Abstract
Aim: Atogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist, is a substrate of OATP and metabolized by CYP3A4. Effect of multiple-dose itraconazole (strong CYP3A4 inhibitor), single-dose rifampin (strong OATP inhibitor) and multiple-dose rifampin (strong CYP3A4 inducer) on single-dose pharmacokinetics (PK) and safety of atogepant were assessed. Methods: Two phase I, open-label, single-center, crossover trials enrolled healthy adults. Results: Cmax and area under the curve of atogepant increased when co-administered with itraconazole. Atogepant systemic exposure increased following co-administration with single-dose rifampin. Atogepant systemic exposure decreased with co-administration of multiple-dose rifampin. Treatment emergent adverse events (TEAEs) were predominantly mild or moderate, and included constipation, dizziness, headache and nauseas. Conclusion: Systemic exposure of atogepant increased significantly when co-administered with a strong CYP3A4 or OATP inhibitor and decreased significantly when co-administered with a strong CYP3A4 inducer.
Plain language summary
Calcitonin gene-related peptide (CGRP) is involved in migraines, a neurological disorder with severe headache and sensory disturbances. A medication called atogepant is a CGRP receptor antagonist that can block the effect of CGRP and is approved by the US FDA for the preventive treatment of migraines. If you take a drug, such as itraconazole, a strong inhibitor of an enzyme called CYP3A4, that metabolizes atogepant, at the same time as atogepant, it can increase the amount of atogepant in your body. OATPs are membrane transport proteins that facilitate liver uptake of atogepant. If you take a drug that is an OATP inhibitor with atogepant, it can increase the amount of atogepant in your body. However, if you take a CYP3A4 inducer such as rifampin, which increases the activity of CYP3A4, it can decrease the amount of atogepant in your body. Your doctor may adjust the dose of atogepant accordingly.
Migraine is a global, highly prevalent condition. The condition may be chronic in some patients [Citation1,Citation2]. Worldwide, migraine is the second leading cause of disability [Citation1]. In 2018, 15.9% of US adults experienced migraines, with more women (21.7%) than men (10.7%) reporting migraine [Citation2]. In the USA, migraine prevalence has been stable from 2005 to 2018 [Citation2]. Migraine continues to be a public health concern as it was the fifth most common reason in the USA for an emergency department visit in 2016 [Citation2]. Migraine may also disproportionately affect people with lower socioeconomic status [Citation2].
Migraine headache is a common neurological disorder described as attacks of throbbing, unilateral headache of moderate or severe pain intensity [Citation3]. It is associated with nausea, vomiting and sensitivity to light and sound. Episodic migraine is characterized by <15 headache days per month, while chronic migraine is defined by the International Classification of Headache Disorders as a headache occurring on 15 or more days per month for more than 3 months, which, on at least 8 days per month, has the features of a migraine headache [Citation3].
Historically, topiramate, a commonly used oral antiepileptic and nonmigraine-specific medications, such as antiepileptics, beta-blockers and antidepressants, have been recommended as preventive treatments for migraine. Migraine specific medications (four monoclonal antibodies and two gepants) that inhibit CGRP pathway were approved for the prophylaxis of migraine in recent years. Although drug–drug interaction (DDI) potential for monoclonal antibodies is low, DDIs with historically used small-molecule antimigraine drugs is well known [Citation4,Citation5].
Atogepant is a calcitonin gene-related peptide (CGRP) receptor antagonist approved for use in the USA for the preventive treatment of migraine in adults [Citation6]. A CGRP is a neuropeptide associated with the pathophysiology of migraine and has become an area of active research for the prevention and treatment of migraine [Citation7–9]. CGRP contributes to the pathogenesis of migraine via the activation of the trigeminovascular system [Citation10]. A CGRP receptor activation leads to vasodilation [Citation11]. Additionally, CGRP concentrations in the serum and saliva of patients with migraine are elevated, and these levels normalize following treatment with triptans [Citation12–14].
