?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: Retrospective insurance claims analysis exploring treatment characteristics in chronic low back pain patients prescribed buprenorphine buccal film (Belbuca®) or transdermal patches. Patients and methods: The first buprenorphine prescription (buccal film or transdermal patch) was an index event. Patients were observed over 6 month pre- and post-index periods. Propensity score matching minimized the selection bias. Results: Buccal film patients had a higher buprenorphine daily dose (501.7 vs 270.9 µg; p < 0.001). The patch-to-film switching rate was higher than vice versa (11.5 vs 3.8%; p < 0.001). The buccal film showed a greater reduction in opioid prescriptions (-1.1 vs -0.7; p = 0.012), daily morphine milligram equivalents (-12.6 vs -7.3; p < 0.001) and opioid treatment duration (-13.4 vs -7.6 days; p = 0.022). Conclusion: Buccal film was associated with higher buprenorphine doses and a greater reduction of opioid burden.
Plain language summary
What is this article about?
The analysis explored treatment patterns in chronic low back pain patients treated with different buprenorphine drugs. The use of other pain medications was also evaluated. Buprenorphine buccal film (Belbuca®) was compared with transdermal patches. This study used commercial insurance data of US patients.
What were the results?
The most relevant findings were:
Patients using buccal film had about two-times higher buprenorphine daily doses.
About 12% of patch patients switched to film, while approximately 4% of film patients switched to patch.
Initiation of both buprenorphine drugs led to reduced usage of opioids and other pain drugs.
Despite a shorter buprenorphine treatment, the film was associated with a greater reduction in opioid use than the patch.
What do the results of the study mean?
The results showed that patients prescribed buprenorphine buccal film would be able to achieve higher daily doses required for appropriate chronic low back pain management. The buccal film will also lead to a great reduction in concomitant opioid use. These advantages may explain why more patients switched from buprenorphine transdermal patch to buccal film than the other way around.
Tweetable abstract
#Lowbackpain patients on #buprenorphine buccal film (#Belbuca) had greater daily doses and reduction in #opioid use and daily #MME than those on buprenorphine #transdermalpatch. More patients switched from patch to film than vice versa.
Chronic low back pain (cLBP) is a commonly diagnosed disease in the US population with a great impact on patients’ quality of life [Citation1,Citation2]. cLBP management requires long-term around-the-clock analgesia, usually achieved with the concomitant use of numerous medications and medical interventions. Due to heavy and prolonged treatment, the condition is associated with a very high economic burden [Citation3–5].
The most recent treatment guidelines in the USA recommend a multidisciplinary approach for cLBP management. Mild-to-moderate cLBP cases should initiate treatment with noninvasive procedures and over-the-counter products such as psychotherapies, clinician-directed exercise programs, lifestyle modifications, topical capsaicin gel and NSAIDs. Severe cLBP requires more optimized therapy and treatment combinations like prescription non-opioid analgesics, short-acting opioids (SAOs) and long-acting opioids (LAOs), adjuvant analgesics (antidepressants, anticonvulsants etc.) and surgical procedures [Citation6–8]. The main goal of cLBP management is finding effective treatment with acceptable safety to improve patient outcomes such as pain relief and physical functioning [Citation9,Citation10]. Opioid medications are commonly required to achieve this goal, especially if patients do not respond well to other non-opioid treatments. Approximately 80% of cLBP opioid users take the medications for at least 1 year, frequently coadministered with other rescue analgesics [Citation11,Citation12]. Although opioids provide efficacy in cLBP relief for many, their use is associated with a greater incidence of treatment-related adverse events. This requires a close monitoring for serious events such as opioid use disorder (OUD) as well as life-threatening conditions (respiratory depression, accidental overdosing etc.) [Citation9,Citation10,Citation13].
Buprenorphine is an atypical opioid approved for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment. Buprenorphine has unique pharmacological characteristics compared with other standard opioid medications due to its partial agonist activity at μ opioid receptors [Citation14,Citation15]. Besides proven efficacy in chronic pain management, buprenorphine was categorized as a Schedule III (CIII) substance by the US Drug Enforcement Administration due to a lower potential for risk and abuse [Citation14,Citation15]. Buprenorphine’s main limitation is low oral bioavailability due to an extensive N-dealkylation by CYP3A4 liver enzymes leading to rapid glucuronidation and inactivity. Novel formulations such as transdermal patch systems and buccal films have been developed to avoid first-pass metabolism and provide long-term analgesia to cLBP patients [Citation14,Citation15]. However, the impact of these formulations on chronic pain management has not been directly compared in the published literature.
