomfort, capsaicin 8% topical system is well tolerated, with no known drug interactions or contraindications, and could offer clinical advantages over oral options. Capsaicin 8% topical system are not for patient self-administration and require incorporation into office procedures, with the added benefit of treatment compliance. This article reviews existing literature and provides comprehensive, practical information regarding the integration of capsaicin 8% topical systems into office procedures.
Plain language summary
Capsaicin 8% topical system is used to treat diabetic nerve pain of the feet. This in-office 30 min application can provide lasting relief of pain (for up to 3 months) by targeting the nerves damaged by diabetes. Since capsaicin acts at the site of diabetic nerve pain without being absorbed into the bloodstream, it is unlikely to interfere with other treatments and has few undesirable effects. Discomfort at the application site is the most commonly reported adverse event. Capsaicin 8% topical system must be applied by a healthcare professional and up to four topical systems can be used per treatment. Incorporating the use of capsaicin 8% topical systems into office procedures can help provide relief for patients living with diabetic nerve pain of the feet and may improve treatment compliance.
Tweetable abstract
This article reviews existing literature and provides comprehensive, practical information regarding the integration of capsaicin 8% topical systems into office procedures.
Painful diabetic peripheral neuropathy
Painful diabetic peripheral neuropathy (PDPN) results from damage to peripheral sensorimotor nerves, including nociceptors (mainly small unmyelinated C fibers, and myelinated Aβ and Aδ fibers) [Citation1–4]. The exact pathophysiological processes involved in the development of PDPN are unclear [Citation5]. The damage to sensory nerves is generally considered to be caused by sustained hyperglycemia and metabolic complications, which injure the nerves and associated microvasculature [Citation4,Citation6]. As sensory nerves are damaged, the density of epidermal nerve fibers (ENFs) gradually decreases [Citation4]. The extent of ENF loss is correlated to the loss of sensitivity to painful stimuli [Citation4].
The development of PDPN varies between patients [Citation4]. It typically begins as distal neuropathy without pain in the feet, develops gradually and symmetrically, and is predominantly sensory, involving numbness, sensitivity, allodynia and hyperalgesia [Citation7–9]. This can further progress to proximal extremities and can be associated with neuropathic pain, burning, allodynia, etc., limiting function, patient satisfaction and quality of life (QoL) [Citation8–12].
In the USA, more than 35 million individuals have diabetes, including more than 5 million with PDPN, of whom >50% have unresolved pain [Citation7,Citation10,Citation11,Citation13]. PDPN is often misdiagnosed or underdiagnosed and can remain untreated for years [Citation14]. Since PDPN is associated with substantial morbidity and adversely affects patient’s QoL [Citation9], it is important that PDPN is recognized and adequately treated.
Treatment of painful diabetic peripheral neuropathy
The goal of treating PDPN is to relieve pain, thereby improving patient function and QoL. When selecting pharmacologic treatments for PDPN, the safety and tolerability of the options should be considered, as these conditions may persist for years, be difficult to treat and primarily affect an older population who generally have other health concerns, additionaly there is the possibility of complicated drug–drug interactions (DDIs). The complex treatment and high-pill burden in diabetic individuals, especially those with PDPN, exacerbates the risk of adverse drug reactions (ADRs), DDIs, contraindications and the need for dose titration and/or adjustment. Most (57.2%) patients with diabetes take 4–6 medications, with a further 16.8% taking ≥7 medications [Citation15] and diabetic patients in the USA take an average of 5.9 medications [Citation16]. Almost all patients with PDPN take systemic pain medications to solely treat PDPN, with 66% taking 1–3 systemic pain medications for PDPN and 17% taking ≥4 medications [Citation10]. Thus, it is important to minimize the risk of DDIs, which can lead to hospital admissions [Citation17,Citation18], ADRs, reductions in therapeutic response [Citation19] and increased mortality [Citation18].
Capsaicin 8% topical system (Qutenza®) is the only topical treatment, and only topical formulation of capsaicin, approved by the US FDA to treat PDPN of the feet [Citation20]. Topical capsaicin is a rational choice for the treatment of PDPN due to its local mechanism of action on relevant skin nociceptors, low potential for systemic effects and DDIs, lack of contraindications and absence of requirements to titrate the dose to achieve optimal effect or adjust the dosage in special populations [Citation3,Citation21–24]. However, clinical experience has shown that low-potency (0.025–0.075%) formulations of topical capsaicin display only modest efficacy, require multiple daily applications and have been associated with topical ADRs and poor compliance [Citation3,Citation21,Citation25,Citation26]. To address these issues, a prescription-strength topical system containing capsaicin 8% was developed using an innovative patch technology, which allows a sufficiently high amount of capsaicin to be released to the skin over a short application time of 30 min, enabling long-term relief of PDPN [Citation20].
Based on its pharmacological properties, clinical effectiveness and favorable safety and tolerability profile, the capsaicin 8% topical system is a recommended first-line option for the treatment of PDPN according to 2022 guidelines from the American Association of Clinical Endocrinology [Citation27] and Clinical Compendia American Diabetes Association (ADA) [Citation28]. In clinical practice, it offers additional advantages, such as ensured compliance due to application by the healthcare professional (HCP), low risk of systemic ADRs and DDIs, and lack of contraindications and titration requirements.
Tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), gabapentinoids and sodium channel blockers are first-line oral options for PDPN according to 2022 ADA [Citation28] and American Association of Clinical Endocrinology [Citation27] guidelines for the management of PDPN (). These are considered to have comparable efficacy based on the clinical evidence supporting their use [Citation28]. Duloxetine (an SNRI) and pregabalin (a gabapentinoid) are the only drugs in these classes currently approved by the FDA to treat PDPN. However, the conventional oral pharmacologic options do not target the specific mechanisms directly associated with PDPN [Citation7,Citation9,Citation29–31]. Moreover, PDPN does not typically respond to traditional analgesics (e.g., acetaminophen, NSAIDs) or weak opioids. Although tramadol extended-release is also FDA-approved to treat PDPN, the use of this dual μ-opioid receptor agonist and norepinephrine reuptake inhibitor is no longer recommended in this indication, due to general concerns regarding the use of opioids [Citation27,Citation28,Citation32]. Importantly, the use of oral therapies for PDPN is limited by their systemic ADRs, potential DDIs, contraindications and the need for dosage adjustments in special populations, such as elderly patients and those with renal or hepatic impairment [Citation33].
Based on 2022 guidelines on the treatment of PDPN from the American Association of Clinical Endocrinology [Citation27] and Clinical Compendia American Diabetes Association [Citation28].
*Indicates FDA-approved for PDPN.
PDPN: Painful diabetic peripheral neuropathy.
![Figure 1. Place of capsaicin 8% topical systems in the pharmacological treatment of painful diabetic peripheral neuropathy of the feet.Based on 2022 guidelines on the treatment of PDPN from the American Association of Clinical Endocrinology [Citation27] and Clinical Compendia American Diabetes Association [Citation28].*Indicates FDA-approved for PDPN.PDPN: Painful diabetic peripheral neuropathy.](/cms/asset/09ab4e06-5777-4239-9dfd-7d2e0ce26e56/ipmt_a_12344556_f0001.jpg)
Subsequent-line therapy for PDPN includes switching to a different first-line therapy [Citation27,Citation32] or trying combinations of pharmacological agents belonging to different drug classes or pharmacological plus non-pharmacological options () [Citation27,Citation28,Citation32]. For chronic intractable pain, neuromodulatory techniques, such as the implantation of an FDA-approved spinal cord stimulation device may be considered () [Citation27,Citation28,Citation32].
The capsaicin 8% topical system
Mechanism of action
Capsaicin is a highly selective agonist for the TRPV1 receptor, an ion channel-receptor complex expressed on nociceptive nerve fibers in the skin with functions widely linked to the generation of pain [Citation21,Citation34,Citation35]. TRPV1 receptors are primarily expressed on small-diameter sensory neurons (mainly C and Aδ) and trigeminal ganglion neurons, but are not expressed on cutaneous neurons involved in tactile or vibratory sensitivity, cold detection and cold pain [Citation35,Citation36]. Activation of TRPV1 receptors by capsaicin and other exogeneous (e.g., heat >43°C; extracellular pH <6) or endogenous stimuli (e.g., anandamide, N-acetyldopamines, leukotriene B4, long-chain unsaturated fatty acids, and 9- and 13-hydroxyoctadecadienoic acid) results in burning, itching, stinging and other physiological responses [Citation3,Citation21,Citation35].
The effects of capsaicin on pain modulation are complex, driven by multiple mechanisms associated with a marked increase in intracellular levels of calcium, and primarily appear to involve a parallel loss of TRPV1-related pain sensation and inactivation of TRPV1-expressing ENFs () [Citation3,Citation37–39]. However, to achieve these effects, low-concentration capsaicin creams must be applied frequently (i.e., three to five times daily for several weeks) [Citation3,Citation37,Citation38]. In contrast, a single application of the capsaicin 8% topical system has been shown to reduce the responsiveness of cutaneous nociceptive sensory nerve fibers and decrease the density of ENFs within 1 week of application [Citation37,Citation39].
Left panel: Upper black arrows indicate capsaicin-insensitive nerve terminals. Lower black arrows indicate nerve terminal retraction due to mitochondrial dysfunction.
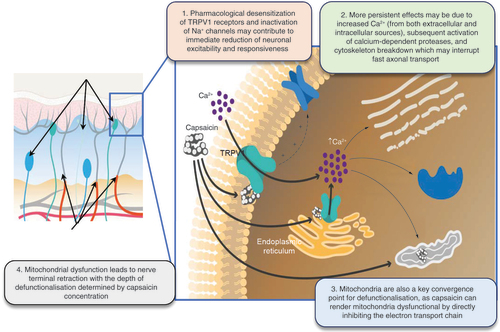
Reductions in TRPV1 receptor sensitivity to painful stimuli
Following initial exposure, capsaicin activates TRPV1 receptors in the same manner as other stimuli, resulting in an influx of calcium ions, the release of vasoactive neuropeptides, and the induction of hot, burning sensations with hyperalgesia [Citation3,Citation21–23]. Prolonged exposure to capsaicin, however, subsequently defunctionalizes and ablates TRPV1-expressing fibers, thereby reducing their responsiveness to painful stimuli and providing pain relief for up to 3 months [Citation3,Citation22,Citation40,Citation41]. High-concentration capsaicin predominantly affects TRPV1-expressing cutaneous fibers with significant (p < 0.05) inhibition of warmth detection, heat pain, neurogenic flare, histamine-induced itch and mechanical pain sensitivity [Citation36]. As expected, non-TRPV1-expressing cutaneous neurons are not affected, with no effects on tactile or vibratory sensitivity, cold detection and cold pain [Citation36].
