Abstract
Background: Infusions with lidocaine or ketamine have been separately established in the treatment of chronic pain. This study aims to retrospectively evaluate the effect of combined infusions of lidocaine and ketamine. Materials & methods: Patient records were screened for receipt of combined ambulatory infusions of lidocaine and ketamine from 2012 through 2021. A scoring system was designed to assess pain response retrospectively. Results: A total of 319 patients were included. Median pain reduction in days was 10.00 (interquartile range: 13.25). Side effects were limited to the acute phase of infusions. A total of 41.4% of patients who received concomitant pain medication reported a dose reduction. Conclusion: Our data support combined infusions as a safe therapy option, with good short-, medium- and long-term reductions in pain and great heterogeneity in treatment response.
Clinical trial registration: ClinicalTrials.gov (NCT05103319)
Tweetable abstract
Combined treatment with intravenous lidocaine and ketamine can be a safe option in the treatment of refractory chronic pain. Prospective data are needed to predict treatment response in a heterogeneous group of pain patients.
Plain language summary
What is this study about?
This study examined data of patients with chronic pain who received an infusion at our hospital with two drugs, lidocaine and ketamine, in an effort to reduce pain. We examined the records of 319 patients and a total of 2995 infusion protocols to gather our data. We wanted to know how much and for how long pain was reduced by these infusions. Additionally, we tried to identify the specific features of patients who profited the most during our infusions. We also had a look at the side effects of the infusions and wanted to know if patients could reduce their daily pain medication intake when receiving infusions.
What were the results?
On average, people had less pain for 10 days after the infusions. Women seemed to benefit more than men. Otherwise, we were unable to identify specific features that predicted how much a patient would benefit. Side effects occurred only during the infusions and for a short period afterward. In addition, 41.4% of patients who took pain medication daily were able to reduce their intake.
What do the results mean?
These results support our clinical experience that infusions with lidocaine and ketamine are safe and can contribute to reduced pain in patients with chronic pain, at least in the short term, and for some patients even longer.
In Europe, 20–30% of the population suffers from chronic pain [Citation1]. Pain that is refractory to first- or even second-line treatment is a well-known problem in pain medicine. The first treatment approach to chronic pain should be its prevention [Citation2]. In chronic pain, specific pharmacological, interventional, surgical and psychosomatic treatments, focused on the pathology, should be the first-line therapy [Citation3]. Treating patients with chronic pain often poses a challenge to the attending physician [Citation4], as a remarkable number of patients do not respond to specific pain treatment or suffer from strong side effects, leading to the discontinuation of therapy [Citation5]. Additionally, some options lose their effectiveness over time [Citation6].
Insufficient treatment of chronic pain not only gives rise to socioeconomic problems, as patients may become unable to work, but is often linked to a variety of impairments, such as reduction in quality of life, sleeping disorders and depression [Citation7–9]. For these patients, treatment options with the ability to reduce the level of daily pain are of great importance, as they improve the quality of life and functioning of the patient in both their work and social environment [Citation10,Citation11].
Commonly prescribed drugs, such as NSAIDs [Citation12], paracetamol [Citation13], metamizole [Citation14], antidepressants [Citation15] and antiepileptics [Citation16], are frequently associated with unwanted and limiting side effects and have thus proven to be less effective. Despite their long history of use in the treatment of chronic pain, the use of opioids has become increasingly concerning [Citation17,Citation18]. Patients with long-term opioid use often require dose escalation, which, in combination with the addictive potential, poses a significant and increasing problem in the management of these patients [Citation6,Citation19,Citation20].
Alternative treatment options, such as the use of infusion therapies with lidocaine [Citation21–24] or ketamine [Citation25,Citation26], have received attention in the treatment of several chronic pain conditions. Studies using lidocaine and ketamine as a treatment approach have shown a benefit with regard to both immediate pain reduction and longer lasting pain relief. With respect to pain, lidocaine was first used to treat neuropathic burning pain [Citation27]. Its advantageous safety profile has made it more favorable than older anesthetic agents and has led to its rapid increase in popularity [Citation28]. Ketamine is primarily used as an anesthetic agent and has rapidly gained in popularity in both clinical use and research [Citation29]. Ketamine has gained further attention as a fast-acting antidepressant [Citation30,Citation31]. Although data on the use of lidocaine and ketamine in chronic pain management as separate agents are extensive [Citation32,Citation33], very few preclinical animal studies [Citation34,Citation35] have investigated the combined use of these analgesics, and only one observational study discussing results of the combined treatment effects has been published [Citation36].
In our pain unit, we have combined the beneficial properties of lidocaine and ketamine via a simultaneous intravenous application for nearly two decades and have achieved good clinical results. The aim of the present study was to retrospectively investigate the response to and safety of the simultaneous application of lidocaine and ketamine in the treatment of various refractory chronic pain conditions. Furthermore, to identify characteristics of treatment of responders to improve patient selection for this treatment and to guide future prospective research in this field.
Materials & methods
This retrospective data analysis was approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz project identifier: 2021–01285) and is registered at ClinicalTrials.gov (NCT05103319). In our pain unit at the University Hospital Basel, a standardized protocol for infusion regimens was established in 2012. Thus, in connection with this study, patient records were analyzed from 2012 through May 2021. Data were subsequently collected from May to August 2021.
