Abstract
Aim: Cryoneurolysis is a potential therapy for peripheral mononeuropathies, but randomized studies of its effects on the duration of pain reduction are lacking. Methods: This retrospective cohort study evaluated the analgesic effects of cryoneurolysis on patients with refractory peripheral mononeuropathy. We included 24 patients who underwent ultrasound-guided cryoneurolysis between June 2018 and July 2022. The daily maximum pain level was recorded using a numerical rating scale before and 1, 3 and 6 months after the procedure. Results: At 1 month, 54.2% of patients reported pain reduction of at least 30%. This percentage was significantly lower at 3 and 6 months (13.8 and 9.1%, respectively). Conclusion: Our results suggest that repeated cryoneurolysis may be a viable treatment for refractory mononeuropathy. Further investigations are needed.
Neuropathic pain has been defined as pain that occurs as a direct result of damage to or lesions of the somatosensory system by the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain [Citation1,Citation2]. Neuropathic pain occurs in 6.9–10% of the population [Citation3]. The symptoms are often refractory and require an interdisciplinary approach that combines pharmacological therapy, occupational therapy measures, psychological coping strategies and interventional procedures [Citation4–6]. Pharmacotherapy includes anticonvulsants (gabapentin/pregabalin), tricyclic/tetracyclic antidepressants and selective serotonin/norepinephrine reuptake inhibitors (duloxetine and venlafaxine). Additionally, local anesthetics (lidocaine) and capsaicin are topically applied. Opioids should be considered based on the side effects of long-term prescription use and the potential for dependency [Citation5,Citation6]. Medical reviews have argued that despite guideline-based treatment, treatment success is often difficult to achieve, with sufficient pain relief experienced by less than 50% of patients [Citation7,Citation8]. If noninvasive options fail, then neuromodulatory [Citation9,Citation10] or neurodestructive invasive therapies [Citation11] can be considered. Cryoneurolysis offers an interesting therapeutic option [Citation12,Citation13].
Cold as a therapeutic tool is a simple and easily accessible element and one of the earliest remedies used by humans. Hippocrates (460–377 BC) used ice and snowpacks for analgesia [Citation14]. During the Napoleonic War, necessary amputations of hypothermic extremities were apparently better tolerated by injured soldiers during freezing temperatures in Russia [Citation15]. In 1962, Irvine developed the first cryoprobe, which used the phase change of liquid nitrogen to decrease temperatures to -196°C. In 1967, Amoils developed a simple, portable cryogenic device that utilized carbon dioxide (CO2) or nitrous oxide (N2O); this was the beginning of modern devices [Citation16]. Furthermore, in 1976, Lloyd et al. reported the clinical application of cryoneurolysis as an analgesic procedure for patients with chronic intercostal neuralgia [Citation17].
The principle of cryoneurolysis is the formation of an ‘ice ball’ at the tip of the probe. Rapid expansion of a gas (N2O or CO2 – Joule–Thomson effect) creates temperatures of -60°C or colder. The application of cold on the nerve leads to a temporary interruption of nerve conduction [Citation18]. The myelin sheaths and axons degenerate after cryoneurolysis (Wallerian degeneration) [Citation19,Citation20]. However, the nerve structure (perineurium, epineurium and basal lamina) remain intact, allowing nerve regeneration and physiological restoration of the neural structure. Neuromas are unlikely to form because of minimal inflammatory reactions, and there is no external nerve damage. The optimal treatment temperature is between -60 and -100°C. Temperatures warmer than -60°C will result in insufficient pain reduction or lesions of the nerve, whereas temperatures colder than -100°C, and especially those colder than -140°C, may be associated with permanent nerve damage [Citation21]. The axonal regeneration rate after cryolesions is between 1 and 3 mm per day; therefore it is believed that there is a direct correlation between the duration of action and the distance from the cryolesion to the pain area [Citation22].
Despite many years of clinical use, randomized studies demonstrating the long-term effects of cryoneurolysis for peripheral mononeuropathies are still lacking. Only a few case reports have shown heterogeneous responses to cryoneurolysis for the treatment of acute and chronic pain [Citation23]. According to Ilfeld et al., cryoneurolysis for the treatment of chronic lower extremity phantom pain did not demonstrate a reduction in pain levels 4 months after the treatment [Citation24], indicating a short duration of the therapy.