Direct & reversible ablation of TRPV1-expressing epidermal nerve fibers
When capsaicin binds to the receptor, the TRPV1 cation channel opens, and calcium enters the intracellular space. When small-diameter sensory neurons are exposed to capsaicin at either low or high concentration, activation of the TRPV1-espressing fibers occurs. However, prolonged exposure to high-concentration capsaicin results in defunctionalization and ablation of the nerve fiber. For this reason, prolonged exposure to high concentrations of capsaicin can provide sustained pain relief by reducing the density of TRPV1-expressing nociceptive ENFs at the site of application [Citation3,Citation37–39,Citation42]. The increased influx of intracellular calcium overwhelms the mitochondria, leading to reversible dysfunction and nerve terminal death. Robust reduction of ENF density after the application of high concentrations of capsaicin in human skin was shown by staining skin biopsies with a pan-axonal marker, protein gene product 9.5 (PGP 9.5) [Citation32,Citation36,Citation39]. This effect is reversible, with eventual regrowth of the ablated neurons and a corresponding return of the ability to detect painful stimuli [Citation39]. For example, in a study in healthy volunteers [Citation39], ENF density at capsaicin 8% topical system application sites was significantly (80%) reduced relative to unexposed sites 1 week after application, which was followed by evidence of ENF regeneration at week 12, and almost full (93%) recovery of ENF at week 24 (Supplementary Figure 1).
As a selective neurolytic agent, the capsaicin 8% topical system inactivates only pain-sensing neurons at the site of application [Citation3,Citation30]. Over several months, painful neuropathy may gradually re-emerge, which is thought to be due to the re-innervation of TRPV1-expressing ENFs in the treated area [Citation3,Citation39], as well as the underlying chronic diabetes and progression of diabetic neuropathy [Citation43,Citation44].
Pharmacokinetic profile
Each 280 cm2 topical system contains a reservoir of capsaicin 179 mg () [Citation20]. Application of the capsaicin 8% topical system results in a favorable pharmacokinetic profile. Unlike conventional transdermal patches, the capsaicin 8% topical system works locally on the skin, with very little systemic absorption [Citation20,Citation21]. During the application period, capsaicin is diffused into the skin at a linear rate [Citation40,Citation41], forming a cutaneous reservoir of bioavailable capsaicin that is sufficient to enable pain relief for up to 3 months [Citation20,Citation45]. Due to the lipophilic properties of the capsaicin-containing solvent, capsaicin is easily absorbed into the layers of the skin, but systemic exposure to capsaicin is transient and low [Citation3,Citation21,Citation46]. Plasma levels of capsaicin peak ≈20 min after removal of the transdermal system, then decline very rapidly, as the small amounts of systemically absorbed capsaicin are rapidly metabolized in the liver by cytochrome P450 (CYP) enzymes, and swiftly eliminated (half-life ≈130 min) by the kidneys [Citation3,Citation21,Citation46,Citation47]. Capsaicin appears to be metabolized very slowly in the skin [Citation48]. Exposure increases with larger treatment areas and with longer treatment duration [Citation46].
The topical system consists of a polyester-film backing layer coated with a matrix composed of capsaicin, a DGME-containing solvent, silicone adhesive mixtures, and other ingredients, and covered with a removable polyester-release liner. As DGME is extremely lipophilic, it is easily absorbed into the epidermal and dermal layers but has little affinity for the blood phase. During application, capsaicin is diffused into the skin via the influx of DGME and efflux of water, forming a reservoir of bioavailable capsaicin [Citation20,Citation45].
DGME: Diethylene glycol monomethyl ether.
![Figure 3. Technology of the capsaicin 8% topical system, showing the transfer of capsaicin into the skin during the application period, enabling long-term (3-month) pain relief.The topical system consists of a polyester-film backing layer coated with a matrix composed of capsaicin, a DGME-containing solvent, silicone adhesive mixtures, and other ingredients, and covered with a removable polyester-release liner. As DGME is extremely lipophilic, it is easily absorbed into the epidermal and dermal layers but has little affinity for the blood phase. During application, capsaicin is diffused into the skin via the influx of DGME and efflux of water, forming a reservoir of bioavailable capsaicin [Citation20,Citation45].DGME: Diethylene glycol monomethyl ether.](/cms/asset/ab2e8526-5252-4f5a-91b5-25defe9611fe/ipmt_a_12344556_f0003.jpg)
Due to the low systemic exposure to capsaicin, as well as the lack of CYP induction and inhibition with capsaicin [Citation20,Citation46], DDIs between capsaicin 8% topical system and systemic medications are unlikely [Citation20], and dosage adjustments are not required in patients with hepatic or renal failure [Citation20,Citation21].
Efficacy & tolerability in clinical trials
STEP trial
The efficacy and safety of a single 30 min application of capsaicin 8% topical system for the treatment of PDPN of the feet were evaluated in STEP, a randomized, controlled, phase 3 trial with a follow-up period of 12 weeks (see Supplementary Table 1 for trial design details) [Citation49].
One 30 min application of the capsaicin 8% topical system provided sustained pain relief for up to 12 weeks in patients with PDPN of the feet (Supplementary Table 1) [Citation49]. Mean average daily pain scores improved from baseline to a significantly greater extent with the capsaicin 8% topical system than with placebo during weeks 2–8 (-27.4 vs -20.9%; p = 0.025; primary end point).
Several secondary outcomes also indicated that the capsaicin 8% topical system was more effective than placebo (Supplementary Table 1) [Citation49]. These included: the mean percent change in average daily pain scores during weeks 2–12 (-28.0 vs -21.0%); the proportion of patients achieving a clinically meaningful reduction in pain (i.e., a ≥30% response) during weeks 2–12 (40.9 vs 31.7%; p = 0.05); the median time to achieve a ≥30% treatment response during weeks 2–8 (19 vs 72 days); and the improvement from baseline in sleep interference scores during weeks 2–8 (-33.1 vs -24.2%; p = 0.03) and weeks 2–12 (-34.0 vs -24.2%; p = 0.02), suggesting rapid and durable improvements [Citation49].
