Abstract
Aim: We aim to show that the use of nondigested micro-fragmented adipose tissue (MFat™, Lipogems®) is a viable alternative for treatment of joint pain and inflammation associated dysfunction in shoulder osteoarthritis (OA). Materials & methods: A total of 25 subjects with OA received an injection of MFat™ and were followed at 6, 18 and 52 weeks intervals. Quantitative analysis of pain and function modalities were performed using the visual analog scale and the disabilities of the arm, shoulder and hand, respectively. Results: All study participants reported significant progressive improvement (p < 0.001) from baseline in visual analog scale and disabilities of the arm, shoulder and hand in shoulder OA cases up to a year post. Conclusion: MFat™ therapy improves pain and function in patients with shoulder OA and can provide a long-term alternative to surgical intervention.
Osteoarthritis (OA) is one of the most common chronic and debilitating conditions seen in the orthopedic setting. This disease results in damage to the articular cartilage and inflammation and successive damage of the joint itself. Shoulder OA can be debilitating with loss of shoulder function leading to depression, anxiety, activity limitations and job-performance problems. Cartilage degradation in shoulder OA can cause subchondral bone remodeling, and ultimate loss in sphericity and congruity of the joint [Citation1]. The joint capsule can thicken, leading to further loss of shoulder rotation [Citation2,Citation3].
Successful treatment of OA remains a challenge particularly due to a lack of blood supply and limited capacity of self-repair in articular cartilage [Citation4,Citation5]. Traditional treatment is aimed at symptom management to control pain and restore function but nothing reparative in nature to alter the progression of disease. Although steroid injections are commonly given to provide pain relief by decreasing joint inflammation, often patients require numerous injections, which has been shown to accelerate OA progression and result in bone loss over time [Citation6]. More advanced cases of OA can develop resistance or are unresponsive to traditional pharmacological methods warranting surgical intervention, which leads many patients searching for less invasive alternative treatment options.
Orthobiologic regenerative medicine approaches have recently emerged as innovative nonsurgical reparative options for the treatment of OA through the use of mesenchymal stem cells (MSCs). The therapeutic use of MSCs and other reparative pericytes is traditionally related to both their anti-inflammatory activity and multilineage differentiation, including their chondrogenic potential [Citation7–11]. Adipose tissue has emerged as an easily accessible, rich source of these reparative cells and can serve as an excellent option in regenerative medicine because of the minimally invasive harvesting procedure and is currently being utilized in several OA studies [Citation12–19]. In this prospective nonrandomized clinical study of 25 patients, we investigate the use of autologous micro-fragmented adipose tissue (MFat™) obtained through a minimal manipulation technique in a closed, full-immersion Lipogems® system and quantified the clinical effectiveness of its use for the treatment of joint pain and inflammation associated dysfunction in patients with OA of the glenohumeral shoulder joint. We propose that the use of this technology improves pain, function and quality of life in patients with mild-to-moderate shoulder OA and can provide a long term alternative to surgical intervention.
Materials & methods
Participants
The current study includes 25 patients with a clinical diagnosis of mild (n = 12) to moderate (n = 13) shoulder OA with no other clinically complicating factors who have completed follow-ups at 6, 18 and 52 week intervals. All participants were submitted to an initial screening visit with a physical examination and shoulder radiography. Inclusion criteria were males and females over the age of 40 with a diagnosis of OA of the shoulder glenohumeral joint and confirmatory radiographs (Kellgren–Lawrence [KL] grade 2–3).
Exclusion criteria were history of immunodeficiency, chronic use of oral corticosteroid or immunosuppressive therapies, history or presence of malignant disorders and/or use of chemotherapy within the last 5 years, except for cutaneous basal cell or squamous cell cancer resolved by excision, signs and symptoms of significant cardiac disease, active use of cigarettes, diagnosis of transient ischemic attack within the last 6 months. Patients with a BMI greater than 45 were not included in the study as a means to control any potential effects of obesity on the effectiveness of the clinical results. We do not anticipate a correlation in BMI to success of these treatments, based on our initial findings. The shoulder joint is not subject to the compressive effects of axial loading like the knee joint, and is therefore unlikely to be affected by BMI.
