Abstract
Aim: To assess whether the use of allogeneic platelet-derived growth factors could serve as a feasible, effective and safe biological therapy for the treatment of medication-related osteonecrosis of the jaw (MRONJ). Materials & methods: Patients with multiple myeloma and MRONJ were included and treated with allogeneic platelet-rich plasma, continued for between 6 and 18 weeks (mean: 9). Results: We observed a treatment success rate of 87.5% (p < 0.05). Assessing the association between healing and treatment duration, we observed a statistically significant relationship (χ2 = 8.00; p = 0.018; Cramer’s V = 1), confirming that healing was very closely related to the duration of the treatment. Conclusion: Allogeneic platelet-rich plasma could be a recommended treatment for MRONJ. Future research with a large sample to validate our findings is required.
Bisphosphonates (a group of drugs that regulate bone metabolism) inhibit osteoclast-mediated bone resorption and hence are widely used for tumor-associated osteolysis and osteoporosis to prevent the skeletal-related events associated with these conditions [Citation1,Citation2]. The use of bisphosphonates as adjuvant treatment in multiple myeloma (MM) is indicated at least in the first 2 years after the diagnosis of the disease [Citation2], and although they are widely known to have various beneficial effects [Citation3], it is recognized that osteonecrosis of the jaw is a potential adverse effect (<5%) in patients on long-term treatment with bisphosphonates [Citation4]. Commonly known as bisphosphonate-induced osteonecrosis of the jaws (BRONJ), this condition tends now to be described as medication-related osteonecrosis of the jaw (MRONJ), reflecting the increasing number of cases of osteonecrosis of the jaw associated with various different antiresorptive and antiangiogenic medications [Citation5]. It can be defined as exposed necrotic bone involving maxillary structures in patients treated with antiresorptive or antiangiogenic agents, and the pathophysiology of this condition remains unclear.
Inhibition of the function of osteoclasts has been observed, although osteoblasts and fibroblasts are also negatively affected in terms of abnormal proliferation [Citation4]. Local treatment with platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) promotes cell adhesion, proliferation and migration thanks to high concentrations of growth factors such as PDGF, TGF and IGF [Citation4]. In the last decade, PRF has been widely used in various fields, particularly in oral and maxillofacial surgery. According to one review, however, there is insufficient scientific evidence to recommend the use of autologous platelet concentrate together with surgical debridement or any other specific treatment protocol for the management of MRONJ, and there is a need for controlled randomized clinical trials of the use of autologous platelet concentrate [Citation6]. In contrast, a more recent review concluded that the scientific evidence from articles published on the use of PRF for bone and soft tissue regeneration in dentistry and maxillofacial surgery shows that PRF is a valuable tool that significantly improves bone and soft tissue regeneration [Citation7]. Nonetheless, the authors also underlined that the scientific community needs PRF protocols to be standardized to allow the benefits of PRF in bone and soft tissue regeneration to be properly assessed in reproducible studies providing stronger scientific evidence.
On the other hand, as an innovative approach to the treatment of MRONJ, it has also been suggested that a procedure involving various different approaches – namely, an Er:YAG laser device (2940 nm; Lightwalker – Fotona®, Lujbljana, Slovenia) (Yttrium, Y and Aluminium, A plus Erbium, Er) to ablate necrotic hard tissue until bleeding bone, in combination with PRP to stimulate healing of hard and soft tissue, and a diode laser (808 nm) for biostimulation of tissue at the surgical site, throughout all the steps in the treatment of this adverse event – may allow faster, less invasive surgery with a more comfortable healing process [Citation8]. In line with this, it was subsequently reported that conservative surgical treatment that combines the use of an Er,Cr:YSGG laser device (2780nm) (Biolase Technology®, CA, USA, EE.UU.) (Yttrium, Y; Scandium, S; & Gallium, G; plus Erbium, Er & Chromium, Cr) and PRP for the treatment of MRONJ in patients with cancer is efficient and effective [Citation9]. Nonetheless, the treatment of MRONJ remains controversial and to date there is insufficient evidence to establish a protocol for the treatment of patients with this condition.
PRP is a biological product that is defined as a part of the plasma fraction of blood that has a higher platelet concentration than that in the original whole blood [Citation10]. Platelet granules contain a mixture of key cytokines and growth factors. The use of PRP has gained remarkable attention in regenerative medicine in general and dentistry in particular [Citation11–13]. The main rationale for the use of PRP is that it acts as a biomaterial to deliver critical growth factors and cytokines from platelet granules to the target area, promoting tissue regeneration in various different types of tissue. Based on greater knowledge of cell signaling and growth factor biology, clinicians have started to use PRP treatment as a novel approach to regenerate damaged tissue, including liver, bone, cartilage, tendons and dental pulp [Citation13]. To improve our understanding of the regenerative effects of PRP in dentistry and oral medicine, there is a need to gather more data on different methods for preparing and applying this biological product, to explain the controversies surrounding its use and to assess future perspectives related to the use of PRP in regenerative dentistry and, by extension, maxillofacial medicine [Citation14].
