Abstract
Background: We have previously shown that subanesthetic ketamine infusions effectively reduce refractory pain. However, the effects of ketamine infusions on comorbid conditions of depression and anxiety have not been explored in this patient population. Methods: We investigated the effects of ketamine on mood and anxiety in patients with refractory chronic pain treated with 7–14 days of subanesthetic continuous intravenous ketamine infusions, using well-validated clinical scales. Results: There was a significant 52% reduction in pain severity and 33% reduction in pain interference scores following ketamine treatment. Ketamine treatment also reduced scores on the depression module of the Patient Health Questionnaire (PHQ-9) by 28% and scores on the Generalised Anxiety and Depression Assessment (GAD-7) by 36%. Conclusion: Multiday subanesthetic ketamine infusions effectively reduce pain, anxiety and depression in patients with complex chronic pain.
Plain language summary
What questions did we seek to answer?
Chronic pain is a common problem with limited effective treatments. We have previously shown that supervised, multiday ketamine treatments given in the hospital can effectively reduce pain levels, with benefit for months for patients with persistent chronic pain. However, pain is quite complex and often co-occurs with other conditions, particularly depression and anxiety. We aimed to investigate how our ketamine infusions would impact these two important classes of disorder. We assessed our patients with chronic pain undergoing ketamine treatments using thoroughly researched clinical measures of pain, mood and anxiety before and after the infusions.
What were the results?
Patients had significant reductions in the standardized measures of pain and anxiety, and improvements in mood. We statistically checked whether these reductions were related, and found that reduced pain levels did not correlate with improvements in mood and anxiety levels.
What do the results suggest?
Ketamine treatments given in a carefully monitored hospital setting are effective in reducing anxiety and improving mood in patients undergoing treatment for chronic pain. The absence of a correlation between the change in pain levels and the change in anxiety and depression levels suggests that ketamine might affect different parts of the brain to achieve these independent effects.
Tweetable abstract
A new study reveals multiday ketamine infusions in hospitals can effectively reduce pain while improving mood and reducing anxiety in patients suffering from refractory chronic pain.
Ketamine is a synthetic anesthetic agent with analgesic and, at higher doses, dissociative effects, first used in 1965 [Citation1]. There has been increasing interest in the last 15 years in using lower-dose ketamine for pain [Citation1–5] as well as for anxiety and depression [Citation5–7]. Currently, oral ketamine has limited benefit for pain, likely in part due to the poor oral bioavailability of ketamine, making intravenous delivery the main therapeutic route of administration [Citation1,Citation8]. We have previously reported our inpatient ketamine infusion program and demonstrated that patients undergoing 10–14 days of ketamine have significant reductions in pain and are able to reduce, or in some cases eliminate, opioid use [Citation9].
Patients with chronic pain are complex and often have medical comorbidities. Anxiety and depression are common comorbidities in patients with chronic pain, although the directional relationship between pain and the comorbidities are not well understood [Citation8]. In one cohort study, patients with chronic pain were more than twice as likely to develop an anxiety disorder and three-times as likely to develop a mood disorder [Citation8]. In fact, most patients referred to chronic pain clinics screen positive for depression using the depression module of the Patient Health Questionnaire (PHQ-9) screening tool [Citation10]. We believe that consideration of the effects of ketamine infusions on these psychiatric comorbidities needs further investigation.
The mechanism driving ketamine’s pain-reducing effects has been established in the literature. The N-methyl-d-aspartate receptor (NMDAR) is upregulated at the dorsal root ganglia of the spine and is a key driver of chronic pain [Citation1]. Ketamine is a potent NMDAR blocker, which is thought to be a major driver of ketamine’s pain-relieving properties. Interestingly, ketamine’s NMDAR blockade may also be important for its anxiolytic and antidepressant effects [Citation1]. However, ketamine’s psychotropic effects are thought to be secondary to its action at the level of the brain, increasing glutamatergic signaling and altering brain electrophysiology [Citation5,Citation7]. Alternatively, the psychoactive effects of ketamine may be driven by an NMDAR-independent induction of brain neuroplasticity, which counteracts pathological cognitive states [Citation6,Citation11]. A systematic review exploring the effects of ketamine on chronic pain and comorbid depression noted high heterogeneity and inconsistent study results, highlighting the challenges in understanding ketamine’s neurobiology [Citation5].