Study design
As part of the standard therapy regimen of the University Hospital Basel Department of Anesthesiology Pain Unit, combined ambulatory infusions of lidocaine and ketamine are offered to patients for treatment of refractory chronic pain. Patients with general contraindications for the use of lidocaine or ketamine, particularly those with unstable psychiatric disorders, are generally not selected to receive combined infusions. As an initial treatment, eligible patients may receive up to three infusions at intervals of 4 weeks. This initial treatment is conducted to accustom the patient to the procedure, perform dose adjustments and determine treatment effects. If a patient reports a distinct improvement in pain or quality of life, with tolerable side effects, they may continue to receive an infusion every 4 weeks. If a patient experiences little to no benefit or suffers from side effects, the therapy is terminated after having received, at most, three infusions. To determine the treatment effect, the pain physician interviews patients prior to every infusion. During treatment, patients are re-evaluated by their specialist pain physician regarding their response in regular intervals of 3–6 months.
Dosing & procedure
The initial dose of lidocaine is 4 mg/kg body weight and usually remains unchanged during the series of infusions. The ketamine dose usually starts at 10 mg and can be adjusted up to 0.5 mg/kg depending on side effects and impact on pain. Patients are required to be in a fasting state for 6 h prior to infusion. Infusions are administered in an outpatient setting over 30 min followed by a 30–45 min postinterventional observation period. Blood pressure, pulse oximetry and heart rate are monitored continuously. Patients are under sustained observation by an anesthesiologist until discharge and are advised to avoid driving and operating heavy machinery for the first 24 h after infusion. Patients are asked to report their level of pain at regular intervals during the infusions according to a numeric rating scale (NRS). Treatment effects of the previous infusion are documented at the protocol of the upcomming infusion (Supplementary Figure 1).
Eligible records were identified by screening the electronic patient records of our pain unit for patients receiving combined lidocaine and ketamine infusions. Demographic data were extracted from patients’ digital records, and data regarding treatments and consultation hours were sampled from patients’ analog records. The following data were extracted and recorded: age, sex, weight, profession, religion, nationality, work status, medical diagnosis, psychiatric diagnosis, total number of infusions received, time frame of infusion therapy, dose of lidocaine, dose of ketamine, other medication received during infusions, pain at the start and end of infusions, blood pressure, pulse, oxygen saturation during infusions, side effects of infusions, duration of pain reduction, activity level, sleep quality, mood, reason for discontinuation of infusions, concomitant therapeutic pain interventions and change in pain medication during therapy.
If patients received more than 12 infusions, data of subsequent infusions were extracted only if one of the following criteria was met: change in dose of lidocaine or ketamine, new side effects or decrease in pain reduction over time. Patients fulfilling all of the inclusion criteria, including an International Classification of Diseases 11th Revision (ICD-11) medical diagnosis of chronic pain and its subcategories, treatment with intravenous lidocaine and ketamine infusions from 2012 to May 2021 at our pain unit at the University Hospital Basel and age at least 18 years at the beginning of treatment, were eligible for the study. Patients with one or more of the following exclusion criteria were denied participation in the study: age less than 18 years at the beginning of treatment, refusal of consent for research and treatment with only lidocaine or ketamine.
Patient consent
In 2016, the University Hospital Basel introduced the so-called research consent concept. By signing this document, patients agree that their anonymized data and sampled biological material may be used for future research. In addition, the local ethics commission approved the use of data of patients who had already completed treatment at our pain unit prior to the existence of the ‘research consent document’. Patients who were still under treatment but did not sign the research consent were asked specifically to consent to inclusion of their data in our study. Data of patients who denied consent were excluded from the study.
Recoding of original pain diagnosis into International Classification of Diseases 11th Revision classification
In January 2022, the new ICD-11 came into effect. Compared with its predecessor, the ICD-11 offers new opportunities to categorize chronic pain conditions. As a result, we have applied these new categories to reclassify our patients into diagnosis groups of similar pathologies to possibly identify patterns of treatment response. Recoding was performed via double check by two separate investigators. In case of disagreement, categorization was adjudicated by a third person.
Primary & secondary objectives
The primary goal of this study was to investigate the response of patients to simultaneous treatment with lidocaine and ketamine, quantified by the amount of pain reduction over time. Secondary objectives included treatment effects independent of the ICD-11 diagnosis group of chronic pain; impact of ketamine dose on outcome measures; number and type of treatment-accompanying pain therapies; impact of infusion therapy on consumption of additionally prescribed pain medication, such as NSAIDs, gabapentinoids and opioids; analysis of side effects associated with infusion therapy; and influence of socioeconomic factors on treatment efficacy.
Pain reduction score
Prior to every infusion, the attending anesthesiologist interviewed the patient about the last infusion. Pain reduction length and quality were assessed. Because of the retrospective nature of this study, the available data were not designed to serve our end points; hence, we had to develop a scoring system to allow for the analysis of descriptive data in a quantitative manner. This scoring system () had to account for length and intensity of pain reduction. Depending on the score, patients were allocated to one of the following groups: increased pain (-1 point), minor impact (1–2 points), moderate impact (3–4 points) and high impact (5–6 points). For example, a patient reporting a pain reduction of 50% (2 points) over less than 2 weeks (1 point) would receive a pain score of 3 points. Thus, the impact on pain would be classified as moderate (3–4 points).
Table 1. Pain reduction score.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 25 (IBM Corporation, NY, USA). Prism 8 (GraphPad Software Inc., CA, USA) was used for data visualization. Patient characteristics and continuous data are reported as numbers or percentages. Measures of central tendencies are reported as mean with standard deviation or median with interquartile range (IQR), if appropriate. Normally distributed data were assessed by reviewing the histograms and by Shapiro–Wilk test. Parametric data were compared using paired t test and nonparametric data were compared using Mann–Whitney U test and Wilcoxon test for dependent variables.