This study aimed to explore the real-world clinical practice of cLBP management among patients treated with buprenorphine buccal film (Belbuca®, Collegium Pharmaceutical, Inc., MA, USA) or buprenorphine transdermal patches by observing opioid (opioids other than CIII buprenorphine, in further text ‘opioid’) and non-opioid rescue medication utilization and CIII buprenorphine treatment characteristics.
Materials & methods
The reporting was aligned with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement checklist for observational cohort studies [Citation16].
Data source
This retrospective cohort study was conducted using US insurance claims data from the IBM MarketScan® Commercial Claims Database. This database consists of medical and drug data for over 215 million individuals, encompassing employees, their spouses and dependents who are covered by employer-sponsored private health insurance in the USA [Citation17]. The study was performed on the insurance data claims captured in a period from 1 January 2018 to 31 December 2021.
Study population
The study population was identified based on the predefined inclusion/exclusion criteria with the aim to ensure a homogeneous pool of cLBP patients prescribed buprenorphine buccal film or transdermal patches.
Inclusion criteria
Patients prescribed buprenorphine buccal film or transdermal patches (regardless of dosage);
Adults ≥18 years of age;
At least two claims with a diagnosis of low back pain based on the International Classification of Diseases – Clinical Modification (ICD-10-CM) codes (Supplementary Table 1) before the first CIII buprenorphine prescription.
Exclusion criteria
A gap in the health-plan or pharmaceutical coverage during the observational period;
Patients with a diagnosis of OUD during the observational period (Supplementary Table 2).
Switching between the buprenorphine buccal film and transdermal patch formulations within the cohorts was allowed during the post-index period.
Study design
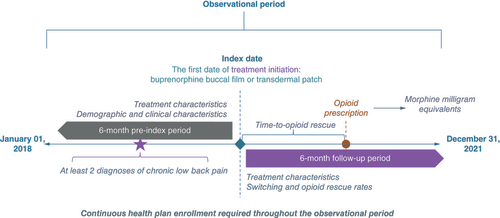
Patients diagnosed with cLBP and treated with buprenorphine buccal film or transdermal patches were captured in the database based on the relevant National Drug Codes (Supplementary Table 3). The date of the first buprenorphine prescription (buccal film or patch) in the database was assigned as the index date. The observational period consisted of 6-month pre-index (prior to the index date) and 6-month post-index periods (after the index date). Patients were required to be without diagnosis of OUD and continuously insured (healthcare and pharmaceutical coverage) during the whole observational period. Demographic characteristics of the study population were assessed on the index date, while clinical characteristics were evaluated during the 6-month pre-index period. Based on the initially prescribed product and the route of administration, patients were stratified in two separate cohorts: those treated with buccal film and those treated with any buprenorphine transdermal patch. The study design is presented in .
Outcome measures
CIII buprenorphine treatment characteristics were assessed during the 6-month follow-up period, while opioid and non-opioid rescue medication utilization trends were evaluated during the 6 month pre-index and 6 month follow-up periods.
CIII buprenorphine treatment characteristics
CIII buprenorphine treatment characteristics consisted of measures related to CIII buprenorphine use such as prescription counts, daily doses, treatment duration and switching outcomes.
The buprenorphine average daily dose (BADD; mcg/day) was calculated based on the formulation strength, number of units dispensed and the number of medication supply days captured in the database. All claims with missing relevant data were excluded from the analysis. The following formulae were employed to calculate the BADD among buccal film- and transdermal patch-treated patients:
where n is the total number of prescriptions per patient.
A treatment switch was defined as an event during the follow-up when patients had a different buprenorphine formulation of interest from the one originally initiated on the index date. In other words, this was an event when a patient in the buccal film cohort had a buprenorphine patch prescription claim or vice versa. Switching rates, time to switch and BADD before the switch were explored and compared between study cohorts.
Rescue medications utilization
Alongside CIII buprenorphine treatment, cLBP patients also used opioid and non-opioid rescue medications. For the purpose of this study, only claims for oral formulations of SAO, LAO and non-opioid rescue medications were identified in the database (Supplementary Table 4). The most relevant non-opioid rescue medications were selected from the cLBP treatment guidelines and included NSAIDs (comprehensively captured in the database using therapeutic class codes; Supplementary Table 5), topiramate, duloxetine, gabapentin and pregabalin (captured with corresponding National Drug Codes) [Citation6–8].