Recent Canadian guidelines do not recommend the use of ketamine for chronic pain conditions [Citation7]. However, a recent meta-analysis into the effectiveness of ketamine for chronic regional pain syndrome (CRPS) showed dramatic reductions in patient pain scores, an effect that was sustained for 3 months [Citation2]. Notably, the US FDA has granted ketamine infusions orphan drug status as a treatment under investigation for CRPS [Citation12]. A second systematic review of ketamine infusions for chronic pain showed that the pain reduction was sustained for up to 6 months [Citation3]. This second systematic review also highlighted evidence of a dose–response effect, with improved responses to cumulative doses of ketamine above 400 mg, although this effect did not reach statistical significance [Citation3]. There is significant heterogeneity in ketamine infusion studies in terms of duration and dose of therapy as well as limited comparison of efficacy of ketamine for different conditions [Citation11,Citation13]. To our knowledge, the effects of ketamine infusions of different durations on pain have not been explored within a single study [Citation13].
In this study we explored the effects of ketamine on well-validated clinical measures of pain, mood and anxiety in a new cohort of patients with refractory chronic pain. The Brief Pain Inventory (BPI) pain severity and pain interference scores were used to measure pain levels, and the PHQ-9 scale to assess mood and the Generalised Anxiety and Depression Assessment (GAD-7) to measure anxiety levels [Citation14–17]. Further, we explored the correlation between the effect of ketamine on these measures of pain and mental health. Lastly, we explored differences in our shorter and longer duration multiday ketamine infusions, ranging from 7 to 14 days.
Patients & methods
We performed a retrospective chart review assessing the effects of multiday ketamine infusions on pain, anxiety and mood. We included all patients from January 2020 to June 2022 admitted to St. Paul’s Hospital in Vancouver, British Colombia, Canada for the inpatient pain program for subanesthetic continuous ketamine infusions to manage chronic, nonmalignant pain. This patient population was not limited by the diagnosis underlying their chronic pain. All data presented in this study are available by request to the corresponding author. The main inclusion criteria were patients with treatment-refractory chronic pain who had not benefited from standard treatments with a high numerical pain rating scale score at baseline (>6). Exclusion criteria included inability to provide consent, pregnancy, past or present substance or alcohol use disorders, psychosis, acute suicidal ideation and abnormal ECG or documented coronary artery disease.
Our protocol for this inpatient subanesthetic ketamine infusion program has previously been published [Citation13]. Briefly, patients discuss the risks and benefits of inpatient ketamine infusion therapy in the outpatient setting prior to enrolling for therapy, and consent is provided to enroll in the study and our retrospective cohort study. Patients have a planned admission of 7–14 days and receive a ketamine intravenous infusion of 5 mg/h, irrespective of weight, which is titrated to a maximum of 30 mg/h as tolerated. Clonidine was prescribed as needed (0.1 mg every 6 h) for elevated blood pressure (>140 systolic), headache or withdrawal symptoms in patients using opioids. Intravenous ondansetron was provided as needed for nausea or vomiting. No other routine analgesic was used in the patients, although chronic treatments (excluding opioids, which were commonly reduced or stopped) were not altered. The duration of treatment was driven primarily by availability of space within our care unit, with longer stays planned for patients with a higher baseline level of pain.