As expected, most treatment-emergent ADRs reported with the capsaicin 8% topical system were application-site reactions (Supplementary Table 1), with no deterioration in sharp, cold or warm sensory perception [Citation49]. Application-site ADRs most commonly involved burning sensations and pain, and were generally transient, self-limiting and typically mild or moderate in intensity [Citation20]. No drug-related ADRs leading to permanent discontinuation, drug-related serious ADRs or deaths were reported with the capsaicin 8% topical system.
PACE trial
The long-term safety and efficacy of up to seven applications of the capsaicin 8% topical system for the treatment of PDPN of the feet was explored in the open-label, randomized PACE trial [Citation42,Citation50]. Patients were randomized to receive 30 min applications of capsaicin 8% topical systems plus standard of care (SoC; i.e., optimized treatment with antidepressants, antiepileptic and/or opioids) or SoC alone for 52 weeks (see Supplementary Table 2 for trial design details) [Citation42,Citation50]. The initial 30 min application of the capsaicin 8% topical system could be followed by up to six subsequent applications at intervals of ≥8 weeks as warranted by the investigators [Citation42,Citation50]. Half (53.8%) of the patients in the capsaicin 8% topical system plus SoC group received seven applications of the capsaicin 8% topical system with a mean between-application interval of 68.4 days [Citation42].
Repeated application of the capsaicin 8% topical system was not associated with deterioration of diabetic neuropathy-related QoL, sensory perception or reflex function at 52 weeks [Citation42]. Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) scores improved from baseline to a greater extent with one to seven applications of the capsaicin 8% topical system plus SoC than with SoC alone at week 52, with a treatment difference of -20.9% (primary end point; Supplementary Table 2). In the subgroup of 84 patients who received seven applications of the capsaicin 8% topical system, the mean change from baseline was -31.2%, with a treatment difference of -24.5% versus SoC alone. Similar improvements were observed across Norfolk subscale scores (small fiber, symptoms, autonomic, physical function, activities of daily living). As indicated by changes from baseline in Utah Early Neuropathy Scale total and subscale scores, slight improvements in sensory function were shown in both treatment groups at 52 weeks (Supplementary Figure 2). No changes in sensory perception (sharp, warm, cold, vibration) and reflex testing were shown in most patients in the capsaicin 8% topical system plus SoC and SoC alone groups (52.7–77.3 vs 54.5–82.5%), with improvements being more common than worsening in the remaining patients [Citation42].
Application of the capsaicin 8% topical system also provided long-term pain relief and improved QoL (secondary end points; Supplementary Table 2) [Citation50]. Patients in the capsaicin 8% topical system plus SoC group showed a reduction in pain as early as the first study assessment (month one), with progressive improvements with subsequent treatment to the end of the study. Changes from baseline in numeric pain rating scale of average daily pain, pain intensity and pain interference scores, the proportions of patients achieving a clinically meaningful reduction in pain (i.e., a ≥30% or ≥50% response) and reporting global impressions of improvements, and improvements in EuroQol5-dimension (EQ-5D) scores were numerically higher in the capsaicin 8% topical system plus SoC group than in the SoC alone group.
In a self-assessment of treatment, more capsaicin 8% topical system plus SoC than SoC alone recipients reported improvements in QoL, as well as improvements in pain and activity levels. In addition, more patients were willing to continue treatment with the capsaicin 8% topical system plus SoC than were willing to continue treatment with SoC alone (73.3 vs 52.3%). A higher proportion of capsaicin 8% topical system plus SoC recipients than SoC alone recipients preferred their study treatment over their previous treatment (63.0 vs 31.1%) [Citation50].
Some patients may require repeated applications of the capsaicin 8% topical system before achieving an initial clinically meaningful reduction in pain. In the post hoc analysis of 313 PDPN patients treated with repeated 30 or 60 min applications of the capsaicin 8% topical system at ≥8-week intervals [Citation51], an initial ≥30% reduction in average pain intensity was attained by 96 patients after the first application, 68 after the second application and 43 after the third application. Of those patients without an initial ≥30% response at 3 months, repeated applications of the capsaicin 8% topical system provided a ≥30% response in 28.1, 42.6 and 45.7% of patients at 6, 9 and 12 months, respectively. Importantly, over time, patients with a slower initial response achieved benefits similar to those with an early response. By month 12, late and early responders achieved similar improvements in pain relief, sleep, QoL and patient satisfaction [Citation51].
Treatment with the capsaicin 8% topical system also appeared to limit the demand for new medications to manage PDPN [Citation50]. A post hoc analysis examined the changes in the proportions of patients using concomitant pain medication over the course of PACE trial. In the capsaicin 8% topical system plus SoC group, the use of concomitant anticonvulsants remained stable (28.2% of patients at baseline vs 29.5% at study end), as did the use of opioids (10.9 vs 11.0%) and antidepressants (10.9 vs 11.0%). In contrast, in the SoC alone group, the use of concomitant anticonvulsants increased by ≈10% (32.3% of patients at baseline vs 43.2% at study end), with increases also being shown in the proportions of patients using opioids (8.4 vs 11.6%) and antidepressants (7.7 vs 15.1%) [Citation50].
Repeated applications of the capsaicin 8% topical system at ≥8-week intervals were generally well tolerated with no new safety concerns [Citation42]. At least one treatment-emergent ADR was reported in 66.7 and 48.4% of patients in the capsaicin 8% topical system plus SoC group and SoC alone groups, respectively, with most being of mild or moderate severity. As expected, application-site ADRs were the most common ADRs reported with the capsaicin 8% topical system (Supplementary Table 2). Drug-related ADRs were reported in 39.7% of capsaicin 8% topical system recipients but did not lead to permanent discontinuation of treatment in any patients.
The results of this open-label study must be interpreted with caution, as they may be confounded by evaluator bias and could represent chance findings. As the secondary and post hoc end points were not powered for statistical analysis, the results of these end points should be considered descriptive only.