The Kruskal–Wallis test was used to test central tendencies of multiple independent variables. All tests were two-sided with a level of significance predetermined at 0.05. In case of multiple group comparisons, a Bonferroni correction was applied. To identify possible predictors of pain reduction, a linear regression model was performed with pain reduction score as the dependent variable and dose of lidocaine and ketamine, NRS at the start and end of infusions, ICD-11 diagnoses, status of disability pension, level of education and depression as a comorbidity as possible predictors.
As our data analysis was retrospective, we had to deal with missing values throughout all categories. The number of missing values barred the application of data imputation models. Therefore, subsets of patients with available data for one category were analyzed. This has to be considered when comparing patient numbers of different analyses and during interpretation and generalization of the results.
Results
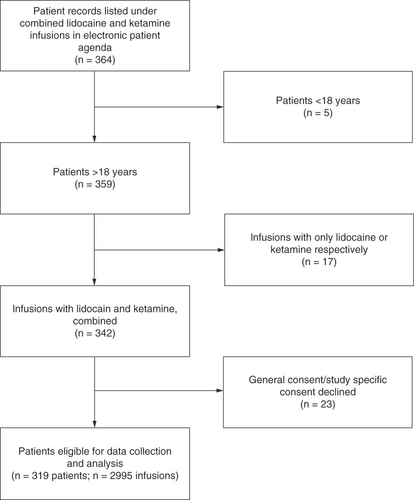
After screening of electronic patient data, 364 patients receiving combined lidocaine and ketamine infusions were initially retrieved (). Excluded patients included five who were underage at the beginning of infusion therapy, 17 who were initially labeled as receiving combined treatment but received only infusions with either lidocaine or ketamine and 23 who declined the research- or study-specific consent.
Baseline characteristics of the included patients (n = 319) are summarized in . Mean patient age was 51.4 years; 52% of patients (n = 166) were male and 48% (n = 153) were female. The majority of patients were of Swiss nationality (61%; n = 195). Only 4.4% (n = 14) of patients reported reported to be in an active employment status at the beginning of infusions. The most common medical ICD-11 diagnosis was chronic neuropathic pain (56.7%; n = 181) followed by chronic secondary muscular pain (48%; n = 153) and chronic postsurgical or post-traumatic pain (34.5%; n = 110). With regard to these diagnoses, multiple entries per patient were possible ().
Table 2. Patient demographics and baseline characteristics.
Table 3. Medical diagnoses.
Depression was the most common psychiatric comorbidity (25.4%; n = 81). Other psychiatric diagnoses occurred only occasionally ().
Table 4. Psychiatric diagnoses.
Infusions
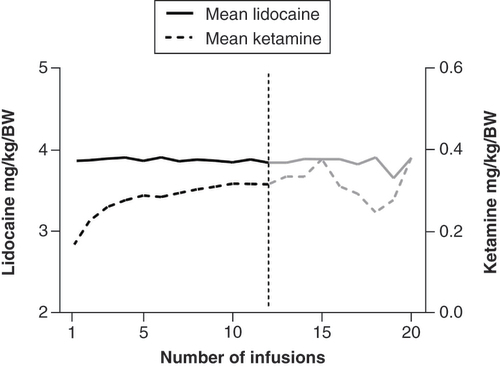
A total of 2995 infusions were examined. The median number of infusions received per patient was three and ranged from one to 116 (corresponding to a maximum of 9.6 years of treatment with a 4-week interval between infusions). Patients received a mean dose of 3.87 ± 0.38 mg/kg lidocaine and 0.22 ± 0.08 mg/kg ketamine, corresponding to an absolute dose of 5–70 mg per infusion. Mean ketamine dose increased from 0.1579 ± 0.06763 mg/kg (n = 317) at the first infusion to 0.31 ± 0.10 mg/kg (n = 66) at the 12th infusion. There was a significant increase in ketamine dose from the first to third infusion (mean dose: 0.16 ± 0.06 to 0.26 ± 0.09 mg/kg; p < 0.0001). In patients receiving more than three infusions, no significant increase or decrease in dose could be detected (mean dose from infusion five to 12: 0.25 ± 0.09 to 0.31 ± 0.11 mg/kg; p > 0.99). Mean lidocaine dose was 3.86 ± 0.34 mg/kg (n = 317) at the first infusion and 3.84 ± 0.28 mg/kg (n = 66) at the 12th infusion. Lidocaine dose showed no significant change overall (p > 0.99) ().
Side effects
In total, 264 patients reported side effects during the infusions. The mean number of side effects per patient was limited to 2 ± 1.2. The most common were dizziness (n = 130) and waking reactions (n = 120), such as hallucinations, mild agitation, confusion and vivid dreams. These side effects were limited to the acute phase of infusion and decayed until discharge.
A total of 54 patients (16.9%) received midazolam at least once during their infusions. Ten patients received regular addition of midazolam to treat respective prevent transient dissociative symptoms or anxiety. A total of 12 (out of 2995) infusions had to be terminated before completion because of intolerable side effects, none of which were classified as serious, and all were temporary ().
Table 5. Side effects.
Short-term effects
Median NRS at the start of the infusion was 6.5 (IQR: 3.0; n = 310 patients), which decreased to a median of 1.0 (IQR: 3.94; n = 288 patients) directly after termination of the infusion. There was no significant difference in the starting NRS from the first infusion (median: 7.0; IQR: 3.00) to the 12th infusion (median: 7.0; IQR: 3.00; p = 0.610). When comparing the NRS at the end of the infusion, the first infusion (median: 2.0; IQR: 4.00) had a lower acute pain reduction compared with the following 11 infusions (median: 1.0; IQR: 3.00; p < 0.0001).