The main study outcome measures were related to opioid use and included opioid prescription counts, opioid treatment duration, daily morphine milligram equivalents (MME), the proportion of patients treated with opioids (opioid rescue rate), time to opioid rescue from the index date and the number of CIII buprenorphine prescriptions before the opioid rescue. The most relevant measures were also explored in SAO and LAO subgroups separately. Additionally, non-opioid rescue medication use outcomes among buprenorphine buccal film and transdermal patch patients were also explored (number of non-opioid prescriptions and non-opioid rescue rates).
MME calculation is based on the opioid daily dose calculated from the strength of opioid medication, the number of opioid units taken daily and the conventional MME conversion factor whose value depends on the generic name of the opioid prescription (sources: CDC and University of Utah; Supplementary Table 6) [Citation9,Citation18]. All claims with missing relevant data were excluded from the analysis. The following formulae were used to estimate the average daily MME in buprenorphine buccal film and transdermal patch cohorts:
where n is the total number of prescriptions per patient.
The impact of CIII buprenorphine initiation on the rescue medication utilization rates was assessed by within-group comparison of average prescription counts, daily MMEs and opioid treatment duration in pre-index (before buccal film and transdermal patch treatment) versus post-index periods (after buccal film and transdermal patch introduction). To fully depict the influence of different buprenorphine formulations on rescue medication use, these changes were compared between the study cohorts.
Statistical analysis
Continuous variables were summarized as means and standard deviations, while categorical variables were summarized as numbers and proportions of the sample. Independent t-test (continuous variables) and χ-square test of independence (categorical variables) were performed to test the difference between the comparable cohorts. A paired-sample t-test was used to assess the differences between the 6 month pre-index period versus the 6 month post-index period within the study cohorts. Between-cohort differences regarding the changes in rescue medication utilization after CIII buprenorphine initiation were tested via independent t-test and validated with mixed model analysis of variance. Statistical significance was determined by two-sided p-values < 0.05 between the cohorts.
Propensity score matching (PSM) with the nearest-neighbor matching algorithm, 1:1 ratio and 0.001 caliper was performed to minimize the selection bias. The demographic characteristics of patients observed on the index date and clinical characteristics of patients observed throughout the pre-index period were used as a basis for the matching process.
All analyses were performed using SPSS® software (v. 23; IBM Corp., NY, USA). The latest version is available for download at the official IBM website [Citation19].
Results
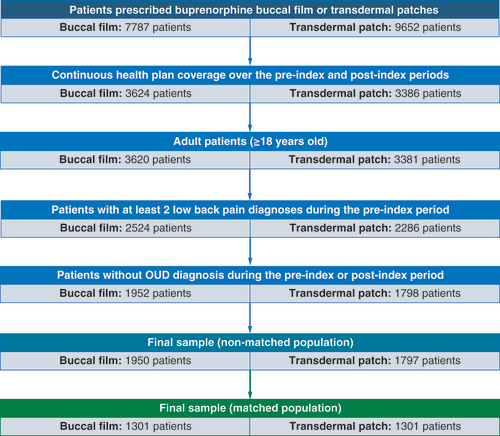
Out of 17,439 patients prescribed buprenorphine buccal film or transdermal patches, 3747 were included in a final nonmatched sample after applying inclusion/exclusion criteria (1950 and 1797 patients in the buccal film and buprenorphine patch cohorts, respectively). After PSM analysis, the final sample of matched patients consisted of 2602 patients (1301 patients in each cohort). The patient selection flow diagram is presented in .
Nonmatched population
The total nonmatched sample consisted of 3747 patients with 1950 patients initially prescribed buccal film and 1797 patients initially prescribed buprenorphine transdermal patches.
There were no significant differences in age (50.1 years in both) and gender (35.9% males in buccal film group and 32.9% males in patch group) characteristics between the study cohorts. The proportion of buprenorphine buccal film patients covered with a comprehensive health plan was lower compared with buprenorphine patch patients (2.4 vs 5.1%; p < 0.001), while the percentage of patients on a consumer-driven health plan was higher (14.4 vs 10.5%; p < 0.001). Also, buccal film patients more commonly resided in south regions (71.1 vs 57.5%; p < 0.001) and were less distributed across north central (13.5 vs 20.1%; p < 0.001) and west regions (8.7 vs 16.3%; p < 0.001). There were no statistical differences between cohorts among other health plans and regions of residence. The full list of demographic characteristics is shown in Supplementary Table 7.