Network meta-analysis
A network analysis provides indirect evidence of the relative efficacy of the capsaicin 8% topical system versus placebo and oral pregabalin, gabapentin and duloxetine in treating PDPN, as no head-to-head studies have been conducted [Citation52]. Of the 25 randomized, controlled trials included in the analysis, STEP was the only trial of the capsaicin 8% topical system. The results of the network analysis should be interpreted with caution, as they are based on indirect comparisons and could represent chance findings.
The likelihood of achieving a ≥30% response with the capsaicin 8% topical system was significantly greater than with placebo (odds ratio (OR) 2.28; 95% CI, 1.19–4.03), and comparable to that with oral treatment with pregabalin (OR 1.83; 95% CI, 0.91–3.34), gabapentin (OR 1.66; 95% CI, 0.74–3.23) and duloxetine (OR, 0.9; 95% CI, 0.5–1.79). However, the capsaicin 8% topical system offered systemic tolerability benefits over these oral agents. Somnolence, dizziness, nausea, diarrhea, fatigue and discontinuation due to ADRs were not reported by any capsaicin 8% topical system recipients. In contrast, pregabalin, gabapentin and duloxetine all significantly increased the risk of somnolence, dizziness and discontinuation due to ADRs relative to placebo, with duloxetine also significantly increasing the risk of nausea, diarrhea and fatigue [Citation52].
Incorporation into office procedures
Capsaicin 8% topical system is an effective option for the treatment of PDPN of the feet, offering the flexibility of being used alone or in combination with other therapies, and the advantages of providing only localized effects, having a low risk of systematic ADRs, a lack of DDIs and contraindications, being a non-opioid option and delivering long-term benefits, without the need for continued administration outside the office or clinic, thereby resulting in ensured patient compliance.
Application of the capsaicin 8% topical system must be incorporated into clinic or office procedures, as it must be administered and handled only by HCPs and cannot be dispensed to patients for self-administration or handling due to its high concentration of capsaicin [Citation20]. Therefore, it is important to understand how to administer and handle capsaicin 8% topical system in the office setting.
In the USA, the capsaicin 8% topical system is FDA-approved to treat PDPN of the feet (applied for 30 min) and neuropathic pain associated with postherpetic neuralgia (applied for 60 min). Up to four capsaicin 8% topical systems applied at each treatment; treatment may be repeated every 3 months or as warranted by the return of pain, but not more frequently than every 3 months [Citation20]. Capsaicin 8% topical system is not indicated to treat patients aged <18 years, as pediatric studies are lacking [Citation20]. Dosage adjustments are not required in any special populations, including the elderly and those with renal or hepatic impairment [Citation20].
Each capsaicin 8% topical system carton contains one or two topical systems plus one 50 g tube of cleansing gel, or four topical systems plus three 50 g tubes of cleansing gel [Citation20]. The cartons should be stored at 68–77°F (20–25°C), with allowed excursions to 59–86°F (15–30°C). Each topical system is packaged in a sealed pouch, which should not be opened until immediately before application.
provides a summary of the instructions for HCPs for applying and handling capsaicin 8% topical system in the office setting. The following precautionary measures should be taken to reduce the potential for capsaicin-related ADRs [Citation20].
Details are based on the US prescribing information [Citation20].
![Figure 4. Summary of the instructions for applying the capsaicin 8% topical system.Details are based on the US prescribing information [Citation20].](/cms/asset/8636641e-d16c-4fec-8ff0-1a375212d51e/ipmt_a_12344556_f0004.jpg)
Avoid unintended exposure to capsaicin
Severe irritation of eyes, mucous membranes, respiratory tract and/or skin can result from accidental exposure to capsaicin and may affect HCPs, patients and other individuals who come into contact with the capsaicin 8% topical system, items used in its application, removal, cleansing, as well as surfaces and items that have been in contact with capsaicin. Follow appropriate precautions while applying the capsaicin 8% topical system (), avoid unnecessary contact with any items in the room, including those that the patient may later have contact with (e.g., horizontal surfaces and bedsheets), and thoroughly clean all areas and items exposed to capsaicin 8% topical systems and dispose of them properly following local biomedical waste procedures. As latex gloves do not provide adequate protection, wear nitrile gloves during the handling, application and cleansing of capsaicin 8% topical systems and other items that come into contact with capsaicin.
Manage unintended exposure to healthcare professionals, patients or others
If skin other than that in the treatment area comes into contact with the capsaicin 8% topical system, apply the supplied cleansing gel for 1 min, wipe off with dry gauze and wash the area with soap and water. If accidental exposure to the eyes or mucous membranes occurs (e.g., from touching the capsaicin 8% topical system or items exposed to capsaicin, then touching the eyes or mucous membrane), flush the irritated eyes or mucous membranes with cool water, and remove the affected individual from the vicinity of the capsaicin 8% topical system. If the respiratory tract is exposed to aerosolized capsaicin (e.g., during the rapid removal of the topical system), irritation of the airways (i.e., coughing, sneezing and shortness of breath) may occur. Provide supportive medical care for shortness of breath. Remove the affected individual from the vicinity of the capsaicin 8% topical system and, if respiratory irritations worsen or do not resolve, do not re-expose them to the capsaicin 8% topical system.
Alleviate application-site pain & burning during application & removal of the capsaicin 8% topical system
Although transient mild-to-moderate application-site ADRs are common following application of the capsaicin 8% topical system, they can be ameliorated by following the appropriate precautions. An optional pretreatment of the affected area with a topical anesthetic can be done to minimize discomfort during the application (). Be prepared to manage application-site pain during and following treatment with local cooling (e.g., cool packs) and/or appropriate analgesics. Application-site pain typically resolves within ≈1.5 h of removal of the topical system and does not return [Citation53]. The capsaicin 8% topical system can usually be applied for the full 30 min treatment period, with only some patients requiring pain relief. For example, in 91 patients with PDPN of the feet, all patients completed ≥90% of the full application duration, and only 14% required medication for treatment-related pain on days zero to five [Citation53].