Given all this, the regenerative potential of PRP is of great interest. Specifically, the main goal in the use of PRP in oral surgery is to regenerate new soft tissue and alveolar bone during the healing process. It has been suggested that platelets in PRP release a set of growth factors that recruit repair cells and promote new tissue during the healing process [Citation15]. Further, PRP is relatively easy to prepare in a dental practice and can be an autologous product [Citation16]. Therefore the application of PRP is of great clinical interest in oral surgery, due to its capacity for tissue regeneration and repair. In line with this, several studies have shown that PRP gel can avoid the development of osteitis and can significantly reduce postoperative pain and discomfort after tooth avulsion [Citation17–20]. Alissa et al. assessed the effect of PRP on the healing of extraction socket [Citation21]. Their findings suggested significantly less postoperative pain and clinically better healing of soft tissue in patients treated with PRP, compared with the control group. In relation to this, assessing patients undergoing third molar extraction, Ogundipe et al. showed that treatment with PRP was associated with significantly less pain, as well as a tendency to less swelling and greater mouth opening [Citation22]. Along similar lines, Ruktowski et al. reported significantly larger increases in radiographic density from baseline after tooth extraction at PRP-treated sites [Citation23], and Prataap et al. [Citation24] indicated that autologous PRP is a biocompatible material that improves soft tissue healing and reduces pain and the rate of alveolar osteitis. However, in all these studies, the exact type of PRP product tested is not clear, attributable to the lack of coherent terminology and associated misunderstandings [Citation21–24]. On the other hand, detailed evaluation of data published indicates that leukocyte- and platelet-rich plasma gels and leukocyte-poor, pure PRP are the products most widely tested in oral and maxillofacial surgery [Citation17]. In relation to this, PRP-related products have been studied in vitro and in vivo in maxillofacial surgery [Citation18–20]. Clinical researchers recommend the use of PRP before or during the placement of dental implants to increase the speed of bone deposition and quality of bone regeneration [Citation25]. As part of a simple and safe therapeutic approach, PRP has proved to be a promising technique in numerous studies on oral and maxillofacial surgery.
Based on whole blood, both autologous (au-PRP) and allogeneic (al-PRP) PRP products can be prepared. In some studies, al-PRP has been found to have a negligible level of immunogenicity and great curative efficacy [Citation26–28], but there has been relatively little research into this type of PRP. At the time of writing, we have not found any studies in humans using single-donor al-PRP with a single freeze–thaw cycle. Our group has accumulated experience over nearly a decade (since January 2012) in the management of PRP obtained from single (healthy) donors [Citation27–29], with promising results; in particular, we have reported the use of ABO-identical single donor platelets for the management of skin ulcers and secondary osteonecrosis of the jaw [Citation27].
Given all this, the objective of our study was to analyze and assess whether the use of al-PRP could serve as a feasible, effective and safe biological therapy for the treatment of osteonecrosis of the jaw. Our hypothesis was that al-PRP is a safe and effective treatment for MRONJ in patients with MM.
Materials & methods
Study design & participants
We conducted a cross-sectional study and report it here in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (www.strobe-statement.org/). However, our sample is very small; for this reason, the findings from the eight patients we present must be considered as preliminary results or the study considered as a pilot study.
Participants were recruited through the transfusion unit of the Complejo Asistencial Universitario de León between January 2017 and February 2021. In our study, seeking to deliver PDGFs, patients with MRONJ were treated with the application of al-PRP obtained by single-donor apheresis. All patients were treated for MM with Zometa®. In all cases, patients were treated using allogeneic platelets, as it was deemed inappropriate to take the blood samples necessary to prepare au-PRP, given the patients’ age, poor venous access and health problems (active cancer, specifically MM, in all cases, together with other conditions such as chronic myeloproliferative syndromes, in some cases).
The type of classification used to assess the severity of osteonecrosis was the one proposed by Ruggiero et al. following the recommendations of the American Association of Oral and Maxillofacial Surgeons (AAOMS) position papers in 2022 [Citation30].
Inclusion & exclusion criteria
All patients had to meet all inclusion criteria. The inclusion criteria were: patients who met the definition criteria of MRONJ proposed by Ruggiero et al. [Citation30]; patients aged 18 or over and having been referred to the hematology unit from other specialities, including maxillofacial surgery and oncology, with osteonecrosis of the jaw, secondary to the use of bisphosphonates (e.g., zoledronic acid, Zometa); adequate level of platelets, both numerically and qualitatively; poor venous access; and with written informed consent.
The exclusion criteria were: having a poor nutritional status (based on severe malnutrition, subclinical malnutrition or body composition indicative of malnutrition); a concomitant serious infection (positive serological results for hepatitis B surface antigen, anti-hepatitis C virus, anti-HIV or syphilis, as well as PCR for hepatitis B or C viruses or HIV, or infected skin ulcers) or lesions secondary to infected osteonecrosis; advanced age (over 90 years old); or functional inability to perform daily wound care alone and no caregiver able to undertake this task (every day for a prolonged period, typically 2–4 months or until wound closure).
Procedure
To provide PDGF for wound treatment, single-donor (apheresis) platelets were obtained strictly following the procedure described by Vidán-Estévez et al. [Citation28]. Specifically, an apheresis machine with a single-step centrifugation process using a mean speed of 2400–2800 rpm enabled us to obtain a concentration of platelets two- to three-fold higher than baseline. For each patient, apheresis platelets were obtained from one individual with the same ABO blood group, aliquoted into 2.5-ml syringes under sterile laminar flow conditions and frozen at -80°C. Patients were given seven vials a week and instructed to use one a day for treating their wounds: first, thawing the aliquot; then, applying the product across the wound; and lastly, covering the area treated with Espongostan® film or with their dental prosthesis itself in such cases. It was assumed that the freeze–thaw process would be sufficient to break down platelets, liberating granules with platelet growth factors involved in the repair of osteonecrosis of the jaw.
Statistical analysis
The sample size estimation was performed using the G* Power 3.1.97 statistical power analysis program (University of Dusseldorf, Dusseldorf, Germany; available at https://es.freedownloadmanager.org/Windows-PC/Gpower-GRATIS.html).
For qualitative variables, frequency and percentage tables were used to describe the data, and contingency tables to explore associations between the variables. Quantitative variables were expressed as mean, median, standard deviation and range. Mann–Whitney U tests were used for comparing the means between numerical variables, and χ-square tests of independence for assessing the relationship between categorical variables.