Lipoaspiration
According to the policies approved by the Institutional Review Boards for the Institute of Regenerative and Cellular Medicine (LG-OA-901), adipose tissue was harvested from 25 patients with mild-to-moderate shoulder OA. Written informed consent was obtained from all study participants. Study participants were taken to a dedicated procedure room and placed in the prone position on the procedure table for aspiration. Under aseptic sterile conditions, stab incisions were made for cannula entry in the abdominal area just below the umbilicus and infiltrated with tumescent anesthesia fluid with 500 ml of saline, 50 ml of 2% lidocaine plus 1 ml of (1:1000) epinephrine. Approximately 15 min following infiltration, 50–70 cc of adipose tissue was aspirated via cannula connected to a VacLock® (Merit Medical, UT, USA) syringe. Following aspiration, steri-strips and waterproof dressings were applied to the area of cannula entry.
Lipoaspirate processing
The harvested fat was immediately processed in the same room via a closed system and the MFat™ was prepared by connecting the lipoaspirate syringe to the Lipogems® device (Lipogems® International, Milan, Italy) and processed as previously described by Bianchi et al. until desired volumes of Lipogems® MFat™ was achieved and the final product was transferred into 10 cc syringes [Citation19].
Intra-articular injections
Immediately following processing and under aseptic conditions, 10 cc of 1% lidocaine was injected into the shoulder(s) under ultrasound guidance. The Terason uSmart 330 Ultrasound System was used to aid in visualization for all injections to ensure precise delivery directly into the glenohumeral joint. A 7 cc of the MFat™ was then injected into the glenohumeral joint of the affected shoulder(s) under ultrasound guidance and gentle range of motion exercises were performed immediately following the injection.
Postoperative & postinjection care
Patients were discharged when stable with post procedure instructions. Prophylactic antibiotics were administered, and patients were monitored for fever and abnormal pain and swelling. Adjunct therapies of supplements and oral cytokines (GUNA® Biotherapeutics, Milan, Italy) were administered to all patients to enhance recovery and healing for a 6 weeks post MFat™ injection. Specifically, supplements including 1000 mg of ProOmega® with 650EPA/450 DHA (Nordic Naturals Mfg, CA, USA) twice daily, 5000 IU of Vitamin D3 once daily, proprietary blends of men or women multivitamins compounded by Covington Orthopedic three-times daily, Inflammove™ herbal supplement that contains turmeric and ginger root three-times daily and Neo40® nitric oxide supplement (Human Power of N™, TX, USA) were included in the post therapy supplement protocol. Additional adjunct therapy of a specific OA protocol of oral growth factors from GUNA® Biotherapeutics were prescribed daily. Four specific cytokines were prescribed as part of the adjunct therapy and included 20 drops of interferon growth factor (IGF) twice daily for neuroendocrine support, 20 drops of anti-IL-1 twice daily for acute pain and fever relief, 20 drops of IL-4 once daily for inflammation and ten drops of FGF once daily for connective tissue support. These drops were administered orally and allowed to absorb buccally for maximum benefit. Patients were followed for a minimum of 1 year post Lipogems® therapy and specifically at 6, 18 and 52 weeks post therapy.
Patient reported outcome measurements
A clinical and functional assessment was performed at each follow-up interval. Patient reported outcomes of pain and function were measured using the visual analog scale (VAS) and the disabilities of the arm, shoulder and hand (DASH), respectively [Citation20–24]. Deviations from baseline conditions were calculated and percentage improvements and/or decline were determined and quantitatively compared for each follow-up time point. Data from patients with baseline pain and function scores less than 15/100 were excluded from the final analysis for this study. Scores less than 15/100 were deemed not active disease and therefore, treatment would be considered prophylactic and not an accurate representation of any restorative effects.