All patients were assessed prior to admission by a pain specialist, the principal investigator of this study. Pain scores were assessed using a numerical rating scale ranging from 0 (no pain) to 10 (unbearable pain) at initial assessments and follow-up appointments. At admission and discharge, patients completed several questionnaires including a BPI [Citation14], PHQ-9 [Citation15,Citation16] and GAD-7 scale [Citation17], all of which have been validated in prior studies. The BPI was used to provide a pain severity score, which was calculated from the average of four ratings of pain severity both in the last 24 h at the time of assessment and in general, and a pain interference score calculated by the average of seven daily activities. The collected data included demographics (age, gender), maximum ketamine dose, total number of infusion days, adverse events and pain scores, and medications at admission, discharge and outpatient follow-up. Routine lab work, including complete white cell count, urine drug screen, renal and liver function, in addition to thyroid stimulating hormone (TSH) and ECG, was completed. Notably, no routine changes were made to any other psychotropic medication, including benzodiazepines, and antipsychotic and antidepressant medications, during the ketamine infusions. Comorbidities of our patient population were determined by chart review, with psychiatric diagnoses made by a physician included in our analysis. This project was approved by the University of British Columbia – Providence HealthCare Research Ethics Board. This manuscript was prepared following the STROBE guidelines [Citation18].
Statistical analysis
We used analysis of variance to test significance. We used Pearson correlations to assess for correlation between variables of interest. A multivariable linear regression was built on Python v. 3.10.9 to compare the change in pain severity, pain interference, PHQ-9 and GAD-7 scores, using pandas v. 1.5.2 to transform the Excel® data and stats models v. 0.13.5 to generate the linear models. In all our analyses our a priori level of significance was p < 0.05, with p < 0.0001-level significance added post hoc. Our hypothesis tests were all two-sided.
Results
Patient characteristics & clinical information
We included 61 patients in our analysis (). All patients received the full dose of ketamine (30 mg/h) and none withdrew from the treatment after initiation. Most of the patients were female (49; 80%). The average age of our cohort was 46.8 years (standard deviation: 8.2). Treatment duration ranged from 7 to 14 days, with an average treatment length of 10.2 days. The most common diagnosis in this patient cohort was fibromyalgia/central sensitization syndrome (n = 40; 66%). Many patients in our cohort had psychiatric comorbidities, with 23 patients (38%) with depression, 14 (23%) with anxiety, 7 (11%) with post-traumatic stress disorder and 3 (4%) with ADHD. Some of these comorbidities were in the same patients; 27 (44%) patients had no psychiatric comorbidities.
Table 1. Patient demographics and pain syndrome diagnosis.
Pain scores
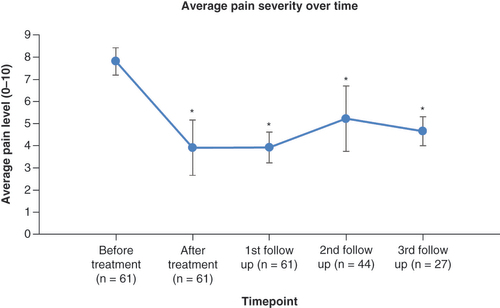
There was a significant reduction in pain severity scores of 52% and pain interference scores of 33% when comparing baseline scores with post-treatment scores (). There were dramatic reductions in the pain scores seen in patients across each category of chronic pain (fibromyalgia/central sensitization syndrome, CRPS, chronic headache and chronic neuropathic pain).
Table 2. Patient pain scores before and after treatment.
We compared the average pain score recorded in the BPI score to an average pain severity rating at follow-ups (while all patients had a first follow-up, only 44 had a second follow-up and 27 have had a third follow-up to date). We noted that pain levels remained significantly reduced from baseline, 2 weeks after discharge at first follow-up, 6 weeks after discharge at second follow-up and 10–12 weeks after discharge at third follow-up ().
Depression & anxiety scores
There was a significant reduction in PHQ-9 scores of 28% and GAD-7 scores of 36% when comparing baseline scores to post-treatment scores. There were dramatic reductions in the pain scores in patients with each category of chronic pain (fibromyalgia/central sensitization syndrome, CRPS, chronic headache and chronic neuropathic pain). The only change that did not reach significance was the reduction in GAD-7 scores of patients with chronic neuropathic pain (p = 0.089), likely due to the small sample size of five patients ().
Table 3. Patient mood and anxiety scores before and after treatment.