Atogepant is a small-molecule CGRP receptor antagonist that is rapidly absorbed after oral administration. It has an elimination half-life of approximately 11 h [Citation15–17]. In phase IIb/III double-blind trials, atogepant significantly reduced the number of days with migraine headache in participants with episodic or chronic migraine [Citation8,Citation18,Citation19]. The most commonly reported side effects in clinical trials were nausea, constipation and upper respiratory tract infections [Citation8].
Atogepant is primarily metabolized by CYP3A4 with a minor contribution from CYP2D6, and is a substrate of several membrane transporters including P-gp and OATP [Citation20]. Combined administration with CYP3A4 inhibitors could increase exposure to atogepant [Citation18]. Itraconazole, a strong inhibitor of CYP3A4, was selected to assess the effect of CYP3A4 inhibitors on the pharmacokinetics (PK) of atogepant [Citation21]. Rifampin is a strong inducer of CYP3A4 and P-gp, and is also an inhibitor of OATP [Citation22,Citation23]. A single dose of rifampin administered to healthy volunteers increased systemic exposure of OATP1B1 substrates by inhibiting hepatocellular uptake via the OATP1B1 transporter [Citation24]. However, after multiple doses, rifampin decreases systemic exposure of CYP3A4 and P-gp substrates [Citation25]. Rifampin, a CYP3A4 inducer and OATP inhibitor, was selected to assess the effects of a strong CYP3A4 inducer (multi-dose rifampin) and a strong OATP inhibitor (single-dose rifampin) on the PK of atogepant.
This series of drug–drug interaction studies evaluated the pharmacokinetics of atogepant co-administration with a CYP3A4 inhibitor (multidose itraconazole) or an OATP inhibitor (single-dose rifampin) or a strong CYP3A4 inducer (multidose rifampin). Secondary objectives were to assess the safety and tolerability of atogepant alone or in combination with itraconazole or rifampin.
Methods
Study design & participants
Two phase I open-label, fixed-sequence, single-center, crossover studies were conducted in healthy volunteers. For both studies A and B, the main eligibility criteria were adults (18–45 years of age) in good health at the time of signing the informed consent. Participants were non-smokers with negative urine drug screens. Participants were excluded if they had a condition or were in a situation that, in the investigator’s opinion, might put the participant at significant risk, confound study results or interfere significantly with study participation. Both studies consisted of a screening visit, two study periods, an end-of-treatment visit and a follow-up visit ().
*Dosing occurred after 10 h fast.
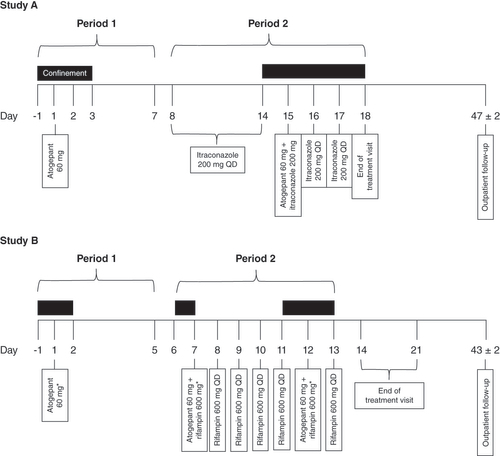
Study A was a phase I open-label, fixed-sequence single-center, crossover trial that enrolled 40 healthy participants (aged 18–45 years) who received single-dose atogepant tablet 60 mg with and without multiple-dose itraconazole capsule 200 mg/day. Participants received 240 ml of water with each dose. When dosing was given under fasting conditions, participants continued their fast and remained seated upright and awake for 4 h post-dose. Participants received two treatments in a fixed-sequence (periods 1 and 2) order with a 7-day washout period:
Period 1 (days -1–7): participants stayed at the study center overnight on days -1, 1, 2 and 3 and received a single 60 mg atogepant dose under fasting conditions on day 1;
Period 2 (days 8–18): participants returned for outpatient visits on days 8 through 13 and received itraconazole 200 mg once daily under fed conditions on days 8 through 14 and did not stay overnight. On day 14, participants returned to stay at the study center overnight on days 14–18. Itraconazole 200 mg was co-administered with 60 mg atogepant on day 15 under fasting conditions. Itraconazole 200 mg once daily under fed conditions was given on days 16 and 17.