Female patients started with a median NRS of 7.0 (IQR: 3.00; n = 150), whereas male patients started with a significantly lower median NRS of 6.0 (IQR: 2.50; n = 160; p = 0.032). Median NRS at the end of infusion did not differ between women (median: 1.0; IQR: 3.50; n = 140) and men (median: 1.5; IQR: 4.00; n = 148; p = 0.581). No difference in NRS at the beginning (p = 0.247) or end (p = 0.188) of infusion could be detected with regard to ICD-11 diagnosis group.
Long-term effects
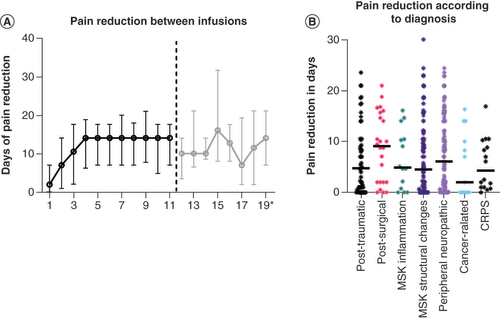
The median pain reduction in days (PRD) overall was 10.00 (IQR: 13.25; n = 783 included infusions). When comparing the impact on pain reduction in between infusions, the results showed that only the median PRD during ketamine dose titration (between the first and second infusion (median: 2.00; IQR: 7.00; n = 182) was significantly shorter compared to the reduction between the following 11 infusions (p < 0.009). With regard to medical diagnoses, most data were acquired from patient groups with chronic peripheral neuropathic pain (PRD: 10.00; IQR: 15.00; n = 408 infusions), chronic secondary musculoskeletal pain associated with structural changes (PRD: 7.00; IQR: 14.50; n = 353 infusions) and chronic post-traumatic pain (PRD: 14.00; IQR: 11.25; n = 97 infusions) ().
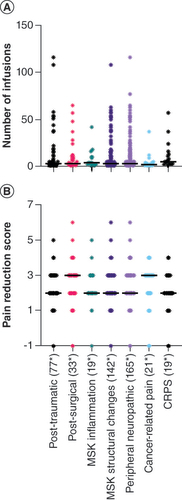
(A) Total number of infusions. (B) Median pain reduction score refered to International Classification of Diseases 11th Revision diagnosis.
CRPS: Complex regional pain syndrome; MSK: Musculoskeletal.
For calculation of the pain reduction score, 1371 infusion protocols provided sufficient data. Patients reported any pain reduction (degree not specified) between two infusions 1241 times, a pain reduction of ≥50% 53 times, a pain-free period 52 times and a period where pain increased after infusion 34 times. Of the 1241 patient reports of any pain reduction, 154 (12.4%) did not state a period, 550 (44.3%) reported a pain reduction of <2 weeks, 496 (40%) reported a pain reduction of ≥2 weeks and 41 (3.3%) reported a pain reduction for the entire time in between infusions.
The median pain reduction score overall was 2.0 (IQR: 1.0; n = 1371 infusions). Throughout the dose-finding process (infusions 1 to 3), the median was 1.0 (IQR: 2.0; n = 614). Considering only the infusions after dose finding (infusions 4 to 12), the median was 3.0 (IQR: 1.0; n = 757 infusions). Pairwise comparison showed that the median pain reduction score between the first four infusions was significantly lower compared to infusion 4 to 12 (p < 0.05 during all single comparisons). We could not detect a difference in pain reduction score in the different pain categories using the Kruskal–Wallis test (p = 0.078). An overview of PRD and ICD-11 diagnoses is presented in .
(A) Over intervals between infusions (e.g., 1 stands for days of pain reduction between first and second infusions). (B) Over International Classification of Diseases 11th Revision diagnosis.
CRPS: Complex regional pain syndrome; MSK: Musculoskeletal.
With respect to sex, female patients had a median pain reduction score of 3.00 (IQR: 1.00; n = 695 infusions) and male patients had a median pain reduction score of 2.00 (IQR: 1.0; n = 676 infusions; p < 0.001). In our study, patients with depression had a higher median pain reduction score (median: 3.00; IQR: 1.00) than patients without depression (median: 2.00; IQR: 1.00), but the difference was not significant (p = 0.072). With regard to the impact of educational level and nationality on pain reduction score, no difference could be detected (p = 0.421 and 0.065, respectively).
Patients with a granted disability pension had a significantly higher median pain reduction score (median: 3.0; IQR: 1.0) than patients who were still in the process of obtaining a disability pension and those rejected for a disability pension (median: 2.0; IQR: 0.0; p = 0.009). To identify possible predictors of our pain reduction score, we performed a linear regression analysis with dose of lidocaine and ketamine, NRS at start and end of infusions, dummy variables of ICD-11 diagnoses, disability pension, level of education and depression as a comorbidity as possible prediction factors of the pain reduction score in addition to the comparative analyses. Only NRS at the end of infusions had a significant impact on the pain reduction score (p < 0.0001), but the regression loop was very low (2%; r2 = 0.019) and thus not clinically relevant.
Treatment adherence
Of the 319 patients initially included, 269 (84.3%) terminated their treatment during the observation period. The most common reason for treatment termination was insufficient impact of infusions on pain (n = 164; 61%). The great majority of these patients quit during the first three infusions (dose-finding period; n = 138; 84.1%). No correlation could be found between early termination and diagnosis group, comorbidities, sex or socioeconomic status. Other reasons for discontinuation were side effects (n = 39; 14.5%), reduced effect on pain reduction over time (n = 17; 6.3%), reduced pain due to a different intervention (n = 8; 3%) and other specified reasons (e.g., moving, absence without excuse; n = 35; 13%). Multiple entries per patient were possible. A total of 27 patients (10%) terminated their treatment for unknown reasons.