Correlation between pain, mood & anxiety scores
We explored the statistical correlation between our two pain scores (pain severity and pain interference) with our psychometric scores and found that these correlated very weakly with our measurements of mood (PHQ-9 scores) and anxiety (GAD-7 scores) both at baseline and following treatment. The change in the pain and psychometric scores after concluding treatment compared with their starting scores, similarly, had a very weak correlation. None of these correlations reached statistical significance. There was a moderate correlation between GAD-7 and PHQ-9 scores at baseline but a very weak correlation following treatment. The changes in PHQ-9 and GAD-7 scores were likewise only weakly correlated (). Notably, like the linear correlations, the only significant correlation identified in multivariable regression models assessing the change in pain severity, pain interference, PHQ-9 and GAD-7 scores was a weak-to-moderate correlation between GAD-7 and PHQ-9 scores.
Table 4. Correlation between baseline and post-treatment scores and changes in pain, anxiety and mood scores.
Response to different durations of ketamine infusion
Although there was a large range in treatment durations (from 7 to 14 days), there were only very weak correlations between pain severity, pain interference, GAD-7 scores and the duration of the ketamine infusion. In contrast, there was a weak but statistically significant correlation between the change in PHQ-9 score and the treatment duration ().
Table 5. Correlation between treatment duration and changes in pain, anxiety and mood scores.
We also compared the changes in scores of the patients who received 14 days of treatment compared with those who received only 7 days of treatment (). These patients all experienced similar reductions in pain severity, pain interference and GAD-7 scores. However, there was a significantly greater reduction in the PHQ-9 scores in the patients who received 14 days of treatment, with about a four-point greater reduction.
Table 6. Change in pain, anxiety and mood scores for 7- or 14-day ketamine infusions.
Opioid usage
Out of the 61 patients included in our study, 26 (43%) had documented opioid use on first admission. Of these initial 26 patients taking opioids, 11 (42%) discontinued all opioids, which was maintained on their first follow-up 2 weeks after discharge. Of the 15 patients who continued to require opioid therapy at the 2 week follow-up, eight (53%) reduced their opioid use compared with their dosage prior to admission for ketamine infusion.
Side effects
In this cohort, there were no serious side effects reported. Mild adverse events included nausea (20 patients), headache (10 patients), flushing (6 patients), chest discomfort (2 patients) and dizziness (1 patient). In total 38 patients (62%) had at least one reported adverse effect, while 23 patients had no adverse events reported. No patients required discontinuation of their ketamine infusions.
Discussion
We demonstrated that a subanesthetic ketamine infusion for 7–14 days in patients with chronic non-cancer-related pain results in clinically and statistically significant decreases in pain scores in addition to decreases in GAD-7 and PHQ-9 scores. There was an average of approximately 50% or four-point reduction in the Brief Pain Inventory (BPI) pain severity score, which is a clinically meaningful difference in a chronic pain population [Citation19]. Similarly, the average reduction of the BPI pain interference score of approximately two points or 33% following ketamine infusion is also clinically significant [Citation20], which suggests reduced burden of disability from pain. We redemonstrated that the pain reduction is persistent at follow-up appointments. We also observed a clinically significant average reduction in both our psychiatric measures, with a reduction in the PHQ-9 scores of approximately six points or 25% [Citation21] and a reduction in GAD-7 scores of approximately five points or 33% [Citation22]. These results using well-validated tools reinforce the existing evidence in the literature that ketamine infusion is a robust antidepressant and anti-anxiety treatment [Citation5,Citation6]. This makes ketamine a powerful treatment, given that patients with chronic pain often have comorbid anxiety and depression. Our patient group undergoing this treatment had a high rate of these two psychiatric diagnoses. In addition, these results build on our previous study to highlight that ketamine infusion not only reduces pain levels but also improves patient functioning [Citation13].