Buccal film was associated with a significantly lower mean Charlson Comorbidity Index (CCI) (0.9 vs 1.1; p = 0.002) and lower proportion of patients with four or more comorbidities from the CCI list (5.6 vs 7.9%; p = 0.006) than buprenorphine patch. Except for myocardial infarction (1.5% in buccal film vs 0.8% in patch; p = 0.042), buprenorphine patch patients were more commonly diagnosed during the pre-index period with peptic ulcer disease (1.7 vs 0.8%; p = 0.011), diabetes without (17.3 vs 14.1%; p = 0.007) and with chronic complications (8.3 vs 6.6%; p = 0.037), diabetic neuropathy (11.4 vs 8.5%; p = 0.003), malignancies (5.8 vs 4.2%; p = 0.021) and metastatic solid tumors (1.4 vs 0.6%; p = 0.016), depression (30.8 vs 25.9%; p = 0.001) and fibromyalgia (16.1 vs 13.6%; p = 0.036) than buccal film-treated patients. The full list of comorbidities captured during the 6-month pre-index period in both cohorts is reported in Supplementary Table 8.
Matched population
A total number of 2602 patients were included in the final sample of matched patients (1301 patients in each cohort). Patients were well balanced between the study cohorts and there were no differences in patients’ characteristics that could impact study outcome measures.
There were no differences observed in the demographic characteristics between the cohorts in the final population sample (Supplementary Table 9). Patients were aged approximately 50 years, with a similar distribution of females in both cohorts (∼68.0%). A majority of patients were insured by preferred provider organization healthcare insurance (60.4% in both cohorts) and resided in the south region (∼69.0%).
Over half of patients (∼60.0%) in both study cohorts had a CCI score of 0, with chronic pulmonary disease and diabetes mellitus without chronic complications reported as the most frequent comorbidities from all CCI components. Of the mental disorders, anxiety and depression were captured as the most commonly occurring complications, and of the other chronic pain comorbidities, almost all patients were reported to have a diagnosis of other spine disorders. The full list of all clinical characteristics is given in Supplementary Table 10.
Patients in the buccal film cohort were treated with a significantly higher BADD than patients treated with buprenorphine patch (501.7 vs 270.9 mcg; p < 0.001). It was demonstrated that there is a longer treatment duration for the buprenorphine patch- versus buccal film-treated patients (91.9 vs 87.2 days; p = 0.043), with a significantly higher number of CIII buprenorphine prescriptions (3.7 vs 3.4; p = 0.011). Otherwise, it was observed that a significantly higher proportion of patients in the buprenorphine patch cohort switched to buccal film treatment than vice versa (11.5 vs 3.8%; p < 0.001). Time to switch was similar among study cohorts, being approximately 70 days from index date to CIII buprenorphine treatment switch ().
Table 1. CIII Buprenorphine treatment characteristics and switching trends in a matched population.
The utilization of opioid and non-opioid rescue medications was assessed during the 6-month pre-index and 6-month follow-up periods to fully depict and examine how they were impacted by CIII buprenorphine treatment initiation.
Patients in the buccal film cohort were heavily pretreated with opioids (both SAOs and LAOs) compared with the buprenorphine transdermal patch cohort (). Buccal film patients were treated with higher daily MMEs during the pre-index period (51.5 vs 39.8 MMEs; p < 0.001), with a significantly higher number of opioid prescriptions (5.7 vs 4.9; p < 0.001) and longer opioid treatment duration (104.2 vs 89.8 days; p < 0.001) versus buprenorphine patch patients. The proportion of patients prescribed opioid treatment during the 6-month pre-index period was similar in both cohorts (86.6 vs 85.5%; p = 0.396). Furthermore, buprenorphine buccal film patients were treated with a significantly higher number of SAOs versus the buprenorphine patch cohort (4.8 vs 4.4; p = 0.005), but the proportion of patients who were prescribed SAOs was equally distributed between the cohorts (84.2 vs 84.6%; p = 0.787). Otherwise, a significantly higher proportion of patients in the buccal film cohort were prescribed LAOs versus the buprenorphine patch cohort (21.2 vs 13.0%; p < 0.001), with a higher number of LAO prescriptions (0.9 vs 0.5; p < 0.001) in the pre-index period.
Table 2. Opioid utilization and treatment characteristics during the 6-month pre-index period in the matched population.