Further, R2 (between 0 and 1) was calculated as a measure of effect size, to allow comparisons between results for the different types of data and statistical tests, using Cohen’s d for comparing means and Cramer’s V coefficient for categorical variables and χ-square tests.
For all the inferential analysis, p-values < 0.05 were considered significant and those < 0.01 highly significant. The statistical analysis was performed using SPSS Statistics® v.25 (IBM Corp., NY, USA), except for effect sizes, which were calculated using G*Power freeware version 3.1.9.7 (available at: https://es.freedownloadmanager.org/Windows-PC/Gpower-GRATIS.html).
Ethical considerations & participant involvement
The study was approved by the Ethics and Clinical Research Committee of the University of León (Spain) (no. COD: ETICA-ULE-004-2016) and all patients gave written informed consent before inclusion. All the procedures were performed in accordance with the principles of the Declaration of Helsinki (2013, revised 5 May 2015) and in compliance with relevant Spanish and European ethical regulations and laws (on biomedical research in humans [14/2007] and on the protection of personal data [15/1999 and 3/2018, as well as the General Data Protection Regulation (EU) 2016/679]). Further, the University of León has a data protection officer, in charge of reporting, assessing and supervising adherence to the legal obligations regarding data protection (Start Up, S.L. contact email: [email protected]).
Regarding the treatment under study, the Spanish Agency of Medicines and Medical Devices (AEMPS) considers PRP to be a medicinal product for human use that can be used under Article 5 of Directive 2001/83/EC of November 6 and the provisions transposing the Directive into Spanish law. Specifically, the autologous plasma and its fractions, components and derivatives have been used in accordance with the provisions in the Resolution of 23 May 2013 and the AEMPS recommendations on the use of these products (www.aemps.gob.es/medicamentosUsoHumano/medSituacionesEspeciales/faqs-terapeutico-plasma-autologo.htm). Further, this study was conducted in strict compliance with all requirements of the current legislation, as well as the standards in terms of quality, efficacy, traceability, information and pharmacovigilance stated in the AEMPS report on the use of PRP (report/V1/23052013) (available at: www.aemps.gob.es/medicamentosUsoHumano/medSituacionesEspeciales/docs/PRP-AEMPS-DEF-mayo13.pdf).
Patient & public involvement
In this research, patients were not invited to comment on the study design, consulted to develop patient-relevant outcomes or interpret the results, or invited to contribute to the editing of this paper.
Results
In this report we present just our preliminary results. A total of eight patients with MM and MRONJ were finally included in the study and treated with al-PRP; other patients were lost due to death during recruitment or lack of treatment follow-up.
None of the patients had high blood pressure or cardiovascular disease, but two had diabetes mellitus; all of them had been treated with zoledronic acid. Regarding the demographic characteristics of the sample, three-quarters of the patients were men (n = 6) and the overall age range was 52–87 years (mean: 76.63 ± 12.33; median: 83), nearly two-thirds (62.5%) of the patients being between 80 and 89 years of age. The mean age of the men was slightly higher than that of the women (78.7 vs 73.0 years), although the difference did not reach significance (Mann–Whitney: ZU = 1.02; p = 0.429).
Regarding the medical history of participants, treatment received for MM was the VMP regimen (bortezomib [Velcade®], melphalan and prednisone) in two cases, cyclophosphamide–prednisone in another two and melphalan–prednisone in one; two patients, the youngest, who were both considered candidates for transplantation, received the VTD regime (bortezomib [Velcade], thalidomide and prednisone) followed by autologous bone marrow transplantation after melphalan conditioning. The last patient was referred from oncology and died due to disease progression during this study; it is known that he had metastatic kidney cancer but we have been unable to access data on the treatment received for this cancer.
Regarding blood group, four patients were group O, and the other four group A; five were Rh-positive and three Rh-negative (62.5 vs 37.5%). The most common group was A+ (three cases) followed by O+ and O- with two cases each, and finally one patient was A-.
shows a descriptive analysis of the sample.
Table 1. Descriptive analysis of the patients.
Reviewing the wounds, they were all located in two regions of the face – inferior border and ramus in the mandible and the inferior border of the mandible – and they ranged from 1 to 5 cm in size (mean: 2.25 cm), the majority being 2–3 cm. Two cases (25%) were classified as stage 1, three (37.5%) as stage 2, one (12.5%) as stage 2–3 and two (25%) as stage 3.
Concerning previous wound treatment, half of the patients (n = 4) had received previous treatment at the hospital (local wound care in two cases and surgery in the other two), while the others had received wound care at home. The previous treatments had lasted for between 1 and 5 months, with a mean of nearly 2 months and half having been treated for more than 3 months. All patients received intravenous bisphosphonates for a minimum of 2 years, because all cases were patients with a recent diagnosis of MM. In all cases the drug used was zoledronic acid (Zometa).
Treatment with al-PRP was continued for between 6 and 14 weeks (mean of 9 weeks). In all five patients treated for 7 weeks or less, complete healing was observed; one wound did not heal with an intermediate length of treatment (between 8 and 12 weeks) and at this stage the patient died due to disease progression; lastly, treatment was continued for more than 13 weeks in the other two patients. shows a descriptive analysis of the wounds. shows an example of a case; one of the patients treated in the current study.
Table 2. Descriptive analysis of the wounds.
At the time of writing, partial healing (50% reduction in wound size) has been achieved in these two patients. Considering together these cases and the five patients in whom complete wound healing has been achieved (wounds requiring no further treatment), we observed a treatment success rate of 87.5% (p < 0.05; ).