These dramatic reductions in pain, anxiety and depression scores were observed across all categories of chronic pain populations, including fibromyalgia/central sensitization syndrome, CRPS, chronic headache and chronic neuropathic pain. We have hypothesized previously that ketamine may be an effective treatment in chronic pain given its mechanism of action via ‘resetting’ of the blockade of NMDAR, which is a driver of central sensitization at the level of the dorsal root ganglion of the spine [Citation1].
Interestingly, the change in our pain scores including both pain severity and pain interference were not significantly correlated with psychometric scores (PHQ-9 and GAD-7). The changes in GAD-7 and PHQ-9 scores, in contrast, were weakly correlated. This lack of correlation between the effect of the ketamine infusion on pain reduction and the effect of the infusion on improving psychiatric measures is suggestive of an independent and different mechanism of action for these two effects. While a weak correlation may have been missed, given the relatively small number of patients studied, we hypothesize that the lack of direct interdependence of ketamine’s psychiatric and pain benefits supports clinical and preclinical data. Other data have indicated that ketamine’s antidepressant effects may be dependent on the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) and serotonin receptors, rather than the NMDAR that is crucial for ketamine’s role in modulating chronic pain [Citation23]. The importance of the serotonin receptor was highlighted in a recent PET study, where the local increase in serotonin receptor binding within the hippocampus was thought to be a key marker of response to ketamine therapy in major depression [Citation23]. Downstream signaling from AMPA receptor potentiation is also thought to be an important effect of ketamine for anxiety [Citation6]. Although the precise mechanisms of action for ketamine’s reduction of chronic pain, anxiety and depression are not fully elucidated, our data suggest that these may be driven by independent biological pathways.
The most common diagnosis in our patient population was fibromyalgia/central sensitization syndrome. A recent systematic review on ketamine infusions for fibromyalgia, which included 84 patients from four small randomized clinical trials, showed that short-term ketamine infusions lasting a maximum of 30 min could decrease pain for hours to days without a long-term reduction in pain scores [Citation24]. Our population had a much longer sustained benefit with the multiday infusion program, in keeping with two case reports showing improved sustained response to ketamine with multiday treatment programs [Citation23,Citation25,Citation26]. Although there is clear benefit of a multiday infusion, a 2-week treatment protocol is likely not necessary for all patients. While there is some benefit with extended ketamine infusions for mood, the shorter-duration ketamine infusion, as short as 7 days, appeared to provide similar reductions in pain and anxiety. These shorter treatments are less resource-intensive. There may be additional benefits for the treatment of comorbid mood disorders with longer treatment courses. Notably, despite the shorter treatment courses than in our past study on ketamine infusions [Citation9], we continued to see a sustained pain-reduction effect at 10–12 weeks’ follow-up. Taken together, our findings and the existing literature highlight that there is significant benefit of a multiday ketamine infusion program over single infusions. Treatment courses of 7 days of infusion are sufficient to achieve maximal and sustained pain reduction.
There was also a dramatic reduction in the number of patients using opioids in our study following the ketamine infusion, despite the reduced time course. Of the patients who were initially taking opioids, 42% eliminated their use of opioids by the time of their discharge from hospital, with a further 52% of patients who continued to use opioids able to maintain a reduced dose. The reduction in opioid use is consistent with our prior report [Citation9] and a systematic review of subanesthetic ketamine infusions for pain [Citation27], which also reported dramatic reductions in opioid use following ketamine infusions.
There were no serious safety concerns in our patient population, although mild adverse events including nausea, headache and flushing were common. The rate of adverse events observed in this study is similar to the rate we identified in a separate cohort of patients in our previous ketamine infusion report [Citation9] and the rate of side effects highlighted in a systematic review of subanesthetic ketamine infusions for pain [Citation27].
We utilized a retrospective cohort analysis as an efficient method for studying ketamine effects. Attempted blinding of participants in ketamine randomized controlled trials appears to be ineffective given that the psychotropic effects of ketamine tend to functionally unblind the patients receiving this therapy [Citation3]. The absence of a control population allowed a larger cohort of patients to use in our analysis, with all patients receiving the improved outcomes associated with this therapy.