Radiologic joint space measurements
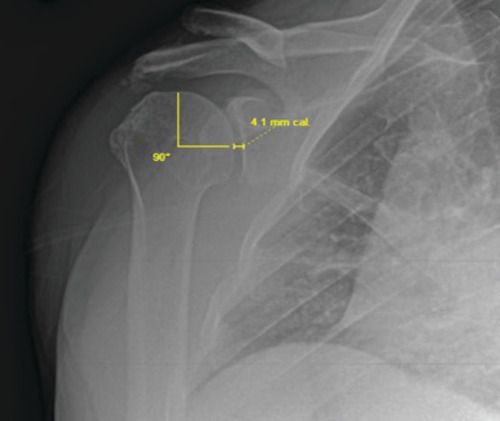
X-rays were taken at each follow-up and radiologic changes in glenohumeral joint spacing were measured as a speculative correlate to articular cartilage growth. An orthopedic surgeon with 24 years of musculoskeletal radiologic reporting experience and board certifications in orthopedic surgery, sports medicine, regenerative medicine and musculoskeletal ultrasound measured all joint space widths with the assistance of a certified radiologic technologist through the use of Merge OrthoPACS™ system (IBM, NY, USA). Quality control of all measurements was conducted by a biomedical engineer to ensure consistency in technique and standards of measurement. The external true anteroposterior projection was used with the standard positioning of the patient supine, slightly turned (20°) to imaged side (a support under the other shoulder) and the arm in the external rotation, palm facing upwards as previously published [Citation25]. Subjects with radiographs that did not allow for a clear visualization of the glenohumeral joint space were excluded from the analysis leaving data from 18 subjects with (n = 8) for mild OA and (n = 10) for moderate OA shoulder cases.
In this position the projection of the joint surface of the humeral head forms a half-circle, the diameter of which is the line joining the two terminal points of the joint surface projection. The mid-point of this line was determined and with a ruler aimed at this point, a 90° angle measurement was established perpendicular to the joint surface of the head of the humerus. The glenohumeral joint space at this site was measured with a ruler from this 90° projection at each time point as indicated in . Deviations from baseline conditions were calculated as normalized changes from preprocedure measurements.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Prism, LLC, CA, USA). The level of significance for all hypothesis tests (p) was set at 0.05. Continuous variables were presented as mean and standard error. Comparisons of shoulder VAS and DASH scores and joint space measurements were independently made with the Kruskall–Wallis test for each dataset. Once the Kruskal-Wallis test showed statistical significance among all normalized timepoints, post hoc analysis was performed using the Wilcoxon signed-rank test to delineate the improvement of measurements between each end point with 95% CIs. Patient reported outcome measurements of DASH scores measured during each follow-up end point for mild and moderate OA shoulder cases were also quantitatively compared utilizing the Mann–Whitney test to reveal differences among KL severity.
Ethical approval
This study was reviewed and approved for human studies by the International Review Board for Cellular Medicine. All patients signed a detailed informed consent, which was also reviewed and approved by the Institutional Review Board. There was no funding provided to the investigator, and no patient compensation for participation.
Results
Patient reported outcomes
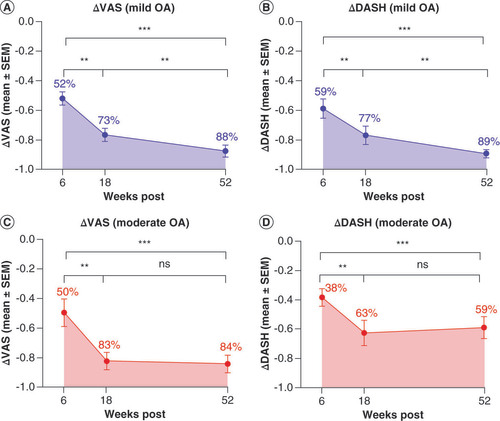
At the 6 week follow-up, all 25 study participants reported significant improvement from baseline with 51.92 ± 4.52% (mean ± standard error of the mean [SEM]) improvement in VAS and 58.78 ± 6.61% improvement in DASH in mild OA cases, and 49.67 ± 9.36% improvement in VAS and 38.39 ± 6.31% improvement in DASH in moderate OA cases. These early results continued to progress in the mild OA group through 18 weeks with 76.56 ± 4.26% improvement in VAS and 76.90 ± 6.30% in DASH and at the yearly follow-up 87.51 ± 4.01% improvement in VAS and 89.31 ± 2.72% improvement in DASH. The patients with moderate OA demonstrated significant functional improvements at the 18 week follow-up with 82.58 ± 6.02% improvement in VAS and 62.61 ± 8.65% improvement in DASH that were maintained at the 1 year follow-up with 84.37 ± 5.97% improvement in VAS and 58.98 ± 7.55% improvement in DASH as illustrated in . Results of Kruskal–Wallis tests of VAS and DASH results revealed statistically significant differences among all time points measured (***p < 0.001) for both mild and moderate OA shoulder cases. Post hoc analysis was performed with the Wilcoxon signed-rank test and showed statistically significant improvement (**p < 0.01) of VAS and DASH scores progressively over time for mild OA shoulder cases as indicated in A&B. Post hoc analysis of data from moderate OA shoulder cases shown in C&D revealed statistical improvement over time through 18 weeks (**p < 0.01), with no significant change after 18 weeks, but rather revealed a maintenance of results from 18 weeks to a year post therapy.