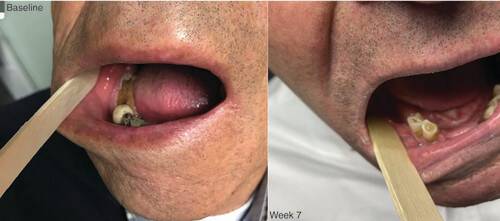
Example of a case of medication-related osteonecrosis of the jaw treated with allogeneic platelet-rich plasma. Left: Before al-PRP treatment (baseline). Right: Control after 7 weeks of treatment.
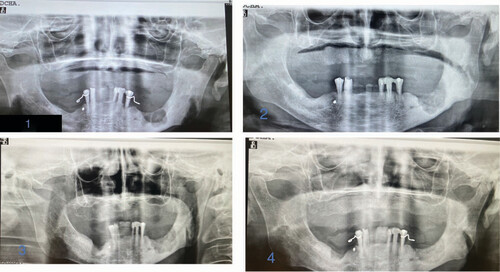
Total weeks of allogeneic platelet-rich plasma treatment = 14. Complete wound healing. (1) Radiological image pre-treatment (baseline). (2) Radiographic control at 15 months from baseline. (3) Radiographic control at 15 months from baseline. (4) Radiographic control at 17 months from baseline.
Total weeks of allogeneic platelet-rich plasma treatment = 6. Complete wound healing. (1) Radiological image pre-treatment (baseline). (2) Radiographic control at 5 months from baseline. (3) Radiographic control at 8 months from baseline.
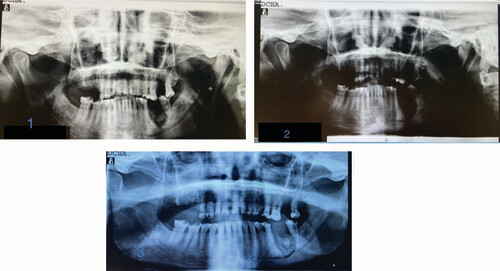
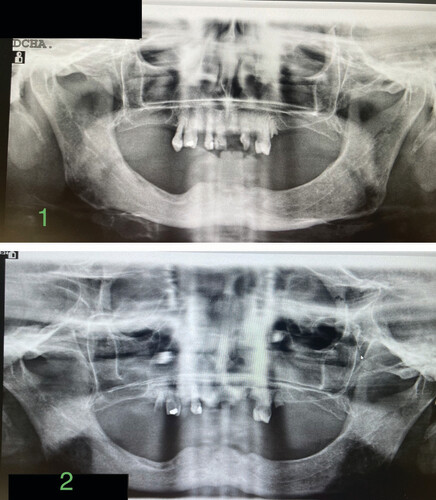
Total weeks of allogeneic platelet-rich plasma treatment = 13. Partial healing. (1) Radiological image pre-treatment (baseline). (2) Radiographic control at 9 months from baseline.
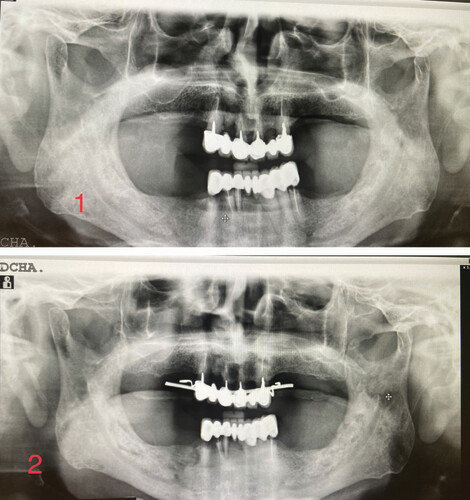
Total weeks of allogeneic platelet-rich plasma treatment = 7. Complete wound healing. (1) Radiological image pre-treatment (baseline). (2) Radiographic control at 10 months from baseline.
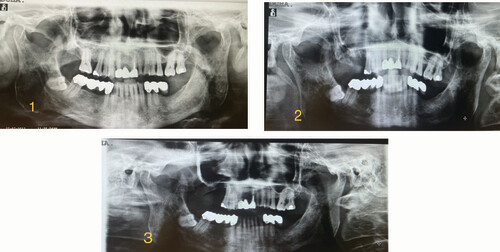
Total weeks of allogeneic platelet-rich plasma treatment = 7. Complete wound healing. (1) Radiological image pre-treatment (baseline). (2) Radiographic control at 6 months from baseline. (3) Radiographic control at 21 months from baseline.
Assessing the association between healing and treatment duration using χ-square tests, we observed a statistically significant relationship (χ2 = 8.00; p = 0.018). The corresponding effect size was very large (as indicated by a Cramer’s V of 1), confirming that healing was very closely related to the duration of the treatment.
Discussion
To our knowledge, this is the first study to provide data on the use of al-PRP prepared with one freeze–thaw cycle for the treatment of MRONJ. Indeed, we have not found any reports of the use of al-PRP in MRONJ in the scientific literature.
In our study, complete healing was observed in nearly two-thirds of cases (five out of eight patients) and partial healing in one-quarter (two further patients). Given this, we can consider PRP treatment to have been a success in 87.5% of cases (seven out eight), and the only other patient died while wound treatment was ongoing. Our analysis confirmed a statistically significant association between healing and treatment duration, and the effect size was very large.