There were several limitations in our study. Although a retrospective cohort analysis is an efficient method for studying the effects of ketamine, it is an uncontrolled study design, which is subject to possible biases. Changes to pain scores may have been influenced by other factors beyond the ketamine, including the impact of a brief hospitalization with close care or concurrent medications administered to patients. Furthermore, we have not administered our pain inventory scales and psychometric assessments on follow-up visits and currently it is not clear whether the beneficial effects of our ketamine infusions, and particularly the beneficial psychiatric effects, are long-standing or transient. Moreover, some patients had missing pain scales for their second and third follow-ups. Lastly, our sample size was relatively small and not selected with a sample size calculation due to the practical constraints of running a resource-intensive inpatient hospital service with limited patient beds available. Patients also had differing underlying diagnoses. Nevertheless, we demonstrate that in our patient population with refractory pain there are significant reductions in pain severity and interference scores in addition to psychometric measures following 7–14 days of intravenous ketamine infusion, using a treatment regimen which was safe and well tolerated. Given the benefits of multiday ketamine infusions in our patient population, we believe performing a prospective double-blind randomized controlled study using our treatment protocol is warranted to better characterize the effects of ketamine on chronic pain, mood and anxiety in a highly controlled setting.
Conclusion
Overall, we highlight that subanesthetic ketamine infusions for 7–14 days significantly reduce measures of pain, anxiety and depression in patients with complex, chronic, non-cancer pain following treatment. The psychiatric and pain-modulating effects of this therapy appear to be independent, which suggests a differing mechanism of action for these therapeutic effects. In addition to effects on pain severity and interference, we found a significant decrease in opioid usage post-infusion. The benefits of the ketamine infusion following treatment were largely independent of the variations in treatment duration in our cohort. We believe a randomized controlled trial of multiday ketamine infusions for chronic pain, particularly in patients with mood or anxiety comorbidities, would be the best next step to assess the efficacy of ketamine.
There is an urgent need to develop new pain therapies that can provide longstanding pain relief and aid in common pain comorbid illnesses like anxiety and depression. We believe ketamine might be one such option that can be used more in the future for severe or refractory cases of chronic pain. Further, we believe understanding ketamine from a mechanistic perspective may be an avenue to develop novel targeted pain, anxiety and depression medications.
We have previously shown ketamine to be effective for the treatment of pain and the reduction of opioid use.
Depression and anxiety are common comorbidities of chronic pain.
There have been concerns raised about the affective side-effect profile of ketamine.
We run a clinic for patients with refractory pain, with a high rate of comorbid depression and anxiety.
Herein, using well-validated clinical tools, we show that ketamine effectively reduces pain, anxiety and depression levels.
In a closely monitored setting as inpatients, our patients did not experience significant adverse effects.
The effects of ketamine on mood and anxiety did not correlate with the response to pain.
The absence of correlation is consistent with different molecular mechanisms of action for the psychological and pain-modulation effects of ketamine.
Author contributions
All authors contributed substantially to the conception and design of this project, including in drafting and revision of the article. All authors granted final approval and are responsible for the content included in the manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors would like to acknowledge the facilities and support provided by St. Paul’s Hospital.
Financial & competing interests disclosure
S Marzoughi received the Ludmila & Henry Zeldowicz Award 2022 from the University of British Columbia in part to support his research efforts. D Ripsman received financial support from the Dr Barbara Allan Scholarship in Medicine 2022 from the University of British Columbia in part to fund his research efforts. This research received no grants from any funding agency in the public, commercial, or not-for-profit sectors. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Noppers I , NiestersM , AartsL , SmithT , SartonE , DahanA. Ketamine for the treatment of chronic non-cancer pain. Expert Opin. Pharmacother.11(14), 2417–2429 (2010).
- Zhao J , WangY , WangD. The effect of ketamine infusion in the treatment of complex regional pain syndrome: a systemic review and meta-analysis. Curr. Pain Headache Rep.22(2), 12 (2018).
- Orhurhu V , OrhurhuMS , BhatiaA , CohenSP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth. Analg.129(1), 241–254 (2019).