Assess reductions in sensory function before application
Assess patients with pre-existing sensory deficits for signs of sensory deterioration/loss before each application of the capsaicin 8% topical system, and reconsider its continued use in those with sensory deterioration/loss or worsening of pre-existing sensory deficit. Application of the capsaicin 8% topical system has been associated with generally minor and temporary reductions in sensory function, with no deterioration of sensory function being shown with repeated use of the capsaicin 8% topical system in PACE [Citation42].
Be aware of the risk of transient increases in blood pressure
Treatment with the capsaicin 8% topical system may cause transient increases in blood pressure (BP; average <10 mm Hg, but may be greater) that may be directly linked to application-site pain caused by capsaicin and not to a pre-existing condition of elevated BP [Citation20]. Monitor BP periodically during and following application of the capsaicin 8% topical system and provide adequate support for treatment-related pain. Before initiating treatment with the capsaicin 8% topical system, consider the presence of factors (e.g., unstable or poorly controlled hypertension, or a recent history of cardiovascular or cerebrovascular events) that may increase the individual’s risk of adverse cardiovascular effects.
Provide the patient with appropriate information
Ensure the patient understands that: accidental exposure to capsaicin can cause severe irritation (advise them not to touch their eyes or other unintended treatment areas, and to notify their physician immediately if irritation of the eyes or airways occurs, or if any ADR becomes severe); acute treatment-related pain may occur during and after application of the capsaicin 8% topical system and may require pain medication; BP will be monitored, as transient increases in BP may result from the treatment-related increases in pain (ask them about any recent cardiovascular events); the treated area may be sensitive to heat (e.g., direct sunlight, hot showers or baths and vigorous exercise) for a few days after treatment; and relief of PDPN will not be immediate, and may take ≈2 weeks to achieve.
Patients should be informed that subsequent or ongoing treatment with the capsaicin 8% topical system may be required. Treatment may be repeated every 3 months or as warranted by the return of pain (not more frequently than every 3 months) [Citation20]. This application schedule potentially provides another benefit over oral therapies, which require daily administration of multiple doses, and lidocaine 5% patches, which require daily application for 12 h. Importantly, the effectiveness and tolerability of the capsaicin 8% topical system are both maintained with subsequent applications, with 1% of patients discontinuing treatment with the capsaicin 8% topical system due to an ADR across all clinical studies.
Case study
The following case study provides an example of the incorporation of the use of the capsaicin 8% topical system into office procedures. A 70 year-old female with non-insulin-dependent diabetes for the past 30 years presented at our office with PDPN of the feet for 15 years and low back pain. She had previously received multiple systemic and topical medications, including antidepressants, opioids and antiepileptics. Upon review, the treatments that had most recently failed were duloxetine, tramadol, pregabalin, amitriptyline, zonisamide and diclofenac gel. Her PDPN and low back pain were currently being treated with gabapentin 800 mg, lidocaine 5% patches (three patches applied for 12 h/day) and a buprenorphine 15 μg/h patch. The patient had the following comorbidities: history of ovarian cancer, heart disease (atrial fibrillation), sleep apnea, obesity, gastro-esophageal reflux disease, depression, chronic obstructive pulmonary disease, hypothyroidism, hyperlipidemia, fibromyalgia, history of pulmonary embolism and irritable bowel disease.
Upon clinical examination, the patient reported an average pain score of 7 out of a maximum of 10 over the past few years, numbness and tingling, intermittent burning and stabbing pain and loss of sensation on the feet.
The clinician decided to initiate treatment with the capsaicin 8% topical system. The patient had a pain score of 7/10 immediately before application. Two capsaicin 8% topical systems were placed to cover the plantar and dorsal surface of each foot (a total of four topical systems). The patient experienced some minor burning near the end of the 30 min application but handled the procedure very well overall. She did not report any significant discomfort following the application. Thereafter, she could feel sensation in her feet, had a decrease in pain score to 4/10, could walk more comfortably throughout the day, and was sleeping better. The patient returned for a second application after 3 months, with a follow-up visit scheduled at 21 days. If deemed necessary, a third application can be given following another 3 month interval. provides the key takeaway messages from the case study.
Table 1. Key messages from the case study of a patient presenting with painful diabetic peripheral neuropathy of the feet.
Conclusion
Capsaicin 8% topical system, a valuable treatment option for PDPN of the feet, may offer clinically relevant benefits over oral therapies. One 30 min application of the capsaicin 8% topical system provides sustained pain relief plus other related benefits. In addition, subsequent applications at ≥3-month intervals continue to provide pain relief without deterioration of sensory function. Due to minimal systemic absorption, topical treatment with capsaicin 8% is well tolerated, with a lack of systemic ADRs, potential DDIs, contraindications and the need for dosage adjustments in special populations.