Strategies for the management of MRONJ
The treatment of MRONJ is still a challenge. It is recognized that MRONJ-associated wounds respond poorly to many traditional treatment approaches (e.g., antibiotics and mouth washes). In particular, as conservative treatment often fails, surgery has been widely recommended for the management of the most advanced cases; furthermore, a workshop of the European Task Force on MRONJ suggested that nonsurgical treatment may lead to disease progression [Citation31]. However, this approach implies more bone involvement and the symptoms associated with major infections due to the risk of bacteremia and sepsis in immunocompromised patients, as well as impairment of patient quality of life [Citation5,Citation32]. In this context, a variety of treatment options have been proposed as adjuvants or alternatives to surgery, seeking to improve outcomes in patients with MRONJ. In the literature, however, strategies proposed for managing this type of osteonecrosis remain a matter of debate and there is a need to identify approaches for treating this condition that have predictable results.
Notably, a Cochrane review published in 2018 found a lack of evidence from randomized controlled trials to guide the treatment of BRONJ [Citation33]. A more recent review assessed the efficacy of hyperbaric oxygen therapy, low-level laser therapy and PRP in the treatment of MRONJ [Citation34]. First, improvement was observed in three-quarters of the 41 patients who received hyperbaric oxygen therapy, with positive effects in terms of faster pain relief and faster and greater reductions in the size and number of lesions; better results were seen when the medication suspected to be the cause of the condition was discontinued. Second, regarding low-level laser therapy, symptoms improved in two-thirds of the 246 patients/sites (cases) treated, and complete healing was observed in 40% of cases. Lastly, with PRP, complete healing was achieved in 65 out of the 81 patients treated (80.2%) and significant improvement in symptoms in a further 14 (17.3%). The authors concluded that all these treatments are safe and effective adjuvant treatments for MRONJ. Nonetheless, as in the Cochrane review, the reviewers concluded that there was a need for more high-quality research.
Stem cells have also been proposed as a treatment for MRONJ. In 2015 Barba-Recreo et al. compared various potential preventive treatments for MRONJ after tooth extractions in animals treated with zoledronic acid and studied the local topical use of different combinations of adipose tissue-derived stem cells (ASCs) with or without prior stimulation with BMP2 and PRP in a MRONJ high-risk model in rats [Citation35]. Their results were very promising, in that the ASC-treated animals had a lower rate of osteonecrosis (14 vs 50%; p = 0.007) and greater bone turnover (p = 0.024) and osteoclast count (p = 0.045) than other animals. Further, it seemed that the combination of ASCs and PRP had a synergic effect and that adding BMP2 might achieve even better results. More recently, other authors have obtained similar results in rats. One study described activation of previously inhibited post-extraction bone remodeling which seemed to be associated with ASC implantation [Citation36]. Another assessed the effects of allogeneic transplantation of bone marrow-derived mesenchymal stem cells (MSCs) in an MRONJ animal model (using Wistar rats) [Citation37]. The authors concluded that allogeneic bone marrow-derived MSCs at extraction sites reduced the rates of MRONJ in rats treated with zoledronic acid, compared with treatment without MSCs. It seems that various cell therapy approaches using MSCs may offer safe, effective treatment options to prevent the development of MRONJ in patients who have to be treated with antiresorptive medication for conditions such as osteoporosis or other metabolic bone diseases/cancer [Citation37]. Nonetheless, further research is necessary to justify the use of in vivo cell therapy using MSCs to treat or even prevent the development of MRONJ in humans.
PRP as an adjuvant in the treatment of MRONJ
In the search for a cure for MRONJ, bone resection combined with the use of growth factors has shown promising results in the treatment of related conditions in animal models [Citation35–39]. For clinical practice, growth factors could be provided by PRP, a product which can be obtained by centrifugation of whole blood, achieving a very high concentration of platelets containing various different growth factors (PDGF, TGF-β, EGF and VEGF).
As well as the aforementioned review [Citation34], various small studies have been reported using au-PRP to treat osteonecrosis of the jaw. For example, Coviello et al. assessed the benefits of PRP for wound healing in patients with MM who developed osteonecrosis of the jaw after surgical tooth extraction [Citation40]. Their study included seven patients (two men and five women) who had been receiving intravenous zoledronic acid or palindromic acid followed by zoledronic acid for an average of 5 years. To treat the osteonecrosis of the jaw, four of the patients only underwent standard surgical debridement and sequestrectomy (excision of ulcerated mucosa and bone margins until cortical bone covered with periosteum is reached), while three received the same surgical treatment and additionally au-PRP. Patients were followed for 3 months, and the findings indicated that the use of PRP to improve wound healing and reduce bone exposure was a good treatment regimen in these patients. However, a recent review reported that there is insufficient evidence to establish the efficacy of the application of autologous platelet concentrates in the prevention and treatment of MRONJ [Citation41].
Overall, numerous laboratory-based studies [Citation35–39] suggest that local topical treatment with au-PRP or al-PRP may be a feasible regenerative treatment for promoting tissue repair, and there have been some promising clinical studies using surgery in combination with PRP, but there is a lack of well-defined protocols for the treatment of MRONJ, hindering the standardization of this treatment approach.
Alternative platelet-rich modalities
Besides PRP, there are a wide range of platelet-rich modalities; these include PRF. In 2017 a study was conducted in humans which recruited 23 patients (15 women and eight men, between 52 and 73 years old) with MRONJ [Citation5]. These patients had a history of various lengths of treatment with bisphosphonates and bone exposed in the maxillofacial area for more than 8 weeks and no history of radiotherapy targeting the maxillary bone. Patients were treated using surgical curettage and PRF. Based on the findings, it was suggested that PRF may act as an effective barrier membrane between the alveolar bone and the oral cavity and could be effective for the closure of bone exposure in patients with MRONJ.