The end-of-treatment visit was conducted on day 18. The follow-up visit was conducted on an outpatient basis on day 47 (±2 days) or 30 (±2) days after the last dose of study treatment if a participant discontinued dosing early.
Study B was a phase I open-label, fixed-sequence single-center, crossover trial that enrolled 32 healthy adults (18–45 years of age) that received the 60 mg single-dose atogepant tablet with and without the 600 mg/day multiple-dose rifampin capsule. During study periods 1 and 2, participants received a total of three treatments with two atogepant washout periods, as described below:
Period 1 (days -1–5): participants stayed at the study center overnight on days -1, 1 and 2 and received a single 60 mg atogepant dose under fasting conditions on day 1;
Period 2 (days 6–14): participants stayed at the study center overnight on days 6 and 7. Participants returned for outpatient visits on days 9 and 10. On day 11, participants returned to stay at the study center overnight on days 11, 12 and 13. In this period, participants received: co-administration of 60 mg atogepant and 600 mg rifampin on day 7 followed by 600 mg rifampin alone, once daily, on days 8, 9, 10 and 11. Participants received rifampin on an outpatient basis on days 9 and 10. On day 12, participants received co-administration of 60 mg atogepant and 600 mg rifampin followed by 600 mg rifampin alone on day 13.
When atogepant was to be administered alone or in combination with rifampin, participants were required to undergo a 10 h overnight fast prior to dosing (beginning on days -1, 6 and 11) and were required to maintain the fast for an additional 4 h following dose administration (on days 1, 7 and 12). When rifampin was to be administered alone (on days 8, 9, 10, 11 and 13), no food should be consumed for 1 h before and after dosing. Participants received 240 ml of water with each dose.
The end-of-treatment visit was conducted on day 14 or within 7 days after day 14 on an outpatient basis, or at the time of early termination. The follow-up visit was conducted on an outpatient basis on day 43 (±2 days) or 30 (±2) days after the last dose of study treatment if a participant discontinued dosing early.
Both studies were conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. Both trials were approved by an Institutional Review Board.
Pharmacokinetic sampling & bioanalytical methods
Blood for plasma PK samples was collected via an indwelling catheter or venipuncture of the antecubital veins from either arm into prechilled 4-ml Vacutainer tubes containing K2EDTA as an anticoagulant. Within 30 min from the time of the blood draw, blood samples for plasma PK were centrifuged at no less than 2500× g for 10 min at approximately 4 °C. After centrifugation, the plasma samples were harvested and transferred into two prechilled, labeled polypropylene tubes. The samples were then flash-frozen in a dry ice and alcohol or acetone bath (with ethanol, isopropanol, acetone or methanol) and stored at approximately -20 °C. The bioanalysis was conducted in compliance with the US FDA Guidance for Industry: Bioanalytical Method Validation dated May 2001.
Study A
Drug concentration
PK venous blood samples for atogepant were collected starting on days 1 and 15 at 0 h (pre-dose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48 and 72 h post-dose. PK venous blood samples for itraconazole were collected pre-dose on days 8, 13, 14 and 15. The following PK parameters were calculated for atogepant from plasma concentration-time data for each participant using Phoenix WinNonlin (Version 6.2) software: Cmax, maximum plasma drug concentration; Tmax, time to reach maximum plasma concentration; AUC0-t, area under the plasma concentration–time curve (AUC) from time 0 to time t; AUC0-∞, AUC from time 0 to infinity; and AUC%, percent of AUC extrapolated from the last measurable concentration to infinity.
The atogepant and itraconazole concentrations in plasma samples were analyzed using validated liquid chromatography tandem mass spectrometry (LC-MS/MS) methods.