Quantitative comparative analysis was performed utilizing the Kruskall–Wallis test, revealing statistically significant changes from baseline value among all data sets for both mild and moderate shoulder OA. Approximate mean percent changes from baseline values are overlaid on plots for clarity. Post hoc analysis was performed with the Wilcoxon signed-rank test and shows statistically significant improvement of VAS and DASH scores progressively over time measured with 95% CI for mild OA as indicated in plots (A & B). Post hoc analysis of data from moderate OA shoulder cases in plots (C & D) reveal statistical improvement over time through 18 weeks, with no significant change after 18 weeks, but rather reveal a maintenance of results from 18 weeks to a year post Lipogems® therapy.
**p < 0.01; ***p < 0.001.
DASH: Disabilities of the arm, shoulder and hand; OA: Osteoarthritis; SEM: Standard error of the mean; VAS: Visual analog scale.
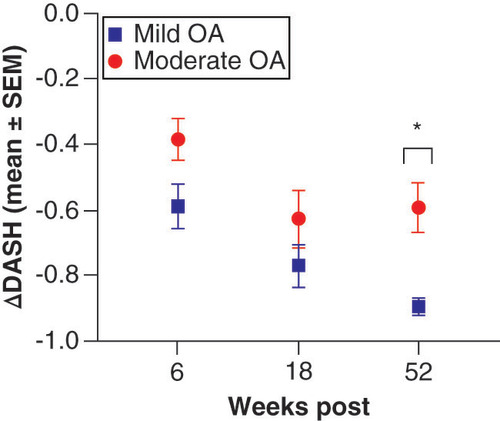
Outcome correlates among groups (mild and moderate OA) were made of the patient reported outcome measurements of DASH scores for each time point measured and were quantitatively compared utilizing the Mann–Whitney test. Results of this analysis shown in showed statistically significant differences in year DASH measurements between mild and moderate OA shoulder cases, with significantly more improvement in function at 1 year post Lipogems® therapy in mild shoulder OA when compared with moderate shoulder OA cases (*p < 0.05).
Results of Mann–Whitney comparisons test among groups at each time point reveals statistically significant differences in DASH scores at the yearly follow-up, with marked improvement in DASH scores at 1 year post Lipogems® therapy for mild OA shoulder cases when compared with that of moderate OA shoulder cases.
*p < 0.05.
DASH: Disabilities of the arm, shoulder and hand; OA: Osteoarthritis; SEM: Standard error of the mean.
Joint space measurements
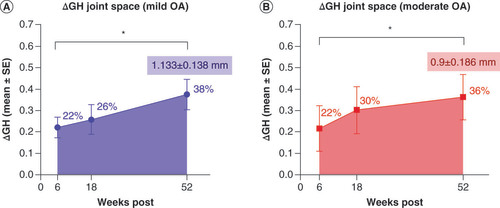
Glenohumeral joint space measurements were recorded for each subject at each time point, an example of which is shown in Supplemental Figure 1. The raw metric joint space measurements for baseline and year time points are provided in Supplementary Table 1 for reference. Normalized deviations from baseline were calculated and quantitatively compared for each follow-up time point as shown in for mild OA shoulder cases and moderate OA shoulder cases (A&B, respectively). At the 6 week follow-up, all included study participants (n = 18) reported significant increases in glenohumeral joint spacing from baseline with 22.19 ± 4.83% (mean ± SEM) increase in mild OA cases and 21.75 ± 10.64% increase in moderate OA cases. Continued joint space increase was revealed at 18 weeks with 25.86 ± 7.04% increase in mild OA cases and 30.39 ± 10.94% increase in moderate OA cases. Yearly follow-ups revealed 37.55 ± 7.19% increase in joint space in mild OA shoulder cases with 36.34 ± 10.61% increase in joint space in moderate OA shoulder cases. Significantly, post hoc analysis of normalized data sets with the Wilcoxon signed rank tests showed significant increases in glenohumeral joint spacing following MFat™ therapy up to 1 year post treatment (*p < 0.05) for both mild and moderate OA shoulder cases with a mean increase in joint spacing of 1.133 ± 0.138 mm in mild OA shoulder cases and 0.9 ± 0.186 mm in moderate OA shoulder cases as illustrated in . No significant difference was found among groups but could be due to smaller sample size and greater variation in results among moderate OA subjects.