More recently, in 2019, Steller et al. reported exposing fibroblasts and osteoblasts treated with zoledronic acid to PRP/PRF [Citation42]. They found significantly greater closure of the scratch area by osteoblasts or fibroblasts treated with PRP or PRF (40.6 and 100.0% by PRP- and PRF-treated osteoblasts; and 100.0% by both PRP- and PRF-treated fibroblasts vs 0.0 and 12.7% in the negative controls, respectively; p ≤ 0.05 in all cases). Further, the negative effect of zoledronic acid on cell migration was generally weakened by using PRP/PRF in both cell lines. After zoledronic acid exposure, cell viability and proliferation decreased, while cell viability improved within 24 h following PRP/PRF application, and zoledronic acid had a notably less negative effect on cell proliferation when PRF was used. Overall, the use of either PRF or PRP seemed to improve the behavior of cells treated with zoledronic acid, and these findings suggested that PRF and PRP may have positive effects in the treatment of BRONJ, although PRF offered some advantages over PRP. Similarly, another recent study obtained significantly better results in terms of recovery and recurrence rates in patients who underwent surgery combined with PRF membrane therapy than in those who underwent traditional surgical treatment alone [Citation43]. Other authors have considered using a second-generation platelet concentrate, such as a natural autologous fibrin matrix (leukocyte and platelet-rich fibrin [LPRF]), which accelerates angiogenesis and has a fibroblast and osteoblast proliferation and an anti-infectious effect through immune regulation [Citation44]; as a consequence, it avoids the exposure of the alveolar bone in the oral cavity, through stimulating the healing of the soft tissues. Specifically, Maluf et al. show two cases of women with breast cancer who were being treated with zoledronic acid and had advanced MRONJ [Citation45]. These cases were treated by surgical resection of the necrotic bone, followed by the placement of an LPRF membrane. Intact mucosal cover and complete wound healing were achieved. In the 2-year clinical and CT scan follow-up, neither patient reported any complaints, and no oral lesions were observed. The authors concluded that this could be a rapid and noninvasive treatment option for managing bone exposure. In particular, the LPRF membrane may help to achieve successful outcomes by serving as a physical barrier against microorganisms and preventing secondary infections. The following year in a study of MRONJ most commonly associated with zoledronic acid and denosumab (60% of the sample) [Citation31], the condition resolved in 78% of the cases (11/14) in which LPRF was used as an adjuvant therapy. Based on these results, it can be suggested that the treatment was effective, and hence was a promising approach [Citation32].
Overall, there is growing evidence that PRF and LPRF are effective in MRONJ. The preparation of these products, with a relatively dense fibrin architecture is, however, somewhat more complex than that of PRP; that is, PRP is arguably a more straightforward option in clinical practice and also seems to be effective, recalling that the aforementioned review found that PRP was associated with complete healing in over 80% of cases and with improvements in most other patients treated [Citation42]. Another issue to consider in practice is that these results refer to au-PRP, and it may not be appropriate to take the blood necessary to prepare this type of PRP from some patients with non-healing wounds. In such cases, al-PRP may be a better option.
Use of al-PRP in the treatment of MRONJ
At the time of writing, we have not found any other studies in humans using single-donor al-PRP in patients with MRONJ, and we believe that our study opens a new avenue of research and provides interesting new data. Specifically, to our knowledge, we report the first scientific evidence of the value of single-donor apheresis platelets prepared using one freeze–thaw cycle for the treatment of MRONJ. Having assessed the current status of our patients, our findings indicate that al-PRP treatment has been successful in 87.5% of the eight cases, healing being significantly related to the duration of this treatment. Notably, our findings are of translational relevance, given that they suggest that treatment based on al-PRP could be sufficiently successful to improve patients’ quality of life.
Implications for practice
In our study, seeking to deliver PDGFs, we applied al-PRP having activated the platelets with a single freeze–thaw cycle and obtained the product using whole blood from a single donor with an apheresis machine and a single centrifugation step (at a speed of 2400–2800 rpm), achieving a platelet concentration two- to three-fold that of baseline. This approach is relatively straightforward, quick and cheap to implement in practice.
Considering these preliminary results together with other available scientific evidence, it is reasonable to suggest that al-PRP could be a promising treatment for MRONJ. If future studies confirm successful healing with this approach, it could be recommended for promoting healing in this type of osteonecrosis. Further, while al-PRP seems to be a successful treatment in advanced wounds, we consider that its preparation is sufficiently straightforward that its use could be recommended at an earlier stage, to prevent the ulceration advancing. Also, the recommendations of the workshop of the European Task Force on MRONJ [Citation31] and the American Association of Oral and Maxillofacial Surgeons position papers in 2022 [Citation30] should be followed for clinicians.
Limitations
In this report we present only our preliminary results. One of the limitations of our study is the small sample size. Future research with larger samples is necessary; similarly, randomized clinical trials are needed to accurately determine the efficacy and effectiveness of the treatment compared with usual care, although, for ethical reasons, it would not be possible to carry out studies compared with placebo. Therefore, in the future, large-scale randomized controlled trials should be conducted to explore the advantages and disadvantages of al-PRP.
It is known that MRONJ healing criteria must be accompanied by a long follow-up period and also by radiological imaging. However, due to advanced age and poor clinical situations, at the present time (September 2022) only one patient lives; the rest have died (87.5%). For this reason, it is not possible for us to have a long-term follow-up of all our patients.
We do not know why complete healing tended to occur at around 7 weeks. In addition to zoledronic acid, some patients were taking other medications, which could have influenced the healing outcome. Thus, this aspect requires further investigation. Future research is necessary to accurately establish the ideal length of treatment. For this reason, we should also carry out a study exploring different cutoff points (e.g., week 7 vs week 13).