Study B
PK venous blood samples for atogepant were collected starting on days 1 and 12: 0 h (pre-dose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36 and 48 h post-dose. On day 7 blood samples were collected at the following time points: 0 h (pre-dose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h post-dose. The following PK parameters were calculated for atogepant from plasma concentration-time data for each participant: Cmax; Tmax; AUC0-24, AUC from time 0 to 24 h; λz terminal elimination rate constant; T½, terminal elimination half-life; CL/F, apparent total body clearance of drug from plasma after extravascular administration; and Vz/F, apparent volume of distribution during the terminal phase after extravascular administration.
Drug concentrations in plasma samples were analyzed using validated liquid chromatography with tandem mass spectrometry detection (LC-MS/MS) methods. The limit of quantitation was 1.00 ng/ml for both atogepant and rifampin.
Safety assessments
Both studies monitored adverse events (AEs), clinical laboratory parameters (hematology, chemistry, coagulation profile, urinalysis, serology, drugs of abuse screen and pregnancy test), vital signs, ECG and physical examinations.
Statistical analysis
Analysis populations
All participants who received at least one dose of atogepant were included in the safety population for both studies A and B. All participants with evaluable PK parameters for atogepant were included in the PK population. Sample size for study A was determined by assuming a within-subject coefficient of variation of 27% for Cmax and AUC of atogepant, and the true ratio of test to reference geometric means was approximately 1, a sample size of 34 participants would provide 90% power to show that the 90% CIs for the point estimate of the ratio of geometric mean PK parameters (Cmax and AUC) of atogepant with relative to and without co-administration of itraconazole were within 80–125%. 40 participants were enrolled to account for possible dropouts. Sample size for study B was determined by assuming a within-participant coefficient of variation of 25% for Cmax and AUC of atogepant and a true ratio of test to reference geometric means of approximately 1, a sample size of 28 participants would provide 90% power to show that the 90% CIs for the point estimate of the ratio of geometric mean PK parameters (Cmax and AUC) of atogepant with and without co-administration of rifampin were within 80–125%. A total of 32 participants were enrolled to account for possible dropouts.
Pharmacokinetics
Plasma concentrations of atogepant for participants in the PK population were summarized by treatment. PK parameters of atogepant were summarized by treatment using descriptive statistics for the PK population. Log-transformed values for Cmax and AUC of atogepant were analyzed using Phoenix WinNonlin (Version 6.2) software and a linear mixed-effects model with treatment as fixed effect and participant as random effect. The two-sided 90% CIs were constructed for the ratio of geometric means of the Cmax and AUC of atogepant between treatments (test vs reference).
Safety
The number and percentage of participants reporting treatment–emergent adverse events (TEAEs) in each treatment were tabulated. Descriptive statistics for clinical laboratory and vital signs were also tabulated.
Results
Participant disposition & baseline demographics
A total of 40 participants entered and completed study A. The mean age was 34.8 years, and 55% of participants were female and 45% were male (see for participant characteristics). A total of 32 participants enrolled in study B. The mean age was 31.3 years, and 59% of participants were female and 41% were male (). A total of 31 (96.9%) participants completed the treatment periods; one participant had a protocol deviation (positive drug screen for cotinine) after receiving atogepant alone and did not complete that treatment period nor the follow-up period. One participant completed all treatment periods but not the follow-up period.
Table 1. Participant characteristics.
Pharmacokinetics
Study A
40 participants completed all planned treatments and were included in the PK population. The mean plasma concentrations of atogepant after the administration of 60 mg either alone or with itraconazole are presented in . The PK parameters of atogepant following administration either alone or in combination with itraconazole are summarized in . The 90% CIs for the geometric mean ratios of Cmax and AUC parameters of atogepant revealed a clinically significant drug interaction. Cmax and AUC parameters of atogepant increased by 2.15- and 5.5-fold, respectively, in the presence of itraconazole. Thus, CYP3A4 inhibition by itraconazole or other strong CYP3A4 inhibitors will result in a clinically significant increase in the exposure of atogepant.
(A) Linear scale, (B) semilogarithmic plot. Error bars represent standard deviation.