Approximate mean percent increases in joint space are overlaid for clarity as well as mean joint space increases (mean ± SE mm) for the 52 week follow-up. Statistically significant increases in glenohumeral joint spacing following micro-fragmented adipose tissue (MFat™) therapy up to 1 year post therapy are indicated in both graphs.
*p < 0.05.
GH: Glenohumeral; OA: Osteoarthritis; SE: Standard error.
Discussion
Traditional treatment in orthopedic medicine is aimed at symptom management to control pain and restore function but no alternative biological treatments have yet been shown to alter the progression of disease. Additionally, there are essentially no effective treatments after conservative therapy has failed for patients suffering with shoulder OA. In this novel pilot study, we use an innovative autologous product as a reparative alternative to such treatment with promising results. Data from mild OA shoulder cases revealed continued progressive improvement over time in both pain and functional scales up to 1 year post Lipogems® MFat™ therapy. Results of analysis of VAS and DASH data from moderate OA shoulder cases revealed statistical improvement over time through 18 weeks, with a maintenance of those results at 1 year post therapy, implicating limitations in improvements of such measurements with increased severity of OA. Additionally, the improvement in DASH scores in moderate shoulder OA cases at 1 year post therapy was statistically less than the improvement in mild OA shoulder cases at 1 year post, suggesting a correlation between the degree of functional restoration to KL severity. This progressive improvement at the yearly follow-up for mild OA shoulder cases is interesting and something that needs to be explored further. While we can consider MFat™ a pain relieving intervention, it is clear that there is a functional benefit of this therapy supporting the reparative nature of MFat™ and likely the reason for the progressive nature of results. Many studies outline the characteristics of the tissue, which include immunomodulation, paracrine signaling and angiogenesis. Based on these results, we speculate that the interaction of the MFat™ within the environmental niche not only provides cushion and support to joints, but also initiates repair of cartilage and bone defects while reducing inflammation resulting in a healing environment that can possibly help prevent future damage. This preliminary data advance our understanding of adipose tissue transfer for shoulder OA and establish the use of micro-fragmented fat as a valuable alternative for the treatment of pain and inflammation associated dysfunction in mild-to-moderate OA in shoulders and demonstrates that this may be an effective treatment for delaying total joint replacement in the future.
Our results are in line with Striano et al. who analyzed the role of MFat™ injection for shoulder pain and arthritis in 20 patients in which they demonstrated significant improvements in pain, function and quality of life as measured by patient reported outcomes up to 1 year post [Citation26]. Additionally, the normalized percentage improvements in pain and function measurements noted in our results suggest greater efficacy and longevity with larger magnitude changes continued over longer periods of time when preliminarily compared with the results reported in previous studies by Zhang et al. examining the use of hyaluronic acid and corticosteroid injections in glenohumeral OA patients, where they showed that neither hyaluronic acid nor corticosteroid injections were significantly better than any other conservative treatment options for shoulder OA [Citation27]. Currently, longer-term progress with larger data sets and the application of such methods in the presence of complicating shoulder pathologies including rotator cuff injury and tendinopathies in the absence and presence of OA are being explored. Preliminary data and anecdotal clinical findings are positive and align with data sets for OA shoulder cases.
Analysis of joint space measurements reveals statistically significant increases in glenohumeral joint spacing following MFat™ therapy up to 1 year post treatment. These novel preliminary results are intriguing and merit further research into the mechanisms involved in these joint space modifications. We anticipate these changes are a result of possible cartilage modification following Lipogems® MFat™ therapy, supporting the reparative capacity of MFat™ therapy. We acknowledge that this analysis of joint space measurements was limited by the removal of the data from patients with radiographs that did not allow for clear visualization of the glenohumeral joint. With that said, we still find the results to be intriguing and significant nonetheless and something to be expanded upon in future studies.
The main limitation in this preliminary study is a lack of formal placebo or control group. It would be difficult to perform an ethical procedure on a control group, if they were to only later receive a SHAM placebo injection. Future studies should include a control group of corticosteroid injections that were followed at the same follow-up time points to make a side by side comparison. Additionally, it is important to consider a placebo effect as a result of patient funded procedures as a possible limitation to this study, but without proper funding for patient compensation, this may be challenging to accomplish. The promising preliminary results warrant further research with larger numbers of study participants. Implementation in other joints is also being explored and evaluated, but this innovative research is promising for the field of orthopedic regenerative medicine and should be a considered a valuable alternative for the treatment of pain and inflammation associated dysfunction in mild-to-moderate OA in shoulders.