Oral systemic treatments for PDPN of the feet often do not provide adequate pain relief. To facilitate the widespread use of capsaicin 8% topical system to treat this common condition, appropriate steps must be taken to integrate its administration by HCPs into the office setting. To protect patients, HCPs, and others from unintentional exposure to capsaicin, instructions for applying the capsaicin 8% topical system must be followed. Appropriate measures must also be taken to ameliorate pain and application-site ADRs, as well as to ensure patients are knowledgeable about this treatment. HCPs should not be concerned about the time required to treat patients with capsaicin 8% topical system in the office setting. According to European pain specialists, the time spent with patients receiving capsaicin 8% for neuropathic pain is comparable to that spent with patients receiving systemic treatment [Citation54]. The time required to apply the capsaicin 8% topical system is offset by the need for fewer follow-up patient visits, and reductions in the time spent renewing prescriptions and counseling patients about treatment changes. Moreover, unlike oral medications, the use of capsaicin 8% topical system is not associated with compliance issues [Citation54]. Most patients preferred the capsaicin 8% topical system over their previous treatments and were willing to continue treatment, with the use of the capsaicin 8% topical system also reducing the high pill burden associated with treating PDPN [Citation50].
The case study presented supports how the incorporation of capsaicin 8% topical system into office procedures provided pain relief in a patient with long-term PDPN despite previous treatment with multiple systemic and topical medications.
Future perspective
Further real-world evidence of the use of capsaicin 8% topical system in the treatment of PDPN of the feet will help identify factors associated with treatment response and will further guide best practices. Analyses of the long-term cost–effectiveness and cost–utility of capsaicin 8% topical system versus other recommended first-line options for PDPN would be of interest to clarify the clinical and QoL benefits of the capsaicin 8% topical system relative to its cost.
Prescription-strength (8%) capsaicin topical system (Qutenza®) is a US FDA-approved therapy for treating painful diabetic peripheral neuropathy of the feet.
The capsaicin 8% topical system offers clinically relevant advantages over oral neuropathic pain medications, as it acts locally to target the source of neuropathic pain with very little systemic absorption of capsaicin.
Application of the capsaicin 8% topical system can provide rapid and sustained pain relief, leading to other pain-related benefits.
The most common adverse reaction is application-site discomfort that can be managed with a cooling pack and/or analgesics during and post application.
Subsequent application of the capsaicin 8% topical system (at ≥3 month intervals) can continue to provide pain relief without deterioration of sensory or reflex function.
Precautionary measures must be taken to reduce the potential for adverse effects, particularly those related to accidental exposure to capsaicin.
The use of capsaicin 8% topical system must be incorporated into office procedures, as they cannot be dispensed to patients for self-administration.
Author contributions
All authors contributed substantially to the conception of the work, reviewed the article for accuracy, and provided constructive feedback. All authors approved the final version of the article and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests disclosure
O Landrum is a consultant/independent contractor for Averitas Pharma, Inc.; L Marcondes and T Egharevba are full-time employees of Averitas Pharma, Inc.; K Gritsenko is a consultant for Averitas Pharma, Inc. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Writing assistance was provided by KA Lyseng-Williamson and DP Figgitt, Content Ed Net, and was funded by Averitas Pharma, Inc.
Ethical conduct of research
Verbal and written informed consent has been obtained from the case-study patient for the inclusion of their medical and treatment history within this work.
Infographic
Download PDF (6.7 MB)Supplemental Information 2
Download MS Word (7.3 MB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pmt-2023-0028
Financial disclosure
The creation of the work was funded by Averitas Pharma, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Colloca L , LudmanT , BouhassiraDet al. Neuropathic pain. Nat. Rev. Dis. Primers.3(16), 17002 (2017).
- Jaggi AS , SinghN. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res.1381, 187–201 (2011).
- Anand P , BleyK. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth.107(4), 490–502 (2011).
- Tesfaye S , BoultonAJ , DyckPJet al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care33(10), 2285–2293 (2010).
- Vincent AM , CalabekB , RobertsL , FeldmanEL. Biology of diabetic neuropathy. Handb. Clin. Neurol.115, 591–606 (2013).
- Tesfaye S , BoultonAJ , DickensonAH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care36(9), 2456–2465 (2013).
- Iqbal Z , AzmiS , YadavRet al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin. Ther.40(6), 828–849 (2018).
- Sloan G , ShilloP , SelvarajahDet al. A new look at painful diabetic neuropathy. Diabetes Res. Clin. Pract.144, 177–191 (2018).
- Javed S , AlamU , MalikRA. Treating diabetic neuropathy: present strategies and emerging solutions. Rev. Diabet. Stud.12(1–2), 63–83 (2015).
- Gore M , BrandenburgNA , HoffmanDL , TaiKS , StaceyB. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J. Pain7(12), 892–900 (2006).
- Gore M , BrandenburgNA , DukesE , HoffmanDL , TaiKS , StaceyB. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manage.30(4), 374–385 (2005).
- Davies M , BrophyS , WilliamsR , TaylorA. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care29(7), 1518–1522 (2006).
- IDF Diabetes Atlas (10th Edition). International Diabetes Federation, Brussels, Belgium (2021). https://diabetesatlas.org/
- Mick G , BaronR , Correa-IllanesGet al. Is an easy and reliable diagnosis of localized neuropathic pain (LNP) possible in general practice? Development of a screening tool based on IASP criteria. Curr. Med. Res. Opin.30(7), 1357–1366 (2014).
- St Onge EL , MillerSA. Pain associated with diabetic peripheral neuropathy: a review of available treatments. P. T.33(3), 166–176 (2008).
- Saydah SH . Medication use and self-care practices in persons with diabetes. In: Diabetes in America.CowieCC, CasagrandeSS, MenkeA ( Eds). National Institute of Diabetes and Digestive and Kidney Diseases (US), MD, USA (2018).
- Dechanont S , MaphantaS , ButthumB , KongkaewC. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf.23(5), 489–497 (2014).
- Aparasu R , BaerR , AparasuA. Clinically important potential drug-drug interactions in outpatient settings. Res. Social. Adm. Pharm.3(4), 426–437 (2007).
- Cascorbi I . Drug interactions--principles, examples and clinical consequences. Dtsch. Arztebl. Int.109(33–34), 546–555 (2012).