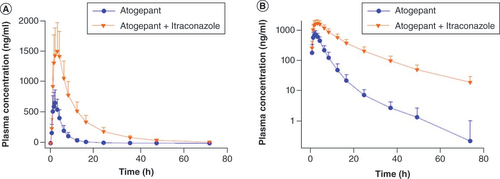
Table 2. Pharmacokinetic parameters of atogepant following administration with and without itraconazole in healthy participants; Study A (N = 40).
Study B
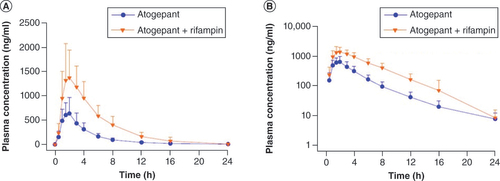
A total of 31 out of 32 participants completed all planned treatments and were included in the PK population. The mean plasma atogepant concentration–time profiles following administration with or without single-dose rifampin, and with or without multiple-dose rifampin are presented in & , respectively. The PK parameters of atogepant following administration either alone or in combination with rifampin are summarized in . A statistically significant increase in atogepant systemic exposure (2.85-fold for AUC0-24 and 2.23-fold for Cmax) was observed following co-administration of single-dose atogepant 60 mg and single-dose rifampin 600 mg compared with administration of single-dose atogepant 60 mg alone. Rifampin is an OATP1B1 inhibitor and atogepant uptake is dependent on its influx into the hepatocyte through the OATP1B1 transporter. The increases in atogepant Cmax and AUC when co-administered with OATP1B1 inhibitors could be clinically significant.
(A) Linear scale, (B) semilogarithmic plot. Error bars represent standard deviation.
(A) Linear scale, (B) semilogarithmic plot. Error bars represent standard deviation.
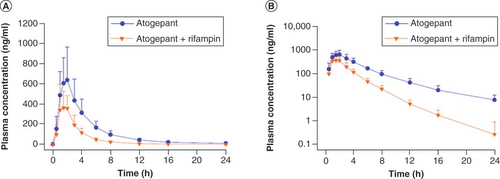
Table 3. Pharmacokinetic parameters of atogepant following administration with and without rifampin in healthy participants; study B (N = 31).
A statistically significant decrease in atogepant systemic exposure (61% for AUC0-∞, 60% for AUC0-t and 30% for Cmax) was observed following co-administration of single-dose atogepant 60 mg and multiple-dose rifampin 600 mg compared with administration of single-dose atogepant 60 mg alone. Given that atogepant is extensively metabolized by CYP3A4 and is also a substrate of P-gp, the decreases in atogepant Cmax and AUC when co-administered with strong CYP3A4 and P-gp inducers could be clinically significant.
Safety assessments
Study A
There were no serious adverse events (SAEs) or discontinuations due to AEs following administration of atogepant 60 mg single dose and itraconazole 200 mg/day to healthy participants alone or in combination. TEAEs are listed in . TEAEs of mild dizziness were experienced by two participants following atogepant alone. TEAEs of mild headache (n = 2) and mild constipation (n = 1) were experienced by participants taking itraconazole alone. All TEAEs were considered related to investigational product administration and resolved within 1 day. Descriptive statistics for laboratory parameters, vital signs and ECGs did not reveal any findings of clinical concern.
Table 4. Number (%) of participants with treatment-emergent adverse events (TEAE) by study treatment, system organ class and preferred term (safety population).
Study B
Following a single dose of atogepant 60 mg alone, co-administration of atogepant 60 mg and single-dose rifampin 600 mg followed by co-administration of atogepant 60 mg and multidose rifampin 600 mg in healthy volunteers, no deaths or other SAEs were reported, and no participants had TEAEs leading to treatment discontinuation. Approximately a third of the participants had TEAEs during the study; overall, the most common TEAEs were nausea and headache (n = 2 for each), . No participants reported TEAEs after administration of atogepant alone. Approximately 16% (5/32) of participants had at least one treatment-related TEAE during the study. All TEAEs were mild or moderate in severity; there were no severe TEAEs reported. There were no notable changes from baseline in clinical laboratory test results, vital signs parameters and ECG findings. One participant had an elevated AST >3 × ULN with a concurrent elevated creatine kinase that was attributed to exertion.