The trend of non-opioid rescue medication prescribing rates during the pre-index period was completely opposite to the trend of opioid utilization (). It was shown that a higher proportion of buprenorphine patch patients utilized non-opioid rescue medications compared with buccal film patients (84.0 vs 80.8%; p = 0.031), with a higher number of prescriptions observed during the pre-index period (5.2 vs 4.8; p = 0.021). More specifically, buprenorphine patch patients were prescribed more NSAIDs (59.6 vs 55.4%; p = 0.029) and gabapentin/pregabalin medications (53.9 vs 49.0%; p = 0.013) than buccal film patients.
Table 3. Non-opioid rescue medication utilization during the 6-month pre-index period in the matched population.
During the 6-month post-index period (), patients in the buccal film cohort were treated with opioids for a significantly longer period (90.8 vs 82.2 days; p = 0.002), with a higher number of opioid prescriptions (4.6 vs 4.2; p = 0.004) and greater daily MMEs (38.9 vs 32.5; p < 0.001) as compared with the buprenorphine patch cohort. The proportion of patients who had opioid treatment (completely abandoned CIII buprenorphine treatment or concomitantly used opioids) was similar between the study cohorts (78.9 vs 76.8%; p = 0.186). Time to opioid treatment and number of CIII buprenorphine prescriptions before opioid treatment were similar between study cohorts. The differences in opioid prescribing rates were mainly driven by a higher proportion of patients prescribed LAOs in the buccal film cohort (13.0 vs 9.4%; p = 0.003), who were treated with a significantly higher number of LAO prescriptions (0.5 vs 0.3; p < 0.001) than buprenorphine patch patients.
Table 4. Opioid utilization and treatment characteristics during the 6-month post-index period in the matched population.
There were no differences noted between study cohorts in the utilization of non-opioid rescue medications during the 6-month post-index period (). The highest proportion of patients in both cohorts utilized NSAIDs (50.9 vs 54.7%; p = 0.050), but there were no differences observed between the cohorts.
Table 5. Non-opioid rescue medication utilization during the 6-month post-index period in the matched population.
Both treatments (buprenorphine buccal film or transdermal patch) demonstrated significant benefits in terms of decreased utilization of opioid and non-opioid rescue medications during the 6-month post-index period compared with the 6-month pre-index period (). Patients in both cohorts were treated with opioids for a significantly shorter period, with a lower number of opioid prescriptions and lower daily MMEs in the 6-month post-index versus 6-month pre-index period (all p-values < 0.05).
Table 6. Changes in rescue medication utilization after CIII buprenorphine treatment initiation in the matched population.
Between-group comparison demonstrated that buccal film treatment achieved greater reductions in opioid utilization during the 6-month post-index period than buprenorphine patch treatment (). It was shown that buccal film treatment caused significantly greater reduction in opioid treatment duration (-13.4 vs -7.6 days; p = 0.022), number of opioid prescriptions (-1.1 vs -0.7; p = 0.012) and daily MME doses (-12.6 vs -7.3; p < 0.001) than buprenorphine patch in the 6-month follow-up period compared with the 6-month pre-index period. More specifically, a greater reduction in the number of LAO prescriptions was noted in the buccal film cohort. There were no significant differences in the non-opioid rescue medication and SAO reduction rate between study cohorts.
Table 7. Between-group comparison of rescue medication utilization changes after CIII buprenorphine initiation in the matched population.
Discussion
This retrospective study demonstrated that cLBP patients in the buccal film cohort were treated with significantly higher CIII buprenorphine daily doses on average (501.7 vs 270.9 mcg; p < 0.001) but received a lower number of CIII buprenorphine prescriptions during a shorter time frame than buprenorphine patch patients. In addition, a significant reduction of opioid and non-opioid rescue medication utilization was observed within both cohorts during the 6-month pre-index versus 6-month post-index periods (all p < 0.05). Comparing these changes between the study cohorts, it was demonstrated that buprenorphine buccal film treatment achieved a greater reduction in utilization of LAOs and opioids in general (regardless of the type) than the buprenorphine patch, with a greater decrease of daily MMEs, number of opioid prescriptions and opioid treatment duration (all p < 0.05).
Based on the publicly available literature, the treatment for cLBP mainly consists of SAOs and LAOs that provide long-term, around-the-clock pain management. A systematic literature review and meta-analysis on opioid use in cLBP concluded that there is moderate-quality evidence of opioid effects in the short term and high-quality evidence in the intermediate term [Citation20]. Altman et al. demonstrated that individualized treatment with oxymorphone, morphine sulfate and oxycodone achieved effective pain relief and improved sleep quality in cLBP patients [Citation21]. Moreover, the latest Cochrane Review reported there was no evidence of superiority in reducing pain intensity for acetaminophen, muscle relaxants and antidepressants compared with placebo, while there was moderate-certainty evidence favoring strong opioids and low-certainty evidence favoring NSAIDs over placebo in cLBP treatment [Citation22].