It is important to note that the term ‘cryotherapy’ is used broadly and encompasses various treatment options. Cryoneurolysis is increasingly being utilized in the management of acute pain across different surgical procedures. The utilization of cryoneurolysis has experienced growth due to the implementation of ultrasound guidance, facilitating accurate nerve targeting. While early reports primarily focused on cryoablation of intercostal nerves during thoracotomy surgery, recent investigations have explored its application in postoperative pain management for mastectomy [Citation25] and total knee arthroplasty [Citation26].
Our study’s novelty lies in evaluating the safety and effectiveness of cryoneurolysis in an office-based setting for treating chronic mononeuropathies. Due to limited research on cryoneurolysis for chronic peripheral neuropathies, our investigation serves as a starting point for future prospective studies using randomized methodologies. Further exploration of cryoneurolysis as a chronic pain management approach is essential, especially for challenging pain conditions. This study aimed to assess the duration and effects of pain reduction with cryoneurolysis, as well as the potential benefits of early intervention for patients with chronic refractory peripheral mononeuropathies.
Patients & methods
Study design
This was a retrospective cohort study of all patients with chronic peripheral mononeuropathy treated with cryoneurolysis between June 2018 and July 2022; data were prospectively collected for quality assurance. Approval was obtained from the Ethics Committee of the Cantonal Ethics Committee for Research, Bern, Switzerland (BASEC no. Req-2023-00082). Because of the retrospective nature of the study, the requirement for written informed consent was waived. Applicable data protection regulations were followed to protect the patients’ informational rights, and the study was carried out according to institutional and good clinical practice guidelines.
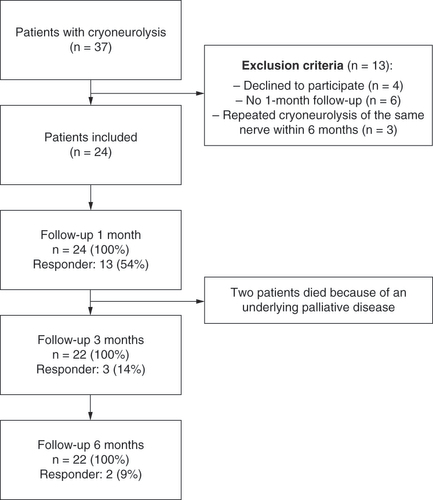
Inclusion criteria were as follows: presence of neuropathic pain attributable to a specific peripheral nerve; nonresponse to noninvasive therapy; and at least 50% pain relief after two ultrasound-guided prognostic differential blocks (first infiltration with lidocaine 2%; second infiltration with ropivacaine 0.5%). Patients with repeated cryoneurolysis of the same nerve within 6 months, those without pain ratings at the 1-month follow-up evaluation and those who refused further use of their data were excluded. shows the study flowchart according to the Strengthening the Reporting of Observational Studies in Epidemiology statement [Citation27]. Pain intensity was recorded using a numerical rating scale (NRS) of 0–10 (0 = no pain; 10 = worst pain imaginable) before surgery as well as 1, 3 and 6 months after surgery. An NRS score reduction of at least 30% was defined as a clinically relevant reduction of pain and the primary outcome of our study [Citation5,Citation28].