Conclusion
OA is a cause of significant morbidity and current conservative management strategies fail to alter the progression of disease. Orthobiologic regenerative medicine approaches have recently emerged as innovative nonsurgical reparative options for the treatment of OA through the use of MSCs. In this novel pilot study, we use an innovative autologous product as a reparative alternative to traditional or surgical treatment of shoulder OA with promising results. We show that the use of Lipogems® MFat™ therapy in combination with adjunct supplements and oral growth factors in mild-to-moderate OA shoulder cases results in clinically significant improvement in both pain and functional scales, signifying Lipogems® as a novel regenerative orthopedic modality for the treatment of degenerative shoulder OA and also as a viable long term alternative to surgical intervention. Our results show that the improvements are not only significant, but also progressive in nature, with optimal results being achieved at up to 1 year post therapy. Future studies will focus on larger randomized control trials to further investigate the clinical efficacy of micro-fragmented adipose tissue with direct comparison against traditional conservative treatments options.
Osteoarthritis (OA) is one of the most common chronic and debilitating conditions seen in the orthopedic setting.
Shoulder OA can be debilitating with loss of shoulder function leading to depression, anxiety, activity limitations and job-performance problems.
Successful treatment of OA remains a challenge particularly due to a lack of blood supply and limited capacity of self-repair in articular cartilage.
Traditional treatment is aimed at symptom management to control pain and restore function but nothing reparative in nature to alter the progression of disease.
Orthobiologic regenerative medicine approaches have recently emerged as innovative nonsurgical reparative options for the treatment of OA through the use of mesenchymal stem cells.
Adipose tissue has emerged as an easily accessible, rich source of these reparative cells because of the minimally invasive harvesting procedure and is currently being utilized in several OA studies.
In this novel pilot study, we use an innovative autologous product as a reparative alternative to traditional or surgical treatment of shoulder OA with promising results.
Quantitative analysis of patient reported outcomes of pain and function measured using the visual analog scale and the disabilities of the arm, shoulder and hand, respectively revealed significant progressive improvement from baseline in visual analog scale and disabilities of the arm, shoulder and hand in shoulder OA cases up to 1 year post injection.
MFat™ therapy improves pain and function in patients with shoulder OA and can provide a long-term alternative to surgical intervention.
This preliminary data advance our understanding of adipose tissue transfer for shoulder OA and establish the use of micro-fragmented fat as a valuable alternative for the treatment of pain and inflammation associated dysfunction in mild-to-moderate OA in shoulders.
Author contributions
H Vinet-Jones contributed to the study design, collection and assembly of data, data analysis and interpretation, statistical analysis and manuscript writing. KF Darr contributed to the conception and design, financial and administrative support, provision of study patients, collection of data and final approval of manuscript.
Ethical conduct of research
This study was reviewed and approved for human studies by the International Review Board for Cellular Medicine. In addition, informed consent has been obtained from the participants involved and was also reviewed and approved by the International Review Board.
Supplemental information
Download Zip (200.6 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/rme-2020-0069
Financial & competing interests disclosure
KF Darr is a consultant for Lipogems® USA, LLC. There was no funding provided to the investigator, and no patient compensation for participation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Ansok CB , MuhSJ. Optimal management of glenohumeral osteoarthritis. Orthop. Res. Rev.10, 9–18 (2018).
- Kerr R , ResnickD , PinedaC , HaghighiP. Osteoarthritis of the glenohumeral joint: a radiologic-pathologic study. Am. J. Roentgenol.144(5), 967–972 (1985).
- Petersson CJ . Degeneration of the gleno-humeral joint: an anatomical study. Acta Orthopaedica Scandinavica54(2), 277–283 (1983).
- Diekman BO , GuilakF. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr. Opin. Rheumatol.25(1), 119–126 (2013).
- Berenbaum F , WalkerC. Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad. Med.132(4), 377–384 (2020).
- Papacrhistou G , AnagnostouS , KatsorhisT. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop. Scand. Suppl.275, 132–134 (1997).