Although opioids are an effective treatment option to cope with cLBP, frequent use can be followed by severe and serious adverse events [Citation23]. The CDC recognized the necessity to optimize opioid utilization in cLBP management with the aim of minimizing treatment-related risks and providing the highest benefits to patients. One of the main goals of the opioid optimization strategy was to reduce the number of persons who develop OUD or experience overdose or other opioid-related adverse events [Citation24]. As an atypical opioid with unique characteristics, CIII buprenorphine has been proven to be an effective cLBP treatment option with substantial safety advantages over standard Schedule II opioids, especially in terms of opioid abuse, poisoning and dependence. Based on the preclinical and clinical evidence, an expert opinion panel supported transitioning strategies from Schedule II opioids to CIII buprenorphine whenever possible [Citation14]. Our study demonstrated the benefits of buccal film treatment by reducing opioid daily MMEs and opioid treatment duration. Therefore, buprenorphine buccal film could be potentially recognized as a valuable tool for opioid optimization strategies.
Several randomized clinical trials of CIII buprenorphine medications in cLBP patients demonstrated dose-dependent analgesia. Gordon et al. observed that patients treated with 20 mcg/h buprenorphine patches had greater pain reduction (measured with a visual analog scale pain intensity score) compared with those receiving 5 mcg/h treatment. Based on the results, the authors pointed out the importance of the titration-to-effect approach and the efficacy advantages of products that are available in wider dosing ranges [Citation25]. Other studies also noted lower pain scores, higher rates of treatment responders and better quality of life among patients treated with higher (20 mcg/h) than lower (5 mcg/h) buprenorphine patch doses [Citation26,Citation27]. Buprenorphine buccal films also demonstrated dose-dependent analgesia in randomized clinical trials of cLBP. Placebo-controlled studies including moderate-to-severe cLBP patients treated with buccal film showed significant analgesic effects of different dosing regimens. Buprenorphine buccal film was superior to placebo in reducing pain severity, with significantly higher rates of treatment responders and lower opioid and non-opioid rescue medication use [Citation28]. The findings from these CIII buprenorphine studies demonstrated the impact of dose-dependent efficacy on cLBP management and patients’ quality of life. They also noted the advantages of products with a wider dosing spectrum in a stepwise dose titration and reaching higher CIII buprenorphine daily doses. Buprenorphine patches are available in 5–20 mcg/h doses on the US market, which equals up to 480 mcg daily CIII buprenorphine dose. On the other hand, buccal film is available in 75–900 mcg doses with recommended twice-daily administration, yielding a greater maximal CIII buprenorphine daily dose than buprenorphine patches. Although the direct head-to-head comparison is essential to draw firm conclusions, buccal film had higher responder rates than transdermal patches in similar clinical trials (64 vs 49% of patients had ≥30% pain score decrease and 40 vs 31% of patients had ≥50% pain score decrease, respectively), most likely due to formulation differences (higher bioavailability and a wider range of available doses) [Citation29].
In our study buccal film had approximately 1.85-times higher BADD than the buprenorphine patch. Therefore, patients more commonly switched from patch to buccal film than vice versa (3.8 vs 11.5%, respectively; p < 0.001) and had a greater reduction in rescue opioid utilization after buccal film treatment initiation, especially LAOs. These findings are most likely due to the flexible dosing with buccal film despite the lower number of CIII buprenorphine prescriptions and shorter CIII buprenorphine treatment duration during the follow-up. Also, regardless of the wider dosing spectrum, the average daily buprenorphine buccal film dose before the treatment switch was similar to that of the buprenorphine patch (381.6 vs 297.1 mcg, respectively; p = 0.059). Given that buccal film patients achieved only 21% of the maximal daily dose, these findings suggest the lack of proper dose titration before switching to buprenorphine patch. This result indicates that these switchers (∼4%) from the buccal film cohort did not have the opportunity to properly titrate the medication and experience the full clinical benefits, which may have led to premature switching to another CIII buprenorphine formulation. Additionally, prior to introducing CIII buprenorphine treatment, patients in the buccal film cohort were more often pretreated with opioids (5.7 vs 4.9 opioid prescriptions and 51.5 vs 39.8 daily MMEs per patient; both p < 0.001), while non-opioid rescue medication use was more common in the buprenorphine patch cohort (4.8 vs 5.2 prescriptions per patient; p = 0.021). This may imply that buprenorphine buccal film was prescribed to more severe cLBP cases that had already failed to control the pain with many opioids.