Procedures
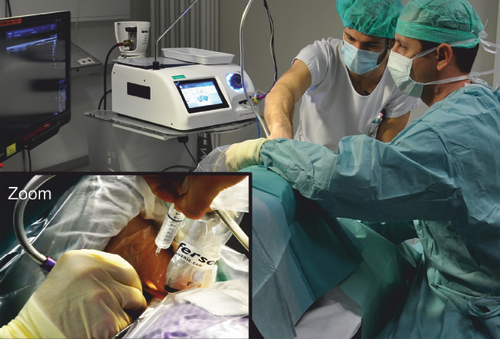
A physical examination, assessment and determination of indications for cryoneurolysis were performed by a certified pain specialist before cryoneurolysis. All treatments were carried out percutaneously using a CRYO-S Electric II® device (18- and 21-gauge CryoFlex probes; Metrum Cryoflex, Mazowieckie, Poland) under ultrasound guidance (). The pain area was palpated and the target nerve was localized using ultrasound and traced proximally. After disinfection and sterile draping, lidocaine 1% was applied to anesthetize the skin and access route to the target nerve. The ablation probe was inserted through a previously placed peripheral venous indwelling catheter (14- to 18-gauge Vasofix® Safety; B. Braun, Melsungen, Germany), directed toward the neural structure under ultrasound guidance, and advanced. The final paraneural positions of the ablation probe and nerve were controlled by electrical neurostimulation (0.5 mA and 1 Hz) eliciting paresthesia in the pain area supplied by the nerve. Lidocaine 2% was administered to anesthetize the nerves before initiating cryoneurolysis. Two to three lesions per nerve were treated for 2 min each at a temperature of -78°C (). To verify the complete coverage of the nerve, we performed ultrasound imaging to visualize the extent of the ice ball surrounding it. After the procedure, ropivacaine 0.5% (2–3 ml) was administered as postinterventional pain therapy. Patients did not receive intravenous or oral analgesia or sedation before, during or after the procedures. All treatments were conducted by a small team of interventionists consisting of three pain specialists. Each specialist had several years of experience in neuromodulation and neurodestructive procedures, including cryoneurolysis, pulsed radiofrequency therapy and continuous radiofrequency therapy.
Zoom indicates the sterile surgical field. The cryoprobe is in the right hand of the operator.
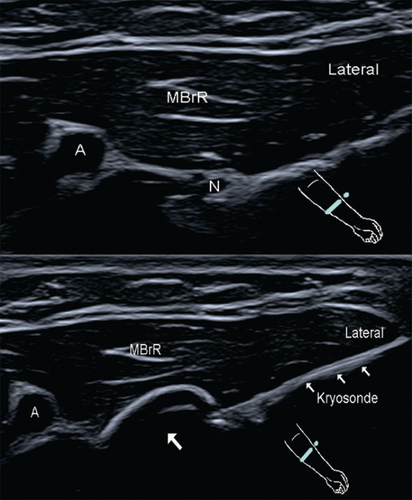
An ‘ice ball’ (arrow) is formed at the tip of the cryoprobe in the area of the superficial radial nerve ramus.
A: Radial artery; MBrR: Brachioradialis muscle; N: Superficial radial nerve.
During follow-up, an interview was performed via telephone 24 h after the intervention to exclude possible procedure-related complications. Patients were reassessed by the physician at the outpatient clinic at 1, 3 and 6 months after the procedure.
Statistical analysis
Demographic data (age, sex, location and type of neuropathy, symptom duration and previous drug therapy) were documented as part of routine clinical practice and included in the analysis (). Wilcoxon rank-sum tests were performed to compare the pain intensity before the intervention and at follow-up. Statistical significance was defined as p < 0.05. All calculations were conducted using R statistical software (v. 4.1.3; R Foundation for Statistical Computing, Vienna, Austria). To evaluate the statistical power of our study and assess the adequacy of our sample size for a pain reduction of 30% or more, a post hoc statistical power analysis was performed using G*Power 3.1 software [Citation29]. An exploratory subanalysis was conducted to investigate the possible advantage of early cryoneurolysis by dividing the patients into two subgroups. Graphs were plotted using GraphPad Prism 8 (GraphPad Software, CA, USA).
Table 1. Patient baseline characteristics and demographics.
Results
Patient cohort
Before the COVID-19 lockdown, we treated an average of two patients per month with cryoneurolysis, including the treatment of genicular nerves, major joints and facet joints. During the pandemic, the frequency of invasive procedures temporarily decreased, which affected our sample size negatively.
Between June 2018 and July 2022, 37 patients were treated for chronic neuropathic pain associated with a specific peripheral nerve using cryoneurolysis. Of these, 24 patients (three of whom had terminal cancer) were included in this retrospective study based on specific inclusion and exclusion criteria. They had a mean age of 58.92 years (standard deviation [SD]: ± 15 years), and 13 (54%) were female. They reported a median of 25.5 months of neuropathic pain before the procedure. Most cases of cryoneurolysis occurred in the intercostal, subcostal, ilioinguinal/hypogastric, superficial peroneal and saphenous nerves. Of the 24 patients included in this study, 17 (71%) received pretreatment with anticonvulsants, 13 (54%) received opioids, 15 (63%) received antidepressants and 13 (54%) received topical lidocaine or capsaicin.