Discussion
CYP3A4-mediated metabolism is a major elimination pathway of atogepant [Citation20]. Although atogepant is effective and safe across a wide dose range of 10 mg to 60 mg QD, quantitative determination of increase/decrease of atogepant systemic exposure due to concomitant administration of CYP3A4 inhibitors/inducers was important to make appropriate dosing recommendations [Citation8,Citation16,Citation18]. Study A assessed the effect of CYP3A4 inhibition by itraconazole, a strong CYP3A4 inhibitor, on the pharmacokinetics of single-dose atogepant in healthy participants [Citation26]. Inhibition of CYP3A4 via administration of 200 mg/day itraconazole for 7 days resulted in a 2.15-fold increase in Cmax and 5.5-fold increase in AUC of atogepant. Increases in atogepant exposure resulting from CYP3A4 inhibition could be clinically significant, and atogepant dose adjustment may be needed when concomitantly administered with strong CYP3A4 inhibitors. The lowest approved dose of 10 mg atogepant is recommended when co-administered with strong CYP3A4 inhibitors. Such a dose adjustment ensures safety and efficacy of atogepant, including in chronic migraine patients for whom the approved dose is 60 mg once daily, when concomitantly administered with strong CYP3A4 inhibitors [Citation6].
Study B was designed to separate the OATP1B1 inhibition effect and CYP3A4 and P-gp induction effect of rifampin on the single-dose pharmacokinetics of atogepant [Citation27]. OATP1B1 inhibition is an immediate effect following single-dose administration of rifampin. Thus, a 2.85-fold increase for AUC and 2.23-fold increase for Cmax were observed following co-administration of atogepant with an OATP1B1 inhibitor. The increases in atogepant Cmax and AUC when co-administered with OATP1B1 inhibitors could be clinically significant and atogepant dose adjustment may be required. An atogepant dose of 10 or 30 mg is recommended when co-administered with strong OATP inhibitors. Such a dose adjustment ensures safety and efficacy of atogepant when concomitantly administered with strong OATP inhibitors. The goal of dose adjustments when atogepant is coadministered with interacting drugs is to maintain systemic exposure within the therapeutic range. Once daily atogepant at 10, 30 and 60 mg doses are approved for the preventive treatment of episodic migraine, and 60 mg once daily atogepant dose is approved for the preventive treatment of chronic migraine [Citation6]. With 2.85-fold increase of atogepant AUC due to OATP inhibition, 10 and 30 mg doses result in exposure already proven to be safe [Citation18]. However, 30 mg atogepant was not recommended when coadministered with strong CYP3A4 inhibitors because 5.5-fold increase results in exposure much higher than the established safe exposure [Citation6].
A decrease in atogepant systemic exposure (61% for AUC0-∞, 60% for AUC0-t and 30% for Cmax) was observed following co-administration with a strong CYP3A4 and P-gp inducer (multiple-dose rifampin). The decreases in atogepant Cmax and AUC when co-administered with strong CYP3A4 and P-gp inducers could be clinically significant and atogepant dose adjustment may be required. Atogepant 30 or 60 mg is recommended when co-administered with strong or moderate CYP3A4 inducers. Such a dose adjustment ensures safety and efficacy of atogepant when concomitantly administered with strong or moderate CYP3A4 inducers [Citation6].
In study A, five TEAEs were reported (two participants experienced dizziness [atogepant alone], two participants experienced headache [itraconazole alone] and one participant experienced constipation [itraconazole alone]). None of the TEAEs were reported as an SAE, and all TEAEs were mild in intensity. No TEAEs led to permanent discontinuation of study treatment. No deaths occurred during the study. There were no post-baseline laboratory findings, vital signs, or ECGs that were of potential clinical significance during the study.