In addition to infusions, 108 patients (33.9%) received concomitant therapeutic pain interventions, including psychosomatic pain therapy (n = 63; 19.7%), operation at site of pain (n = 22; 6.9%), intra-articular injection of cortisone or local anesthetic (n = 16; 5%), trigger point infiltration (n = 4; 1.3%) and otherwise classified interventions (n = 46; 14.4%). Patients with concomitant pain therapies did not differ in pain reduction score (p = 0.232) compared with patients without therapies.
Accompanying pain medication
At the beginning of infusion therapy, 145 patients (45.5%) reported a daily intake of pain medication. A total of 120 patients (37.6%) used gabapentinoids; 95 (29.8%) used NSAIDs, paracetamol or metamizole; and 43 (13.5%) used opioids. The most common medication combination was gabapentinoids and NSAIDs (n = 35; 11%) followed by gabapentinoids and opioids (n = 17; 5.3%), opioids and NSAIDs (n = 16; 5%) and a combination of all three (n = 6; 1.9%).
A dose reduction in daily pain medication throughout the infusion therapy was reported in 60 of 145 patients (41.4%). We registered 34 reductions in opioids, 17 reductions in gabapentinoids and 15 reductions in NSAIDs, paracetamol and/or metamizole. Multiple entries per patient were possible. We documented 32 patients with an increase in daily intake of pain medication. A total of 24 patients started a new pain medication during infusion therapy. An overview of medication changes is provided in .
Table 6. Pain medication.
A total of 43 patients were taking opioids prior to their first infusion. Of these patients, four remained at a constant dose throughout the infusions, 34 were able to reduce their daily intake and five had to increase their dose throughout the infusions. Patients started with a median dose of 77.25 mg (IQR: 97.5) of morphine equivalents per day and were able to reduce to a median dose of 43.5 mg (IQR: 104.25) of morphine equivalents per day. The median dose reduction was 30 mg (IQR: 45.50) of morphine equivalents, which corresponded to a 50% dose reduction based on Wilcoxon test of difference between starting and ending dose (p = 0.014). Eight patients were able to discontinue their opioids completely.
We registered two gabapentinoids: gabapentin and pregabalin. Median doses of gabapentin and pregabalin at the beginning of infusions were 1200 mg (IQR: 1275; n = 50) and 200 mg (IQR: 225; n = 69), respectively. On average, patients remained at a stable dose level throughout the infusions (gabapentin: p = 0.842; pregabalin: p = 0.415). A total of 95 patients reported a daily intake of NSAIDs, paracetamol and/or metamizole. Given the vast variety of NSAIDs, a deeper analysis of specific drugs was not possible, and descriptive data are presented. The majority of patients taking NSAIDs, paracetamol and/or metamizole remained at a constant dose level during infusion therapy.
Discussion
This retrospective single-center study examined the response to combined lidocaine and ketamine infusions with regard to pain intensity in patients with treatment-refractory chronic pain. We aimed to address the safety profile of the combined treatment procedure, the short- and long-term response as well as possible development of treatment tolerance, the potential for reduction in opioids and other pain medications and the classification of responder/nonresponder patterns. The finding that a majority of patients were not engaged in active employment at the beginning of infusion therapy as well as the high pain score is a strong indication that patients were suffering as a result of chronic pain. This is consistent with the literature, which shows the heavy impact of chronic pain on daily routines.
Rationale for combining lidocaine & ketamine
Since its development in the USA in 1962, ketamine has been used primarily as an anesthetic [Citation29]. Nevertheless, it shows promising benefits in pain therapy [Citation32] and the treatment of depressive disorders. Although several routes of administration are possible, intravenous administration is the most common [Citation37]. Ketamine is known to affect many sites of action, the most important being the N-methyl-D-aspartate (NMDA) receptor [Citation38,Citation39]. With respect to pain, the NMDA receptor is involved in pain regulation and the development of chronic pain through increased central sensitization and windup by increasing neuronal excitability during nociceptive conditioning [Citation39]. Other sites of action of ketamine are nicotinic and muscarinic acetylcholine receptors, opioid receptors, monoamine receptors and voltage-sensitive sodium channels [Citation39,Citation40]. Sleigh et al. have suggested that ketamine may suppress pain signal transmission by inhibiting astrocyte and microglial activation [Citation41]. Ketamine has shown beneficial effects in studies investigating its use in chronic regional pain syndrome [Citation26], fibromyalgia [Citation42], chronic phantom limb pain [Citation25], postherpetic neuralgia [Citation43] and neuropathic pain [Citation44].
When it comes to high-dose opioid administration, opioid-induced hyperalgesia is a well-known problem. One theory suggests that overactivation of NMDA receptors leads to the development of this phenomenon, which might explain the positive effect of ketamine on this condition, particularly in chronic pain patients on opioids. The mechanism behind ketamine’s effect on depression is not yet completely understood. It has been stated that ketamine can help to restore synapses in the prefrontal cortex and hippocampus and reverse some of the negative impacts of depression on brain structures [Citation37]. This is of interest because depression is one of the most common comorbidities in chronic pain patients. In addition to its effect on pain and depression, recent studies have shown that ketamine affects the synthesis of proinflammatory mediators. A preclinical study on gonarthrosis in rabbits showed a diminished inflammatory response after administration of ketamine [Citation45]. When given intraoperatively, ketamine can reduce the postoperative inflammatory response by inhibiting IL-6 [Citation46].