Pain reduction
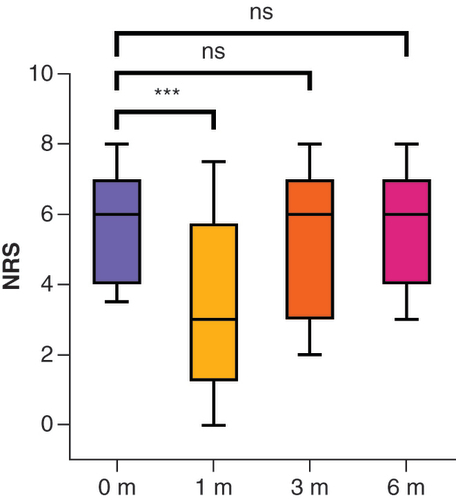
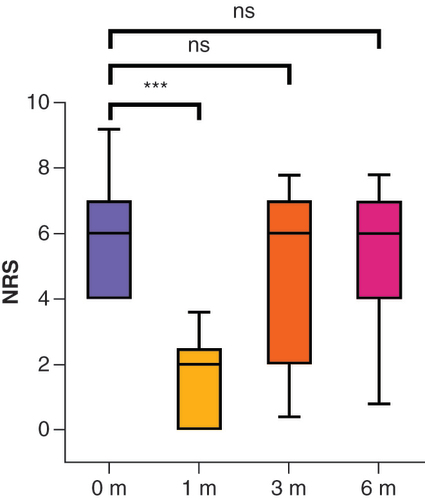
The mean pain scores were 5.8 (SD: ± 1.8) before intervention and 3.4 (SD: ± 2.6; z = -3.16; p < 0.001; r = 0.65), 5.4 (SD: ± 2.1; z = -0.34; p = 0.371) and 5.5 (SD: ± 2.0; z = -0.22; p = 0.414) at 1, 3 and 6 months after the intervention, respectively ( & ). At 1 month, 13 of 24 patients (54.2%) responded to the treatment () and reported pain at a mean NRS score of 1.5 (SD: ± 1.3; z = -4.27; p < 0.001; r = 1.18). At 3 months, the responder rate decreased to three of 22 patients (13.6%) and at 6 months to two of 22 patients (9.1%). Pain relief was no longer significant at these time points. The post hoc power analysis showed that the sample size of the present study was sufficient to find larger effects. At the 1-month follow-up evaluation, the statistical power was 0.99 (effect size of r = 0.65; α = 0.05; n = 24). However, smaller effects, such as those expected at the 3- and 6-month follow-up evaluations, would not be observable with the available sample size: At the 3-month follow-up evaluation, the statistical power was 0.4 (effect size of r = 0.07; α = 0.05; n = 22). At the 6-month follow-up evaluation, the statistical power was 0.24 (effect size of r = 0.04; α = 0.05; n = 22).
Table 2. Pain level before and after cryoneurolysis during the 6-month observation period.
0 m = before, 1 m = after 1 month, 3 m = after 3 months, 6 m = after 6 months. The box and whisker plots show the median, first and third quartiles and 10–90% percentiles. Wilcoxon rank-sum test to compare groups.
***p < 0.001.
m: Months; NRS: Numerical rating scale, ns: Not significant.
0 m = before, 1 m = after 1 month, 3 m = after 3 months, 6 m = after 6 months. The box and whisker plots show the median, first and third quartiles and 10–90% percentiles. Wilcoxon rank-sum test to compare groups.
***p < 0.001.
m: Months; NRS: Numerical rating scale, ns: Not significant.
Two patients died independently of the treatment because of an underlying disease receiving palliative treatment; therefore follow-up of these patients at 3 months and 6 months was no longer possible. Both patients experienced significant pain relief (>50%) during the observation period. Another patient developed painful vascular occlusion of the radial artery caused by the progression of an underlying malignancy. Therefore the assessment of treatment success based on the NRS score after cryoneurolysis of the suprascapular nerve was limited in this case.
In a subgroup of patients (n = 12) with symptoms for up to 24 months, an exploratory sub-analysis found a trend of a better response to cryoneurolysis at 1 month (median NRS score [interquartile range] decreased from 6.5 [1] to 2.5 [4]) compared with patients having symptoms longer than 24 months (decrease from 4.0 [2] to 3.5 [2]), with no statistical significance and no advantage in terms of the symptom relief duration.