- Nava S , SordiV , PascucciLet al. Long-lasting anti-inflammatory activity of human microfragmented adipose tissue. Stem Cells Int.2019, 5901479 (2019).
- Paolella F , ManferdiniC , GabusiEet al. Effect of microfragmented adipose tissue on osteoarthritic synovial macrophage factors. J. Cell. Physiol.234(4), 5044–5055 (2019).
- Vezzani B , ShawI , LesmeHet al. Higher pericyte content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl. Med.7(12), 876–886 (2018).
- Mamidi MK , DasAK , ZakariaZ , BhondeR. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthr. Cartil.24(8), 1307–1316 (2016).
- Carelli S , MessaggioF , CanazzaAet al. Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant.24(7), 1233–1252 (2015).
- Russo A , ScrepisD , DiDonato SL , BonettiS , PiovanG , ZorziC. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: an update at 3 year follow-up. J. Exp. Orthop.5(1), 52 (2018).
- Minteer D , MarraKG , RubinJP. Adipose-derived mesenchymal stem cells: biology and potential applications. Adv. Biochem. Eng. Biotechnol.129, 59–71 (2013).
- Emadedin M , GhorbaniLiastani M , FazeliRet al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch. Iran. Med.18(6), 336–344 (2015).
- Jo CH , LeeYG , ShinWHet al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells32(5), 1254–1266 (2014).
- Shin YS , YoonJR , KimHS , LeeSH. Intra-articular injection of bone marrow-derived mesenchymal stem cells leading to better clinical outcomes without difference in mri outcomes from baseline in patients with knee osteoarthritis. Knee Surg. Relat. Res.30(3), 206–214 (2018).
- Tremolada C , ColomboV , VenturaC. Adipose tissue and mesenchymal stem cells: state of the art and lipogems(R) technology development. Curr. Stem Cell Rep.2, 304–312 (2016).
- Ceserani V , FerriA , BerenziAet al. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc. Cell8, 3 (2016).
- Bianchi F , MaioliM , LeonardiEet al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant.22(11), 2063–2077 (2013).
- Angst F , SchwyzerHK , AeschlimannA , SimmenBR , GoldhahnJ. Measures of adult shoulder function: disabilities of the arm, shoulder, and hand questionnaire (DASH) and its short version (QuickDASH), shoulder pain and disability index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, constant (Murley) score (CS), simple shoulder test (SST), Oxford shoulder score (OSS), shoulder disability questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care Res. (Hoboken)63(Suppl. 11), S174–S188 (2011).
- Dixon D , JohnstonM , McqueenM , Court-BrownC. The disabilities of the arm, shoulder and hand questionnaire (DASH) can measure the impairment, activity limitations and participation restriction constructs from the International Classification of Functioning, Disability and Health (ICF). BMC Musculoskelet. Disord.9, 114 (2008).
- Dominguez-Gasca LG , Chico-CarpizoF , Magana-ReyesJ , Dominguez-CarrilloLG. Shoulder injuries in the elderly and their functional impact on the DASH scale. Acta Ortop. Mex.32(1), 13–16 (2018).
- Germann G , WindG , HarthA. The DASH (disability of arm-shoulder-hand) questionnaire--a new instrument for evaluating upper extremity treatment outcome]. Handchir. Mikrochir. Plast. Chir.31(3), 149–152 (1999).
- Jester A , HarthA , WindG , GermannG , SauerbierM. Disabilities of the arm, shoulder and hand (DASH) questionnaire: determining functional activity profiles in patients with upper extremity disorders. J. Hand Surg. Br.30(1), 23–28 (2005).
- Lehtinen JT , LehtoMU , KaarelaK , KautiainenHJ , BeltEA , KauppiMJ. Radiographic joint space in rheumatoid glenohumeral joints. A 15-year prospective follow-up study in 74 patients. Rheumatology (Oxford)39(3), 288–292 (2000).
- Striano Rd , MalangaG , BilboolN , AzatullahK. Refractory shoulder pain with osteoarthritis, and rotator cuff tear, treated with micro-fragmented adipose tissue. J. Orthopaedics Spine Sports Med.2(1), 14–19 (2018).
- Zhang B , ThayaparanA , HornerN , BediA , AlolabiB , KhanM. Outcomes of hyaluronic acid injections for glenohumeral osteoarthritis: a systematic review and meta-analysis. J. Shoulder Elbow Surg.28(3), 596–606 (2019).