- Balachandran A , TassoneVK , AdamsahibFet al. Efficacy of ketamine for comorbid depression and acute or chronic pain: a systematic review. Front. Pain Res.3, 1022767 (2022).
- Tully JL , DahlénAD , HaggartyCJ , SchiöthHB , BrooksS. Ketamine treatment for refractory anxiety: a systematic review. Br. J. Clin. Pharmacol.88(10), 4412–4426 (2022).
- Marcantoni WS , AkoumbaBS , WassefMet al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019. J. Affect. Disord.277, 831–841 (2020).
- Tran K , McCormackS. Ketamine for Chronic Non-Cancer Pain: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines.Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada (2020). www.ncbi.nlm.nih.gov/books/NBK564230/
- Pereira FG , FrançaMH , PaivaMCA , AndradeLH , VianaMC. Prevalence and clinical profile of chronic pain and its association with mental disorders. Rev. Saude Publica51, 96 (2017).
- Chebini A , MarzoughiS , RandhawaJet al. The effects of a multiday (10–14 days) subanesthetic dose IV ketamine infusion in the treatment of refractory chronic pain. Pain Manag.12(3), 337–346 (2022).
- Rayner L , HotopfM , PetkovaH , MatchamF , SimpsonA , McCrackenLM. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. Pain157(7), 1472–1479 (2016).
- Falk E , SchlieperD , van CasterPet al. A rapid positive influence of S-ketamine on the anxiety of patients in palliative care: a retrospective pilot study. BMC Palliat. Care19(1), 1 (2020).
- US Food and Drug Administration . Orphan drug designations and approvals: ketamine (2022). www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=883122
- Maher DP , ChenL , MaoJ. Intravenous ketamine infusions for neuropathic pain management: a promising therapy in need of optimization. Anesth. Analg.124(2), 661–674 (2017).
- Tan G , JensenMP , ThornbyJI , ShantiBF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J. Pain5(2), 133–137 (2004).
- Kroenke K , SpitzerRL , WilliamsJB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med.16(9), 606–613 (2001).
- Seo JG , ParkSP. Validation of the Patient Health Questionnaire-9 (PHQ-9) and PHQ-2 in patients with migraine. J. Headache Pain16, 65 (2015).
- Löwe B , DeckerO , MüllerSet al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med. Care46(3), 266–274 (2008).
- Vandenbroucke JP , von ElmE , AltmanDGet al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLOS Med.4(10), e297 (2007).
- Mease PJ , SpaethM , ClauwDJet al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res.63(6), 821–826 (2011).
- Dworkin RH , TurkDC , WyrwichKWet al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain9(2), 105–121 (2008).
- Löwe B , UnützerJ , CallahanCM , PerkinsAJ , KroenkeK. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med. Care42(12), 1194–1201 (2004).
- Toussaint A , HüsingP , GumzAet al. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J. Affect. Disord.265, 395–401 (2020).
- Tiger M , VeldmanER , EkmanCJ , HalldinC , SvenningssonP , LundbergJ. A randomized placebo-controlled PET study of ketamine’s effect on serotonin1B receptor binding in patients with SSRI-resistant depression. Transl. Psychiatry10(1), 159 (2020).
- Pastrak M , Abd-ElsayedA , MaF , VroomanB , VisnjevacO. Systematic review of the use of intravenous ketamine for fibromyalgia. Ochsner J.21(4), 387–394 (2021).
- Zanicotti CG , PerezD , GlueP. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J. Palliat. Med.15(4), 400–403 (2012).
- Amin P , RoelandE , AtayeeR. Case report: efficacy and tolerability of ketamine in opioid-refractory cancer pain. J. Pain Palliat. Care Pharmacother.28(3), 233–242 (2014).
- Zekry O , GibsonSB , AggarwalA. Subanesthetic, subcutaneous ketamine infusion therapy in the treatment of chronic nonmalignant pain. J. Pain Palliat. Care Pharmacother.30(2), 91–98 (2016).