Complications
One complication (a minor adverse event) occurred in a patient with cryoneurolysis of the saphenous nerve, after which he experienced new dysesthesia in the knee region after the intervention. These symptoms could not be assigned with certainty to the area supplied by the nerve, and they disappeared spontaneously within 3 months.
Discussion
Cryoneurolysis resulted in a clinically relevant and statistically significant decrease in pain intensity at 1 month in just over half of the patients with chronic refractory neuropathic pain. At the 3- and 6-month follow-up evaluations, no significant difference from baseline was observed.
Cryoneurolysis of the peripheral nerves is used as interventional pain therapy to relieve refractory pain [Citation13]. Unfortunately, randomized trials addressing the effectiveness of cryoneurolysis for chronic peripheral mononeuropathies are lacking. To date, randomized controlled trials have only been published for the treatment of cervicogenic headache and gonarthrosis in non-cancer pain conditions [Citation12]. That said, cryoneurolysis has been described as safe, well-tolerated and effective for treating nociceptive knee pain [Citation30,Citation31]. In another investigation addressing cervicogenic headache, the effect was temporary, with a comparable increase in pain at 6–7 weeks after the intervention [Citation32]; this result corresponds to the approximate duration of the effect we observed.
Yoon et al. evaluated 22 patients (of 144 patients) with refractory chronic peripheral neuropathy who underwent cryoneurolysis during a prospective case study. They observed pain relief for up to 12 months after the procedure, considering 11/22 patients receiving repeated cryoneurolysis within 12 months. However, significantly lower temperatures (-135 to -160°C) were used than those usually applied [Citation13]. Although colder temperatures are associated with the risk of permanent nerve damage [Citation21,Citation33], the authors reported no relevant complications. Additionally, they performed cryoneurolysis under sedation or with additional pain medication before, during and after the intervention, which may have introduced an additional bias and limited the assessability. These practices were avoided in our center according to our standard operating procedures.
The response rate of 58.3% for cryoneurolysis during this study despite a positive response after two prognostic nerve blocks was similar to the expected response rate. Furthermore, other neurodestructive procedures like medial branch thermoablation had response rates of approximately 50–80% [Citation34,Citation35].
Similar to other invasive procedures, the complications of cryoneurolysis include nerve damage, postprocedural pain, infection, bleeding, skin damage and temporary or permanent loss of sensation at treatment location and distribution area of a specific nerve. Pain intensity can be severe and last for several days or weeks. Furthermore, skin lesions can be observed because of the superficial position of the needle. Although considered an uncommon adverse event, pneumothorax is a potential complication of cryoneurolysis around the pleural space [Citation12]. All the aforementioned complications are rare, and ultrasound-controlled cryoneurolysis of a peripheral nerve in particular is very safe in contrast to chemical neurolysis or thermal neurolysis with heat, which can lead to anesthesia dolorosa [Citation36].
Cryoneurolysis resulted in pain reduction of more than 50% for two patients with palliative-stage tumor disease; therefore such treatment could also be preferentially considered in the context of palliative treatment. The course of a patient with neuropathic pain in the auricularis magnus nerve after extirpation of thyroid carcinoma, local radiotherapy and skin transplantation is exemplary of the diagnostic procedure and therapeutic possibilities of cryoneurolysis. Complete freedom from pain was observed during the prognostic nerve block. One series (five infiltrations with local anesthesia using lidocaine 2%) led to short-term pain improvement for approximately 4 days. Capsaicin therapy (8%) had no effect on pain scores. Pulsed radiofrequency therapy resulted in a 50% improvement in pain for 2 weeks. Finally, cryoneurolysis of the auricular nerve resulted in freedom from pain for 1 month and a significant reduction in oral analgesia. After 3 months, the symptoms increased. However, opioid therapy could still be avoided. An appointment for a second cryoneurolysis session was scheduled.