Strengths & limitations
This is the first US retrospective real-world evidence analysis of cLBP treatment characteristics among patients prescribed buprenorphine buccal film or transdermal patches. However, several study limitations should be noted alongside the results interpretation. First, the main limitation is related to the nature and characteristics of real-world data and coding system restrictions. The claims are primarily collected for billing purposes; therefore, data entry errors and miscoding, duplicates, or negative-input claims may have occurred. This has been overcome by employing data cleaning, performing a careful patient selection process and using complex methods to minimize selection bias and balance the differences between study cohorts, such as PSM. Second, there is a lack of data related to low back pain chronicity and severity. Chronicity was assumed if patients had at least two low back pain claims prior to buccal film or buprenorphine patch initiation. However, the potential risk that study cohorts consisted of a heterogeneous sample of patients in terms of pain level could not be diminished. Third, the defined duration of pre- and post-index periods is relatively short due to the criterion that required continuous healthcare and pharmaceutical coverage during the observational period. This criterion is required to ensure realistic and credible observation, while pre- and post-index durations had to be equal (6 months) to provide a trustworthy comparison of rescue medication utilization changes prior to and after CIII buprenorphine introduction. Therefore, using a longer observational period in the analysis would substantially reduce the study population size and additionally limit the application of findings in real-world clinical practice. Finally, the fourth study limitation is related to the generalizability of the study conclusions, as this retrospective claims analysis was conducted in a sample of commercially insured patients.
Conclusion
Buprenorphine buccal film was associated with a significantly greater reduction in concomitant oral opioid use than buprenorphine transdermal patches after treatment initiation, despite the lower average number of prescriptions and treatment duration. Additionally, there was a higher rate of patients who switched from buprenorphine patch to buccal film than vice versa. Pre-index treatment characteristics also imply buccal film was prescribed to more severe cases as they had the higher opioid burden and lower use of non-opioid analgesics before initiating CIII buprenorphine treatment than the patch cohort.
The findings are based on the real-world data from commercially insured chronic low back pain (cLBP) patients treated with buprenorphine.
Index date was the first buprenorphine buccal film or buprenorphine transdermal patch prescription; patients were observed during the 6-month pre- and post-index periods.
Cohorts were defined based on the formulation of index buprenorphine treatment and matched using demographic and clinical characteristics.
Patients achieved higher daily doses using buccal film than transdermal patches (501.7 vs 270.9 mcg; p < 0.001).
More patients switched from patches to film than vice versa (11.5 vs 3.8%; p < 0.001).
Buccal film patients had higher opioid and lower non-opioid rescue medication burden prior to buprenorphine initiation.
Starting buprenorphine treatment significantly decreased opioid and non-opioid rescue medication utilization in both cohorts.
Despite shorter buprenorphine treatment duration (87.2 vs 91.9 days; p = 0.043) and fewer buprenorphine prescriptions (3.4 vs 3.7; p = 0.011), the buccal film led to a greater decrease in prescription counts (-1.1 vs -0.7; p = 0.012), treatment duration (-13.4 vs -7.6 days; p = 0.022) and daily morphine milligram equivalents (-12.6 vs -7.3; p < 0.001) of concomitant opioids.
Author contributions
F Stanicic, D Grbic, D Vukicevic and V Zah contributed equally to this research. All authors were responsible for conception and design of the work; the acquisition, analysis and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; and providing the final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Writing disclosure
No writing assistance was funded for manuscript preparation.
Supplemental Appendix
Download MS Word (51.9 KB)Acknowledgments
All steps in conducting this research were supervised and critically reviewed by Todd Kunkel Scientific Communications (Collegium Pharmaceutical, Inc.).
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/pmt-2023-0124
Financial disclosure
This study was funded by Collegium Pharmaceutical, Inc. ZRx Outcomes Research Inc. received financial support for conducting the research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Shmagel A , Foley R , Ibrahim H . Epidemiology of chronic low back pain in US adults: data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res. 68(11), 1688–1694 (2016).
- Manchikanti L , Singh V , Falco FJE , Benyamin RM , Hirsch JA . Epidemiology of low back pain in adults. Neuromodulation 17(Suppl. 2), 3–10 (2014).