- Qutenza® (capsaicin) topical system: US prescribing information. Averitas Pharma, Inc, NJ, USA (2022).
- Baranidharan G , DasS , BhaskarA. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther. Adv. Neurol. Disord.6(5), 287–297 (2013).
- Leppert W , Malec-MilewskaM , ZajaczkowskaR , WordliseckZ. Transdermal and topical drug administration in the treatment of pain. Molecules23(3), 681 (2018).
- Frias B , MerighiA. Capsaicin, nociception and pain. Molecules21(6), 797 (2016).
- Moran MM , SzallasiA. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br. J. Pharmacol.175(12), 2185–2203 (2018).
- Zilliox LA . Neuropathic pain. Continuum (Minneap Minn).23(2, Selected Topics in Outpatient Neurology), 512–532 (2017).
- Derry S , MooreRA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev.12(9), CD010111 (2012).
- Blonde L , UmpierrezGE , ReddySSet al. American Association of Clinical Endocrinology Clinical Practice Guideline: developing a diabetes mellitus comprehensive care plan—2022 update. Endocr. Pract.28(10), 923–1049 (2022).
- Pop-Busli R , AngL , BoultonAJMet al. Diagnosis and treatment of painful diabetic peripheral neuropathy. American Diabetes Association, Inc, VA, USA (2022).
- Pickering G , MartinE , TiberghienF , DelormeC. Localized neuropathic pain: an expert consensus on local treatments. Drug Des. Devel. Ther.11, 2709–2718 (2017).
- Finnerup NB , AttalN , HaroutounianS , McNicolE. Pharmacotherapy for neuropathic pain in adults: a systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol.14(2), 162–173 (2015).
- Cruccu G , TruiniA. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther.6, 35–42 (2017).
- Price R , SmithD , FranklinGet al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: Report of the AAN Guideline Subcommittee. Neurology98(1), 31–43 (2022).
- Stillman M . Clinical approach to patients with neuropathic pain. Cleve. Clin. J. Med.73(8), 726–739 (2006).
- Qutenza® (capsaicin) topical system: US prescribing information. Averitas Pharma, Inc, NJ, USA (2022).
- Benítez-Angeles M , Morales-LázaroSL , Juárez-GonzálezE , RosenbaumT. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci.21(10), 3421 (2020).
- Lo Vecchio S , AndersenHH , Arendt-NielsenL. The time course of brief and prolonged topical 8% capsaicin-induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging. Exp. Brain Res.236(8), 2231–2244 (2018).
- Malmberg AB , MizisinAP , CalcuttNA , von SteinT , RobbinsWR , BleyKR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain111(3), 360–367 (2004).
- Nolano M , SimoneDA , Wendelschafer-CrabbG , JohnsonT , HazenE , KennedyWR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain81(1–2), 135–145 (1999).
- Kennedy WR , VanhoveGF , LuSP , TobiasJ. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J. Pain.11(6), 579–587 (2010).
- Noto C , PappagalloM , SzallasiA. NGX-4010, a high-concentration capsaicin dermal patch for lasting relief of peripheral neuropathic pain. Curr. Opin. Investig. Drugs.10(7), 702–710 (2009).
- Premkumar LS , SikandP. TRPV1: a target for next generation analgesics. Curr. Neuropharmacol.6(2), 151–163 (2008).
- Vinik AI , PerrotS , VinikEJet al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol.16(1), 251 (2016).
- Feldman EL , CallaghanBC , Pop-BusuiRet al. Diabetic neuropathy. Nat. Rev. Dis. Primers5(1), 41 (2019).
- Yang H , SloanG , YeYet al. New Perspective in Diabetic Neuropathy: from the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front. Endocrinol. (Lausanne).10, 929 (2019).
- Wohlrab J , NeubertRH , HeskampML , MichaelJ. Cutaneous drug delivery of capsaicin after in vitro administration of the 8% capsaicin dermal patch system. Skin Pharmacol. Physiol.28(2), 65–74 (2015).
- Babbar S , MarierJF , MouksassiMSet al. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther. Drug Monit.31(4), 502–510 (2009).
- Blair HA . Capsaicin 8% dermal patch: a review in peripheral neuropathic pain. Drugs78(14), 1489–1500 (2018).
- Chanda S , BashirM , BabbarS , KogantiA , BleyK. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos.36(4), 670–675 (2008).
- Simpson DM , Robinson-PappJ , VanJet al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J. Pain18(1), 42–53 (2017).
- Vinik AI , PerrotS , VinikEJet al. Repeat treatment with capsaicin 8% patch (179 mg capsaicin cutaneous patch): effects on pain, quality of life, and patient satisfaction in painful diabetic peripheral neuropathy: an open-label, randomized controlled clinical trial. J. Curr. Med. Res. Opin.2(12), 388–401 (2019).
- Freynhagen R , ArgoffC , EerdekensM , EngelenS , PerrotS. Progressive response to repeat application of capsaicin 179 mg (8% w/w) cutaneous patch in peripheral neuropathic pain: comprehensive new analysis and clinical implications. Pain Med.22(10), 2324–2336 (2021).
- van Nooten F , TreurM , PantiriK , StokerM , CharokopouM. Capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy: a systematic literature review and network meta-analysis. Clin. Ther.39(4), 787–803.e18 (2017).
- Peppin JF , MajorsK , WebsterLR , SimpsonDM. Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. J. Pain Res.4, 385–392 (2011).
- Wagner T , Roth-DaniekA , SellA , EnglandJ , KernK-U. Capsaicin 8% patch for peripheral neuropathic pain: review of treatment best practice from ‘real-world’ clinical experience. Pain Manag.2(3), 239–250 (2012).