No additional effect was found during further follow-up; however, the significance was limited because of the small number of cases. It is conceivable that the lack of statistically significant differences in the 3- and 6-month follow-up results was attributable to the low test power. Another explanation could be the wide variety of different nerves that had been treated; it may be the case that a subset of these profits more or less from the treatment. Moorjani et al. observed evidence of reversible Wallerian degeneration as well as demyelination of the nerve during histological sectioning of the nerve after cryoneurolysis [Citation19,Citation20]. Because of the current understanding of the physiological changes that occur in nerves after the procedure, the need for repeated treatments is not unexpected. Currently, there is no evidence indicating that the effect of cryoneurolysis diminishes with repeated use, or that it is associated with an increased risk [Citation37]. The influence of repeated cryoneurolysis on the duration of this effect is an interesting topic that requires further evaluation.
The optimal timing of cryoneurolysis remains unknown. For our patients, all noninvasive treatment attempts were performed without satisfactory pain relief. However, cryoneurolysis was performed after a mean of 38.3 months (SD: ± 50.9) because of delays in therapy and latencies in patient referral times. It is possible that the response rate is greater with more rapid treatment, as has been demonstrated previously. Studies of preoperative and perioperative cryoneurolysis of intercostal nerves with thoracic surgery resulted in pain improvement and reduced postoperative opioid use [Citation38–40]. Similar findings were observed by Ilfeld et al.: percutaneous cryoneurolysis was found to significantly improve pain relief and reduce the need for opioids without causing any systemic side effects or complications in patients undergoing both unilateral and bilateral mastectomy [Citation25]. Kumar et al. reported a success rate of more than 85% for neuropathic pain treatment when a neuromodulation system was implanted within 24 months of symptom onset [Citation41]. We also observed a similar trend among the subgroup of patients with shorter symptom durations (≤24 months) and lower pain levels at the 1-month follow-up evaluation after cryoneurolysis. The patients included in the study had already experienced chronic and treatment-resistant pain. By intervening at an earlier stage, it may be possible to prevent the progression to chronic pain and achieve better outcomes, potentially before attempting various antineuropathic medications with uncertain effectiveness [Citation42].
To ensure effective management, it is crucial to recognize that different types of neuropathic pain (e.g., acute nerve-root pressure pain, chronic nerve-root pressure pain and phantom pain) are distinct entities that need to be differentiated. While all pain is ultimately processed by the brain, these types of pain exhibit distinct characteristics, arise from different sources and may respond differently to treatment. Neuropathic pain, for example, can vary in its sensory qualities, intensity and therapeutic outcomes, emphasizing its diverse and multifaceted nature. In future studies, these different qualities could be assessed before cryoneurolysis to identify positive predictive factors and enhance pre-test probability.
Conclusion
In patients with chronic refractory peripheral mononeuropathies, cryoneurolysis is a viable treatment option and associated with very few adverse events.
Further investigations would involve conducting a prospective, controlled randomized study to determine the optimal temperature and to explore the value of early cryoneurolysis, prior to assessing the potential benefits of repeated cryoneurolysis.
Neuropathic pain is a type of pain caused by damage to the somatosensory system, affecting 6.9–10% of the population.
The symptoms are often refractory. Treatment options for neuropathic pain include pharmacological therapy, occupational therapy, coping strategies and interventional procedures.
Medical reviews have shown that despite guideline-based treatment, the effects are often inadequate, with sufficient pain relief experienced by less than 50% of patients.
Cryoneurolysis, a procedure that temporarily interrupts nerve conduction using cold, has been used for treating chronic pain for many years.
The principle of cryoneurolysis is to form an ‘ice ball’ at the tip of the probe, which degenerates myelin sheaths and axons but leaves the nerve structure intact for regeneration.
While the exact optimal temperature during cryoneurolysis remains unknown, the literature predominantly suggests treatment temperatures above -100°C.
Cryoneurolysis resulted in a significant decrease in pain intensity at 1 month, but no significant difference was observed at 3- and 6-month follow-ups.
The duration of therapeutic effect is limited by physiological regeneration of the nerve.
The study concludes that cryoneurolysis is an additional therapy that can alleviate severe chronic neuropathic pain safely and effectively.
The procedure could be considered in the context of palliative treatment.