Conclusion
Given the results observed in our patients over weeks of treatment with al-PRP – specifically, single-donor (apheresis) platelets prepared as described herein with one freeze–thaw cycle – we conclude that al-PRP could be a recommended treatment for MRONJ. With our knowledge at the present time, it is preliminary to say whether the application of PDGF in the treatment of MRONJ will be adequate in clinical settings. Future research is needed to perform in vitro characterization; in addition, more studies with a large cohort of patients are required to validate our findings.
The treatment of medication-related osteonecrosis of the jaw (MRONJ) with platelet-rich plasma (PRP) remains controversial and to date there is insufficient evidence to establish a protocol for the treatment of patients with this condition.
This is the first study to provide data on the use of allogeneic PRP prepared with one freeze–thaw cycle for the treatment of MRONJ.
We can consider PRP treatment to have been a success in 87.5% of cases.
Our analysis confirmed a statistically significant association between healing and treatment duration, and the effect size was very large.
Allogeneic PRP could be a recommended treatment for MRONJ.
While allogeneic PRP seems to be a successful treatment in advanced wounds, we consider that its preparation is sufficiently straightforward that its use could be recommended at an earlier stage, to prevent the ulceration advancing.
Future research with larger samples is necessary to validate these results.
Author contributions
Conceptualization: J Vidán-Estévez and J Seco-Calvo. Data curation: J Vidán-Estévez, F Barrrigon and J Seco-Calvo. Formal analysis: J Seco-Calvo. Funding acquisition: J Vidán-Estévez and J Seco-Calvo. Investigation: J Vidán-Estévez, F Barrrigon, S Sanchez-Herraez and J Seco-Calvo. Methodology: J Vidán-Estévez, F Barrrigon, S Sanchez-Herraez and J Seco-Calvo. Project administration, resources, software, supervision, validation, visualization, writing (original draft): J Seco-Calvo. Writing (review and editing): J Vidán-Estévez, F Barrrigon, S Sanchez-Herraez and J Seco-Calvo.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval from the Ethics Committee of the University of Leon (ref: COD: ETICA-ULE-004-2016) and have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- ChienHI , ChenLW , LiuWCet al.Bisphosphonate-related osteonecrosis of the jaw. Ann. Plast. Surg.86(25), S78–S83 (2021).
- MarinoS , PetruscaDN , RoodmanGD. Therapeutic targets in myeloma bone disease. Br. J. Pharmacol.178(9), 1907–1922 (2021).
- GozzettiA , GennariL , MerlottiDet al.The effects of zoledronic acid on serum lipids in multiple myeloma patients. Calcif. Tissue. Int.82(4), 258–262 (2008).
- StellerD , HerbstN , PriesR , JuhlD , HakimSG. Impact of incubation method on the release of growth factors in non-Ca2+-activated PRP, Ca2+-activated PRP, PRF and A-PRF. J. Craniomaxillofac. Surg.47(2), 365–372 (2019).
- InchingoloF , CantoreS , DipalmaGet al.Platelet rich fibrin in the management of medication-related osteonecrosis of the jaw: a clinical and histopathological evaluation. J. Biol. Regul. Homeost. Agents.31(3), 811–816 (2017).
- Lopez-JornetP , SanchezPerez A , AmaralMendes Ret al.Medication-related osteonecrosis of the jaw: is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J. Craniomaxillofac. Surg.44(8), 1067–1072 (2016).
- GhanaatiS , Herrera-VizcainoC , Al-MaawiSet al.Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery: how high is the level of scientific evidence?J. Oral. Implantol.44(6), 471–492 (2018).
- FornainiC , CellaL , OppiciAet al.Laser and platelet-rich plasma to treat medication-related osteonecrosis of the jaws (MRONJ): a case report. Laser Ther.26(3), 223–227 (2017).
- MauceriR , PanzarellaV , ManiscalcoLet al.Conservative surgical treatment of bisphosphonate-related osteonecrosis of the jaw with Er,Cr:YSGG laser and platelet-rich plasma: a longitudinal study. Biomed. Res. Int.2018, 3982540 (2018).
- AnituaE. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implants14, 529–535 (1999).
- AgrawalAA. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World. J. Clin. Cases5(5), 159–171 (2017).
- MurrayPE. Platelet-rich plasma and platelet-rich fibrin can induce apical closure more frequently than blood-clot revascularization for the regeneration of immature permanent teeth: a meta-analysis of clinical efficacy. Front. Bioeng. Biotechnol.6, 139 (2018).
- XuJ , GouL , ZhangPet al.Platelet-rich plasma and regenerative dentistry. Aust. Dent. J.65(2), 131–142 (2020).
- AttiaS , NarberhausC , SchaafHet al.Long-term influence of platelet-rich plasma (PRP) on dental implants after maxillary augmentation: retrospective clinical and radiological outcomes of a randomized controlled clinical trial. J. Clin. Med.9(2), 355 (2020).
- YangLC , HuSW , YanMet al.Antimicrobial activity of platelet-rich plasma and other plasma preparations against periodontal pathogens. J. Periodontol.86(2), 310–318 (2015).
- ZhuW , ZhuX , HuangGTet al.Regeneration of dental pulp tissue in immature teeth with apical periodontitis using platelet-rich plasma and dental pulp cells. Int. Endod. J.46(10), 962–970 (2013).
- DelCorso M , VervelleA , SimonpieriAet al.Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol.13(7), 1207–1230 (2012).
- MasukiH , OkuderaT , WatanebeTet al.Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent.2(1), 19 (2016).
- FranchiniM , CrucianiM , MengoliCet al.The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfus.17(5), 357–367 (2019).
- FanY , PerezK , DymH. Clinical uses of platelet-rich fibrin in oral and maxillofacial surgery. Dent. Clin. North Am.64(2), 291–303 (2020).