In study B, no deaths or SAEs occurred during the study; no participants had TEAEs that led to discontinuation. The safety results observed in this study of atogepant alone or atogepant co-administered with rifampin are similar to the safety profile observed with atogepant in other clinical studies. No new safety concerns were identified.
The strengths of these studies include crossover designs, within participant comparisons, and serial PK sampling. Participants were healthy volunteers with no other medication usage or confounding factors that could affect the results. These design features suggest that these studies provided accurate quantification of the effect of CYP3A4 inhibition or induction or OATP inhibition.
Limitations
The study had a small sample size, but this is a general limitation of phase I studies. Only healthy participants were included while the approved indication is for migraine. The short duration of the study does not allow for long-term analysis.
Conclusion
Atogepant co-administered with strong CYP3A4 inhibitors should be administered at the lowest approved dose of 10 mg to account for increased exposure. Atogepant co-administered with strong or moderate CYP3A4 inducers should be administered at 30 or 60 mg to account for decreased exposure. Atogepant co-administered with strong OATP inhibitors should be administered at 10 or 30 mg to account for increased exposure. The safety results observed with atogepant alone or atogepant co-administered with rifampin or itraconazole are similar to the safety profile observed with atogepant in other clinical studies.
Calcitonin gene-related peptide (CGRP) is a neuropeptide associated with the pathophysiology of migraine and has become an area of active research for the prevention and treatment of migraine.
Atogepant, a CGRP receptor antagonist, was recently approved for the preventive treatment of migraine in adults.
Atogepant is a substrate of OATP and metabolized by CYP3A4.
Itraconazole, a strong inhibitor of CYP3A4, was selected to assess the effect of CYP3A4 inhibitors on the pharmacokinetics (PK) of atogepant. Systemic exposure of atogepant increased significantly when co-administered with a strong CYP3A4 inhibitor.
Rifampin, a CYP3A4 inducer and OATP inhibitor, was selected to assess the effects of a strong CYP3A4 inducer (multi-dose rifampin) and a strong OATP inhibitor (single-dose rifampin) on the PK of atogepant. Systemic exposure of atogepant increased significantly when co-administered with single-dose rifampin and decreased significantly when co-administered with multi-dose rifampin.
Atogepant 10 mg is recommended when co-administered with strong CYP3A4 inhibitors.
Atogepant 30 or 60 mg is recommended when co-administered with strong or moderate CYP3A4 inducers.
Atogepant 10 or 30 mg is recommended when co-administered with strong OATP inhibitors.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors thank the study investigators, participants and clinical sites for their contributions to various aspects of the studies.
Financial & competing interests disclosure
This work was supported by AbbVie. AbbVie participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review and approval of the publication. R Boinpally, D McGeeney and JM Trugman are employees of AbbVie Inc. and may hold AbbVie stock or stock options. W Chen is a former employee of AbbVie Inc. and may hold AbbVie stock or stock options. This work was previously presented at the 2022 American Headache Society Annual Scientific Meeting. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/
Additional information
Funding
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392(10159), 1789–1858 (2018).
- Burch R , RizzoliP , LoderE. The prevalence and impact of migraine and severe headache in the United States: updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache61(1), 60–68 (2021).
- Katsarava Z , ManackA , YoonMSet al. Chronic migraine: classification and comparisons. Cephalalgia31(5), 520–529 (2011).
- Maideen NMP , RajkapoorB , MuthusamyS , RamanathanS , ThangaduraiSA , SughirAA. A review on pharmacokinetic and pharmacodynamic drug interactions of adrenergic β-blockers with clinically relevant drugs - an overview. Curr. Drug Metab.22(9), 672–682 (2021).
- AbbVie Canada . UBRELVY product monograph. https://www.abbvie.ca/content/dam/abbvie-dotcom/ca/en/documents/products/UBRELVY_PM_EN.pdf (Accessed 20May2023).