Author contributions
Z Nemecek takes responsibility for the integrity of this study. He designed the study together with C Sturm and M Harnik, acquired the data, conducted the main analyses, wrote the interpretation, drafted the article, implemented revisions by other authors and approved the current version. C Sturm takes responsibility for the integrity of this work. She designed the study together with Z Nemecek and M Harnik, acquired the data, conducted the main analyses, wrote the interpretation, drafted the article, implemented revisions by other authors and approved the current version. A Rauen participated in the conception of the study and helped analyze and interpret data. She critically revised and approved the revised version of the manuscript. F Reisig acquired data, helped with data interpretation, critically revised the article and approved the current version. K Streitberger participated in the study design, critically revised the manuscript and approved the current version. M Harnik takes responsibility for the integrity of this work. He designed the study together with C Sturm and Z Nemecek, acquired the data, conducted the main analyses, wrote the interpretation, drafted the article, implemented revisions by other authors and approved the current version.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Financial & competing interests disclosure
Institutional funding of this study was provided only by the Department of Anesthesiology and Pain Medicine, Inselspital, University of Bern. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- International Association for the Study of Pain . IASP Taxonomy. Pain terms. Neuropathic pain. www.iasp-pain.org/resources/terminology/#Neuropathicpain (Accessed 10May2023).
- Finnerup NB , HaroutounianS , KamermanPet al. Neuropathic pain: an updated grading system for research and clinical practice. Pain157(8), 1599–1606 (2016).
- van Hecke O , AustinSK , KhanRA , SmithBH , TorranceN. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain155(4), 654–662 (2014).
- Attal N , BouhassiraD. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms?Pain156(4), S104–S114 (2015).
- Attal N . Pharmacological treatments of neuropathic pain: the latest recommendations. Rev. Neurol. (Paris)175(1–2), 46–50 (2019).
- Bates D , CarstenSchultheis B , HanesMCet al. A comprehensive algorithm for management of neuropathic pain. Pain Med.20(Suppl. 1), S2–S12 (2019).
- O’Connor AB . Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics27(2), 95–112 (2009).
- O’Connor AB , DworkinRH. Treatment of neuropathic pain: an overview of recent guidelines. Am. J. Med.122(Suppl. 10), S22–S32 (2009).
- Attal N , BouhassiraD. Advances in the treatment of neuropathic pain. Curr. Opin. Neurol.34(5), 631–637 (2021).
- Mekhail NA , ChengJ , NarouzeS , KapuralL , MekhailMN , DeerT. Clinical applications of neurostimulation: forty years later. Pain Pract.10(2), 103–112 (2010).
- Walsh T , MalhotraR , SharmaM. Radiofrequency techniques for chronic pain. B. J. A. Educ.22(12), 474–483 (2022).
- Goyal S , KumarA , SharmaRS , GoyalD , SinghGK. Efficacy of cryoneurolysis in the management of chronic non-cancer pain: a systematic review and meta-analysis. Indian J. Anaesth.66(7), 485–497 (2022).
- Yoon JHE , GrechushkinV , ChaudhryA , BhattacharjiP , DurkinB , MooreW. Cryoneurolysis in patients with refractory chronic peripheral neuropathic pain. J. Vasc. Interv. Radiol.27(2), 239–243 (2016).
- Hippocrates . Volume IV: Nature of Man. Regimen in Health. Humours. Aphorisms. Regimen I–III. Dreams. In: Hippocrates, Heracleitus, Nature of Man. Regimen in Health. Humours. Aphorisms. Regimen 1-3. Dreams. Heracleitus: On the Universe. Loeb Classical Library, London, UK(1931).
- Wang H , OliveroW , WangD , LanzinoG. Cold as a therapeutic agent. Acta Neurochir. (Wien)148(5), 565–570 (2006).
- Amoils SP . The Joule Thomson cryoprobe. Arch. Ophthalmol.78(2), 201–207 (1967).
- Lloyd JW , BarnardJD , GlynnCJ. Cryoanalgesia. Lancet308(7992), 932–934 (1976).
- Katz J , NelsonW , ForestR , BruceD. Cryoanalgesia for post-thoracotomy pain. Lancet315(8167), 512–513 (1980).
- Moorjani N , ZhaoF , TianY , LiangC , KalubaJ , MaiwandMO. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur. J. Cardiothorac. Surg.20(3), 502–507 (2001).
- Bittman RW , BehbahaniK , GonzalezF , PrologoJD. Interventional cryoneurolysis: what is the same, what is different, what is new?Semin. Intervent. Radiol.36(05), 374–380 (2019).