Lidocaine was first synthesized in 1963. Its most well-known mechanism of action is the nonselective, reversible binding at voltage-gated sodium channels on the internal surface of nerve cell membranes [Citation27,Citation47]. This prevents the closing of these channels and leads to a decrease in nerve depolarization. Lidocaine not only binds to sodium channels but also affects a variety of other pathways. Among these are voltage-gated potassium channels, which are known to impact tonic firing neurons, and an intracellular increase in calcium, leading to a modification of T-type calcium channels involved in pain signaling. By inhibiting voltage-gated, hyperpolarization-activated cyclic nucleotide channels in the CNS, lidocaine reduces the excitatory inward current of cations [Citation48,Citation49]. To attenuate the pain response even more, lidocaine can enhance the inhibitory effect of glycine channels [Citation49]. Lidocaine also interacts with ligand-gated ionotropic glutamate receptors, leading to a reduction of excitatory glutamate in the presynaptic cell.
Similar to ketamine, lidocaine inhibits rapid excitatory neurotransmission by blocking NMDA receptors through various mechanisms [Citation50,Citation51]. Two experimental studies have implied that lidocaine acts on a different site of action on NMDA receptors than ketamine [Citation51,Citation52]. With regard to the inflammatory response, lidocaine decreases the synthesis of proinflammatory mediators such as IL-1β, IL-6 and TNF-α [Citation49,Citation53]. Chronic pain conditions that have been shown to be responsive to lidocaine are fibromyalgia, chronic regional pain syndrome, diabetic neuropathy, opioid-refractory cancer pain, spinal cord injury-associated neuropathic pain and postherpetic neuralgia.
Lidocaine and ketamine have proven their beneficial impact as intravenous infusions on multiple chronic pain conditions in various studies. Ketamine, in particular, has become a target of increasing interest, and numerous studies have been published over the last few years. Both drugs have a broad but specific influence on pain processing, which seems to render combined administration useful. Unfortunately, the combination of both medications remains poorly investigated and to date has been explored only in preclinical studies [Citation34,Citation35]. Only one prospective observational study on the combined effect of lidocaine and ketamine supports the safe and effective use in therapy-refractory neuropathic pain [Citation36].
When compared directly, one study found the effect of ketamine on pain to be superior to that of lidocaine in patients with spinal cord injury [Citation54]. Another study comparing these two agents found that ketamine as well as lidocaine reduced postoperative opioid consumption and that lidocaine, but not ketamine, reduced the development of postoperative neuropathic pain at 3 months [Citation55]. In our study, the significant positive effect of increasing ketamine doses during the first three infusions on effect strength and duration reflects the benefit of combination therapy, as lidocaine resulted in reduced pain, but maximal effects were reached after dose finding of ketamine.
Treatment adherence & safety
The majority of patients terminating therapy because of insufficient pain reduction did so during the test phase of the first three infusions (84.1%). Beyond this phase, pain reduction and treatment adherence were quite stable. All side effects were temporary, and the median number of side effects was two. Nevertheless, 12.23% of patients discontinued therapy for this reason. In conclusion, limiting side effects seemed infrequent, and no serious adverse events were registered throughout 2995 infusions, rendering it a safe procedure.
Difference in sex
Female patients showed a significantly higher starting NRS as well as a significantly higher pain reduction score compared with male patients. According to the literature, females are at greater risk of chronic pain, and some studies suggest that females express more severe clinical pain than males [Citation56,Citation57]. However, there exists some inconsistency in the literature, especially concerning sex differences in pain therapy, as there seems to be a difference between the sexes with regard to pain therapy response, but this is highly dependent on the specific treatment [Citation58,Citation59]. Similar to preceding studies, there was a sex difference with respect to the therapeutic effect in our study, although no distinct cause could be identified.
Change in pain medication
Regarding patients with a daily intake of pain medication, 41.4% were able to reduce their dose. This could be attributed to the pain-reducing qualities of infusion therapy leading to less need for pain medication. Ketamine has already been proven to be a useful tool to save opioids after surgery and has been used as an effective agent in opioid withdrawal [Citation60]. In our study, patients who reported a reduction in daily opioid consumption under infusion therapy had a mean dose reduction of 50%. This is clinically relevant, especially with regard to opioid-induced hyperalgesia. However, this effect might be biased since one of the rationales for applying combined infusions in our pain unit is to reduce daily opioid intake. Still, our data support this approach.
Strengths & limitations
Because of the retrospective nature of our study, most of our outcomes are exploratory, and missing data in a relevant number of cases only allowed analyses of patient subgroups without corrections for the missing data. This limits generalization to a certain degree. Recoding to new ICD-11 diagnosis groups and socioeconomic data did not reveal patterns of responders. Thus, we are unable to explain the high interindividual variability in treatment response.
With our pain score, we are likely to underestimate the true effect on pain reduction since the scoring group with no quantifiable time received the least amount of points regarding length of pain reduction. It is possible that patients within this group had a much longer pain reduction effect than stated and thus received an inappropriately low score. By contrast, as physicians tend to note a positive treatment effect, there is a potential to overestimate the observed effect on pain reduction. Because of the large number of investigated patients and infusions, we have good evidence supporting the safety of infusion therapy. Patients responding to infusion therapy did not develop tolerance. This is reflected in the stable dose of lidocaine and ketamine.
Conclusion
Based on our data, combined ambulatory infusion of lidocaine and ketamine can be regarded as a safe procedure. Chronic pain patients refractory to other pain therapies showed good short-term benefits with regard to pain reduction, and moderate long-term pain reduction was observed in a subset of patients, with high interindividual variability. Future prospective studies investigating predictors of treatment response and direct treatment effects and efficacy (e.g., biomarkers, imaging techniques) are needed, especially to minimize the high rate of treatment failure and to translate combined infusion therapies from the trial-and-error principle to a specific treatment option for chronic pain patients.
This retrospective study investigated the effects of ambulatory infusions of lidocaine and ketamine on chronic pain patients.