- AlissaR , EspositoM , HornerKet al.The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur. J. Oral Implantol.3(2), 121–134 (2010).
- OgundipeOK , UgbokoVI , OwotadeFJ. Can autologous platelet-rich plasma gel enhance healing after surgical extraction of mandibular third molars?J. Oral Maxillofac. Surg.69(9), 2305–2310 (2011).
- RutkowskiJL , JohnsonDA , RadioNMet al.Platelet rich plasma to facilitate wound healing following tooth extraction. J. Oral Implantol.36(1), 11–23 (2010).
- PrataapN , SunilPM , SudeepCBet al.Platelet-rich plasma and incidence of alveolar osteitis in high-risk patients undergoing extractions of mandibular molars: a case–control study. J. Pharm. Bioallied Sci.9(5), S173–S179 (2017).
- SanchezAR , SheridanPJ , KuppLI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int. J. Oral Maxillofac. Implants18, 93–103 (2003).
- ScevolaS , NicolettiG , BrentaFet al.Allogenic platelet gel in the treatment of pressure sores: a pilot study. Int. Wound J.7(3), 184–190 (2010).
- Vidán-EstévezJ , EscalanteF , EscribanoPet al.Uso de los factores de crecimiento derivados de las plaquetas en úlceras cutáneas y osteonecrosis mandibular secundarias: una terapia eficaz. Presented at: LV Congreso Nacional de la Sociedad Española de Hematología y Hemoterapia.Seville, Spain, 17–19 October, 2013.
- Vidán-EstévezJ , Sánchez-HerráezS , Escalante-BarrigónFet al.Healing of chronic wounds with platelet-derived growth factors from single donor platelet-rich plasma following one freeze–thaw cycle. a cross-sectional study. J. Clin. Med.10, 5762 (2021).
- Seco-CalvoJ , Vidán-EstévezJ , Sánchez-HerráezS. Successful healing of non-healing surgical wound based on the release of platelet-derived growth factors from single donor allogeneic platelet-rich plasma with one freeze-thaw cycle: a case report after a 1-year follow-up. Transl. Med. Commun.7, 14 (2022).
- RuggieroSL , DodsonTB , AghalooTet al.American Association of Oral and Maxillofacial Surgeons’ position paper on medication-related osteonecrosis of the jaws – 2022 update. J. Oral Maxillofac. Surg.80(5), 920–943 (2022).
- SchiodtM , OttoS , FedeleSet al.Workshop of European Task Force on medication-related osteonecrosis of the jaw – current challenges. Oral Dis.25(7), 1815–1821 (2019).
- ValenteNA , ChatelainS , AlfonsiFet al.Medication-related osteonecrosis of the jaw: the use of leukocyte-platelet-rich fibrin as an adjunct in the treatment. J. Craniofac. Surg.30(4), 1095–1101 (2019).
- RollasonV , LaverrièreA , MacDonaldLCet al.Interventions for treating bisphosphonate-related osteonecrosis of the jaw (BRONJ). Cochrane Database Syst. Rev.2, CD008455 (2016).
- deSouza Tolentino E , de CastroTF , MichellonFCet al.Adjuvant therapies in the management of medication-related osteonecrosis of the jaws: systematic review. Head Neck41(12), 4209–4228 (2019).
- Barba-RecreoP , DelCastillo Pardo de Vera JL , Georgiev-HristovTet al.Adipose-derived stem cells and platelet-rich plasma for preventive treatment of bisphosphonate-related osteonecrosis of the jaw in a murine model. J. Craniomaxillofac. Surg.43(7), 1161–1168 (2015).
- Alonso-RodriguezE , González-Martín-MoroJ , Cebrián-CarreteroJLet al.Bisphosphonate-related osteonecrosis. Application of adipose-derived stem cells in an experimental murine model. Med. Oral Patol. Oral Cir. Bucal.24(4), e529–e536 (2019).
- Rodríguez-LozanoFJ , Oñate-SánchezR , Gonzálvez-GarcíaMet al.Allogeneic bone marrow mesenchymal stem cell transplantation in tooth extractions sites ameliorates the incidence of osteonecrotic jaw-like lesions in zoledronic acid-treated rats. J. Clin. Med.9(6), 1649 (2020).
- CardosoCL , CurraC , CuriMMet al.Treatment of bisphosphonate-related osteonecrosis using platelet-rich plasma: microtomographic, microscopic, and immunohistochemical analyses. Braz. Oral Res.33, e050 (2019).
- ToroLF , de Mello-NetoJM , SantosFFVDet al.Application of autologous platelet-rich plasma on tooth extraction site prevents occurence of medication-related osteonecrosis of the jaws in rats. Sci. Rep.9(1), 22 (2019).
- CovielloV , PelusoF , DehkharganiSZet al.Platelet-rich plasma improves wound healing in multiple myeloma bisphosphonate-associated osteonecrosis of the jaw patients. J. Biol. Regul. Homeost. Agents26(1), 151–155 (2012).
- FortunatoL , BennardoF , BuffoneCet al.Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg.48(3), 268–285 (2020).
- StellerD , HerbstN , PriesRet al.Positive impact of platelet-rich plasma and platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep.9, 8310 (2019).
- SzentpeteriS , SchmidtL , RestarLet al.The effect of platelet-rich fibrin membrane in surgical therapy of medication-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg.78(5), 738–748 (2020).
- GiudiceA , BaroneS , BennardoF. Effect of platelet-rich fibrin in surgical treatment of medication-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg.78(10), 1659 (2020).
- MalufG , CaldasRJ , SilvaSantos PS. Use of leukocyte- and platelet-rich fibrin in the treatment of medication-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg.76(1), 88–96 (2018).