- Dahlhamer J , Lucas J , Zelaya C et al. Prevalence of chronic pain and high-impact chronic pain among adults – United States, 2016. MMWR Morb. Mortal. Wkly Rep. 67(36), 1001–1006 (2018).
- Stevans JM , Delitto A , Khoja SS et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw. Open. 4(2), e2037371 (2021).
- Kim LH , Vail D , Azad TD et al. Expenditures and health care utilization among adults with newly diagnosed low back and lower extremity pain. JAMA Netw. Open. 2(5), e193676 (2019).
- Qaseem A , Wilt TJ , Mclean RM et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 166(7), 514–530 (2017).
- North American Spine Society (NASS) . Evidence-based clinical guidelines for multidisciplinary spine care: diagnosis and treatment of low back pain 2020). (Accessed May 2023). www.spine.org/Portals/0/assets/downloads/ResearchClinicalCare/Guidelines/LowBackPain.pdf
- Department of Veteran Affairs (VA) and Department of Defense (DoD) . VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain v3.0 (2022). (Accessed May 2023). www.healthquality.va.gov/guidelines/pain/lbp/
- Dowell D , Ragan KR , Jones CM , Baldwin GT , Chou R . CDC clinical practice guideline for prescribing opioids for pain – United States, 2022. MMWR Recomm. Rep. 71(3), 1–95 (2022).
- Dowell D , Haegerich TM , Chou R . CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm. Rep. 65(1), 1–49 (2016).
- Fine PG , Mahajan G , Mcpherson ML . Long-acting opioids and short-acting opioids: appropriate use in chronic pain management. Pain Med. 10(Suppl. 2), S79–S88 (2009).
- Shmagel A , Ngo L , Ensrud K , Foley R . Prescription medication use among community-based US adults with chronic low back pain: a cross-sectional population based study. J. Pain 19(10), 1104–1112 (2018).
- White AP , Arnold PM , Norvell DC , Ecker E , Fehlings MG . Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine 36(Suppl. 21), S131–S143 (2011).
- Webster L , Gudin J , Raffa RB et al. Understanding buprenorphine for use in chronic pain: expert opinion. Pain Med. 21(4), 714–723 (2020).
- Elkader A , Sproule B . Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin. Pharmacokinet. 44(7), 661–680 (2005).
- STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement Checklist (2023). (Accessed 13 November 2023 ). www.strobe-statement.org/
- Merative . Merative™ MarketScan® Research Databases (2023). (Accessed 15 April 2023 ). www.merative.com/real-world-evidence
- The University of Utah . Opioid oral morphine milligram equivalent (MME) conversion factors (2022). (Accessed 15 April 2023 ). https://medicaid.utah.gov/Documents/files/Opioid-Morphine-EQ-Conversion-Factors.pdf
- IBM Corporation . IBM SPSS Statistics (2023). (Accessed 13 November 2023 ). www.ibm.com/products/spss-statistics
- Abdel Shaheed C , Maher CG , Williams KA , Day R , Mclachlan AJ . Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern. Med. 176(7), 958–968 (2016).
- Altman RD , Smith HS . Opioid therapy for osteoarthritis and chronic low back pain. Postgrad. Med. 122(6), 87–97 (2010).
- Cashin AG , Wand BM , O’Connell NE et al. Pharmacological treatments for low back pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst. Rev. 4(4), Cd013815 (2023).
- Chou R , Turner JA , Devine EB et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention workshop. Ann. Intern. Med. 162(4), 276–286 (2015).
- Substance Abuse and Mental Health Services Administration (SAMSHA) – Center for Behavioral Health Statistics and Quality . Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health (2021). (Accessed 20 April 2023 ). www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
- Gordon A , Rashiq S , Moulin DE et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res. Manag. 15(3), 169–178 (2010).
- Steiner D , Munera C , Hale M , Ripa S , Landau C . Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J. Pain 12(11), 1163–1173 (2011).
- Miller K , Yarlas A , Wen W et al. Buprenorphine transdermal system and quality of life in opioid-experienced patients with chronic low back pain. Expert Opin. Pharmacother. 14(3), 269–277 (2013).
- Rauck RL , Potts J , Xiang Q , Tzanis E , Finn A . Efficacy and tolerability of buccal buprenorphine in opioid-naive patients with moderate to severe chronic low back pain. Postgrad. Med. 128(1), 1–11 (2016).
- Pergolizzi JV Jr , Raffa RB . Safety and efficacy of the unique opioid buprenorphine for the treatment of chronic pain. J. Pain Res. 12, 3299–3317 (2019).