The primary objective was the degree and duration of pain reduction.
Secondary objectives included safety profile, classification of responder/nonresponder patterns and impact of infusions on use of pain medication.
A total of 319 patients with an average of three infusions were included.
A total of 2995 infusions were examined.
Median pain reduction in days was 10.00 (interquartile range: 13.25), with significantly higher pain reduction scores in females (p < 0.001).
Side effects were limited to the acute phase of infusions, and 41.4% of patients with daily use of pain medication reported a dose reduction; however, no patterns in treatment response could be identified.
Our data support combined infusions as a safe therapy option, with good short- and medium long-term effects on pain in a subset of patients and high interindividual variability.
Author contributions
All authors contributed to the conception of the study. The data collection was executed by J Striebel. Both T Schneider and J Striebel contributed to the writing process. W Ruppen revised the manuscript critically.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data. Deidentified, individual data that underlie the results reported in this article (text, tables, figures and appendices) along with the study protocol will be available indefinitely to any researcher who wants access to them.
Acknowledgments
The authors thank A Dwileski (scientific assistant), Clinic for Anesthesia, Intermediate Care, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel, Basel, Switzerland, for providing editorial assistance.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/pmt-2023-0037
Financial disclosure
Support for this study was provided solely by the Pain Unit, Clinic for Anesthesia, Intermediate Care, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel, Basel, Switzerland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with anyorganization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Breivik H , EisenbergE , O’BrienT. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health13, 1229 (2013).
- Kress L , UceylerN. Pain prevention: what is in the pipeline?Schmerz35(1), 53–58 (2021).
- Vardeh D , MannionRJ , WoolfCJ. Toward a mechanism-based approach to pain diagnosis. J. Pain17(Suppl. 9), T50–T69 (2016).
- Mao J . Challenges of managing chronic pain. BMJ356, j741 (2017).
- Lovejoy TI , MorascoBJ , DemidenkoMI , MeathTHA , FrankJW , DobschaSK. Reasons for discontinuation of long-term opioid therapy in patients with and without substance use disorders. Pain158(3), 526–534 (2017).
- Ballantyne JC , MaoJ. Opioid therapy for chronic pain. N. Engl. J. Med.349(20), 1943–1953 (2003).
- Mills SEE , NicolsonKP , SmithBH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth.123(2), e273–e283 (2019).
- Patel AS , FarquharsonR , CarrollDet al. The impact and burden of chronic pain in the workplace: a qualitative systematic review. Pain Pract.12(7), 578–589 (2012).
- Duenas M , OjedaB , SalazarA , MicoJA , FaildeI. A review of chronic pain impact on patients, their social environment and the health care system. J. Pain Res.9, 457–467 (2016).
- Paz MGD , SouzaLAF , TatagibaBet al. Factors associated with quality of life of older adults with chronic pain. Rev. Bras. Enferm.74(Suppl. 2), e20200554 (2021).
- Steenstra IA , MunhallC , IrvinEet al. Systematic review of prognostic factors for return to work in workers with sub acute and chronic low back pain. J. Occup. Rehabil.27(3), 369–381 (2017).
- Melcarne L , Garcia-IglesiasP , CalvetX. Management of NSAID-associated peptic ulcer disease. Expert Rev. Gastroenterol. Hepatol.10(6), 723–733 (2016).
- Freo U , RuoccoC , ValerioA , ScagnolI , NisoliE. Paracetamol: a review of guideline recommendations. J. Clin. Med.10(15), 3420 (2021).
- Andrade S , BartelsDB , LangeR , SandfordL , GurwitzJ. Safety of metamizole: a systematic review of the literature. J. Clin. Pharm. Ther.41(5), 459–477 (2016).
- Braund TA , TillmanG , PalmerDM , GordonE , RushAJ , HarrisAWF. Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: an iSPOT-D report. Transl. Psychiatry11(1), 417 (2021).
- Quintero GC . Review about gabapentin misuse, interactions, contraindications and side effects. J. Exp. Pharmacol.9, 13–21 (2017).
- Lee M , SilvermanSM , HansenH , PatelVB , ManchikantiL. A comprehensive review of opioid-induced hyperalgesia. Pain Physician14(2), 145–161 (2011).
- Hauser W , MorlionB , VowlesKEet al. European* clinical practice recommendations on opioids for chronic noncancer pain – part 1: role of opioids in the management of chronic noncancer pain. Eur. J. Pain25(5), 949–968 (2021).
- Compton WM , VolkowND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend.83(Suppl. 1), S4–S7 (2006).
- Compton WM , VolkowND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend.81(2), 103–107 (2006).
- Williams DR , StarkRJ. Intravenous lignocaine (lidocaine) infusion for the treatment of chronic daily headache with substantial medication overuse. Cephalalgia23(10), 963–971 (2003).
- Viola V , NewnhamHH , SimpsonRW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J. Diabetes Complications20(1), 34–39 (2006).
- Kastrup J , PetersenP , DejgardA , AngeloHR , HilstedJ. Intravenous lidocaine infusion – a new treatment of chronic painful diabetic neuropathy?Pain28(1), 69–75 (1987).
- Grigoras A , LeeP , SattarF , ShortenG. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin. J. Pain28(7), 567–572 (2012).
- Eichenberger U , NeffF , SveticicGet al. Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesth. Analg.106(4), 1265–1273, (2008).
- Schwartzman RJ , AlexanderGM , GrothusenJR , PaylorT , ReichenbergerE , PerreaultM. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain147(1–3), 107–115 (2009).
- Kandil E , MelikmanE , AdinoffB. Lidocaine infusion: a promising therapeutic approach for chronic pain. J. Anesth. Clin. Res.8(1), 697 (2017).