- Zhou L , ShaoZ , OuS. Cryoanalgesia: electrophysiology at different temperatures. Cryobiology46(1), 26–32 (2003).
- Evans PJ . Cryoanalgesia. The application of low temperatures to nerves to produce anaesthesia or analgesia. Anaesthesia36(11), 1003–1013 (1981).
- Biel E , ArokeEN , MayeJ , ZhangSJ. The applications of cryoneurolysis for acute and chronic pain management. Pain Pract.23(2), 204–215 (2023).
- Ilfeld BM , SmithCR , TuranAet al. Ultrasound-guided percutaneous cryoneurolysis to treat chronic postamputation phantom limb pain: a multicenter randomized controlled trial. Anesthesiology138(1), 82–97 (2023).
- Ilfeld BM , FinneranJJ , SwisherMWet al. Preoperative ultrasound-guided percutaneous cryoneurolysis for the treatment of pain after mastectomy: a randomized, participant- and observer-masked, sham-controlled study. Anesthesiology137(5), 529–542 (2022).
- Swisher MW , BallST , GonzalesFB , CidambiKR , TrescotAM , IlfeldBM. A randomized controlled pilot study using ultrasound-guided percutaneous cryoneurolysis of the infrapatellar branch of the saphenous nerve for analgesia following total knee arthroplasty. Pain Ther.11(4), 1299–1307 (2022).
- von Elm E , AltmanDG , EggerM , PocockSJ , GøtzschePC , VandenbrouckeJP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol.61(4), 344–349 (2008).
- Finnerup NB , AttalN , HaroutounianSet al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol.14(2), 162–173 (2015).
- Erdfelder E , FaulF , BuchnerA , LangAG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods41(4), 1149–1160 (2009).
- Radnovich R , ScottD , PatelATet al. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthritis Cartilage25(8), 1247–1256 (2017).
- Diep D , MittalN , SanghaH , FaragJ. Cryoneurolysis for non-cancer knee pain: a scoping review. Interv. Pain Med.2(2), 100247 (2023).
- Kvarstein G , HögströmH , AllenSM , RoslandJH. Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study. Scand. J. Pain20(1), 39–50 (2019).
- Cheng J-G . Cryoanalgesia for refractory neuralgia. J. Perioper. Sci.2(2), 1–8 (2015).
- Cohen SP , DoshiTL , ConstantinescuOCet al. Effectiveness of lumbar facet joint blocks and predictive value before radiofrequency denervation. Anesthesiology129(3), 517–535 (2018).
- Curatolo M , BogdukN. Diagnostic blocks for chronic pain. Scand. J. Pain1(4), 186–192 (2010).
- Steiger H-J , HorstmannG , FreynhagenR. Current treatments for trigeminal neuralgia – a surgical approach. Dtsch. Arztebl.104(39), 2655–2661 (2007).
- Hsu M , StevensonFF. Wallerian degeneration and recovery of motor nerves after multiple focused cold therapies. Muscle Nerve51(2), 268–275 (2015).
- Clemence J , MalikA , FarhatLet al. Cryoablation of intercostal nerves decreased narcotic usage after thoracic or thoracoabdominal aortic aneurysm repair. Semin. Thorac. Cardiovasc. Surg.32(3), 404–412 (2020).
- Pilkington M , HarbaughCM , HirschlRB , GeigerJD , GadepalliSK. Use of cryoanalgesia for pain management for the modified Ravitch procedure in children. J. Pediatr. Surg.55(7), 1381–1384 (2020).
- Park R , CoomberM , GilronI , ShanthannaH. Cryoanalgesia for postsurgical pain relief in adults: a systematic review and meta-analysis. Ann. Med. Surg.69, 102689 (2021).
- Kumar K , FrcsC , RizviSet al. Impact of wait times on spinal cord stimulation therapy outcomes. Pain Pract.14(8), 709–720 (2014).
- Giménez-Campos MS , Pimenta-Fermisson-RamosP , Díaz-CambroneroJI , Carbonell-SanchísR , López-BrizE , Ruíz-GarcíaV. A systematic review and meta-analysis of the effectiveness and adverse events of gabapentin and pregabalin for sciatica pain. Aten. Primaria54(1), 102144 (2022).