- AbbVie . Prescribing Information: qulipta (atogepant) tablets, for oral use (2023). https://www.rxabbvie.com/pdf/QULIPTA_pi.pdf
- Charles A , Pozo-RosichP. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet394(10210), 1765–1774 (2019).
- Ailani J , LiptonRB , GoadsbyPJet al. Atogepant for the preventive treatment of migraine. N. Engl. J. Med.385(8), 695–706 (2021).
- Cohen F , YuanH , SilbersteinSD. Calcitonin gene-related peptide (CGRP)-targeted monoclonal antibodies and antagonists in migraine: current evidence and rationale. BioDrugs36(3), 341–358 (2022).
- Villalón CM , OlesenJ. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol. Ther.124(3), 309–323 (2009).
- Edvinsson L . The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache57(Suppl. 2), 47–55 (2017).
- Alpuente A , GallardoVJ , AsskourL , CaronnaE , Torres-FerrusM , Pozo-RosichP. Salivary CGRP and erenumab treatment response: towards precision medicine in migraine. Ann. Neurol.92(5), 846–859 (2022).
- de Vries Lentsch S , GarreldsIM , DanserAHJ , TerwindtGM , MaassenVanDenBrinkA. Serum CGRP in migraine patients using erenumab as preventive treatment. J. Headache Pain.23(1), 120 (2022).
- Goadsby PJ , EdvinssonL. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol.33(1), 48–56 (1993).
- Min KC , KraftWK , BondiskeyPet al. Atogepant is not associated with clinically meaningful alanine aminotransferase elevations in healthy adults. Clin. Transl. Sci.14(2), 599–605 (2021).
- Boinpally R , McNameeB , YaoLet al. A single supratherapeutic dose of atogepant does not affect cardiac repolarization in healthy adults: results from a randomized, single-dose, phase I crossover trial. Clin. Pharmacol. Drug Dev.10(9), 1099–1107 (2021).
- Boinpally R , JakateA , ButlerM , BorbridgeL , PericlouA. single-dose pharmacokinetics and safety of atogepant in adults with hepatic impairment: results from an open-label, phase I trial. Clin. Pharmacol. Drug Dev.10(7), 726–733 (2021).
- Goadsby PJ , DodickDW , AilaniJet al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase IIb/3 trial. Lancet Neurol.19(9), 727–737 (2020).
- Pozo-Rosich P , AilaniJ , AshinaMet al. Atogepant for the Preventive Treatment of Chronic Migraine: Results From the PROGRESS Phase 3 Trial. Lancet DOI: 10.1016/S0140-6736(23)01049-8 (2023) ( Online ahead of print).
- Rustichelli C , AvalloneR , FerrariA. Atogepant: an emerging treatment for migraine. Expert Opin. Pharmacother.23(6), 653–662 (2022).
- Vossen M , SevestreM , NiederaltC , JangIJ , WillmannS , EdgintonAN. Dynamically simulating the interaction of midazolam and the CYP3A4 inhibitor itraconazole using individual coupled whole-body physiologically-based pharmacokinetic (WB-PBPK) models. Theor. Biol. Med. Model.4, 13 (2007).
- Vavricka SR , Van MontfoortJ , HaHR , MeierPJ , FattingerK. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology36(1), 164–172 (2002).
- Venkatesan K . Pharmacokinetic drug interactions with rifampicin. Clin. Pharmacokinet.22(1), 47–65 (1992).
- Lau YY , HuangY , FrassettoL , BenetLZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin. Pharmacol. Ther.81(2), 194–204 (2007).
- Backman JT , LuurilaH , NeuvonenM , NeuvonenPJ. Rifampin markedly decreases and gemfibrozil increases the plasma concentrations of atorvastatin and its metabolites. Clin. Pharmacol. Ther.78(2), 154–167 (2005).
- US Food and Drug Administration . Drug Development and drug interactions | table of substrates, inhibitors and inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (Accessed 18July2023).
- Zheng HX , HuangY , FrassettoLA , BenetLZ. Elucidating rifampin’s inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin. Pharmacol. Ther.85(1), 78–85 (2009).