- Calatayud J , GonzalezA. History of the development and evolution of local anesthesia since the coca leaf. Anesthesiology98(6), 1503–1508 (2003).
- Le Dare B , PelletierR , MorelI , GicquelT. History of ketamine: an ancient molecule that is still popular today. Ann. Pharm. Fr.80(1), 1–8 (2022).
- Zarate CA Jr , NiciuMJ. Ketamine for depression: evidence, challenges and promise. World Psychiatry14(3), 348–350 (2015).
- Lener MS , KadriuB , ZarateCAJr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs77(4), 381–401 (2017).
- Niesters M , MartiniC , DahanA. Ketamine for chronic pain: risks and benefits. Br. J. Clin. Pharmacol.77(2), 357–367 (2014).
- Mayhew A , ArgaezC. Intravenous Lidocaine for Chronic Pain: A Review of the Clinical Effectiveness and Guidelines. Canadian Agency for Drugs and Technologies in Health (2018).
- Daradka M , IsmailZB. Evaluation of the clinical and analgesic effects of subarachnoid ketamine–lidocaine administration in goats undergoing mastectomy. Vet. Med. (Auckl.)5, 35–39 (2014).
- Kaka U , SaifullahB , AbubakarAAet al. Serum concentration of ketamine and antinociceptive effects of ketamine and ketamine–lidocaine infusions in conscious dogs. BMC Vet. Res.12(1), 198 (2016).
- Safakish R , BabazadehS, Emadi T, SohanpaI. Ambulatory infusions of lidocaine and ketamine for management of chronic pain: an observational prospective cohort study. J. Anesth. Pain Res.5, 138 (2022).
- Abdallah CG , AdamsTG , KelmendiB , EsterlisI , SanacoraG , KrystalJH. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress. Anxiety33(8), 689–697 (2016).
- Mion G , VillevieilleT. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther.19(6), 370–380 (2013).
- Cohen SP , BhatiaA , BuvanendranAet al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med.43(5), 521–546 (2018).
- Peltoniemi MA , HagelbergNM , OlkkolaKT , SaariTI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin. Pharmacokinet.55(9), 1059–1077 (2016).
- Sleigh J HM , VossL , DennyB. Ketamine - more mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care4(2-3), (2014).
- Graven-Nielsen T , KendallSA , HenrikssonKGet al. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain85(3), 483–491 (2000).
- Kim YH , LeePB , OhTK. Is magnesium sulfate effective for pain in chronic postherpetic neuralgia patients comparing with ketamine infusion therapy?J. Clin. Anesth.27(4), 296–300 (2015).
- Webster LR , WalkerMJ. Safety and efficacy of prolonged outpatient ketamine infusions for neuropathic pain. Am. J. Ther.13(4), 300–305 (2006).
- Lu W , WangL , WoC , YaoJ. Ketamine attenuates osteoarthritis of the knee via modulation of inflammatory responses in a rabbit model. Mol. Med. Rep.13(6), 5013–5020 (2016).
- Dale O , SomogyiAA , LiY , SullivanT , ShavitY. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth. Analg.115(4), 934–943 (2012).
- Lauretti GR . Mechanisms of analgesia of intravenous lidocaine. Rev. Bras. Anestesiol.58(3), 280–286 (2008).
- Estebe JP . Intravenous lidocaine. Best Pract. Res. Clin. Anaesthesiol.31(4), 513–521 (2017).
- Yang X , WeiX , MuY , LiQ , LiuJ. A review of the mechanism of the central analgesic effect of lidocaine. Medicine (Baltimore)99(17), e19898 (2020).
- Muth-Selbach U , HermannsH , StegmannJUet al. Antinociceptive effects of systemic lidocaine: involvement of the spinal glycinergic system. Eur. J. Pharmacol.613(1–3), 68–73 (2009).
- Sugimoto M , UchidaI , MashimoT. Local anaesthetics have different mechanisms and sites of action at the recombinant N-methyl-D-aspartate (NMDA) receptors. Br. J. Pharmacol.138(5), 876–882 (2003).
- Kurabe M , FurueH , KohnoT. Intravenous administration of lidocaine directly acts on spinal dorsal horn and produces analgesic effect: an in vivo patch-clamp analysis. Sci. Rep.6, 26253 (2016).
- Dunn LK , DurieuxME. Perioperative use of intravenous lidocaine. Anesthesiology126(4), 729–737 (2017).
- Kvarnstrom A , KarlstenR , QuidingH , GordhT. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol. Scand.48(4), 498–506 (2004).
- Jendoubi A , NaceurIB , BouzouitaAet al. A comparison between intravenous lidocaine and ketamine on acute and chronic pain after open nephrectomy: A prospective, double-blind, randomized, placebo-controlled study. Saudi J. Anaesth.11, 177–84 (2017).
- Fillingim RB , DoleysDM , EdwardsRR , LoweryD. Clinical characteristics of chronic back pain as a function of gender and oral opioid use. Spine (Phila. Pa 1976)28(2), 143–150 (2003).
- Tang YR , YangWW , WangYL , LinL. Sex differences in the symptoms and psychological factors that influence quality of life in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol.24(6), 702–707 (2012).
- Niesters M , DahanA , KestBet al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain151(1), 61–68 (2010).
- Bartley EJ , FillingimRB. Sex differences in pain: a brief review of clinical and experimental findings. Br. J. Anaesth.111(1), 52–58 (2013).
- Quinlan J . The use of a subanesthetic infusion of intravenous ketamine to allow withdrawal of medically prescribed opioids in people with chronic pain, opioid tolerance and hyperalgesia: outcome at 6 months. Pain Med.13(11), 1524–1525 (2012).