Abstract
Aims: Given the logistical issues surrounding intramyocardial cell delivery, we sought to address the efficacy of the simpler, more accessible intracoronary route by re-evaluating REGENERATE-DCM and REGENERATE-IHD (autologous cell therapy trials for heart failure; n = 150). Methods: A retrospective statistical analysis was performed on the trials’ combined data. The following end points were evaluated: left ventricular ejection fraction (LVEF), N-terminal pro brain natriuretic peptide concentration (NT-proBNP), New York Heart Association class (NYHA) and quality of life. Results: This demonstrated a new efficacy signal for intracoronary delivery, with significant benefits to: LVEF (3.7%; p = 0.01), NT-proBNP (median -76 pg/ml; p = 0.04), NYHA class (48% patients; p = 0.01) and quality of life (12 ± 19; p = 0.006). The improvements in LVEF, NYHA and quality of life scores remained significant compared to the control group. Conclusion: The efficacy and logistical simplicity of intracoronary delivery should be taken into consideration for future trials.
Tweetable abstract
The efficacy and logistical simplicity of intracoronary delivery should be considered when planning cell therapy trials.
Graphical abstract
Plain language summary
Trials of cell therapy for heart failure have not clearly identified the best method to deliver the cells to the heart. A small proportion of these studies have used the intracoronary method (which infuses the cells into the heart’s arteries) as it was thought to be less effective. However, this is the simplest method and uses widely accessible techniques and equipment. By combining data from two previous heart failure trials, we sought to look for an efficacy signal for the intracoronary method in a larger sample size. We found that the intracoronary route demonstrated improvements in patients’ heart function and symptoms. Although it may require a larger number of patients to show efficacy, this signal, alongside the intracoronary route’s relative simplicity, should be taken into consideration when future trials of cell therapy for heart failure are planned.
Heart failure remains a major cause of death worldwide. Despite improvements in treatment, the prognosis for symptomatic heart failure is poor, with 5- and 10-year survival rates of approximately 50% and 10%, respectively [Citation1,Citation2]. Stem cell therapies have shown potential benefit for heart failure patients with no further treatment options [Citation3,Citation4,Citation5,Citation6]. Autologous bone marrow-derived cells (BMCs) have been the most utilized cell type in cell therapy trials due to their accessibility and low cost of isolation [Citation7]. To date, most studies of cell therapy for heart failure have used the intramyocardial route to deliver cell therapy [Citation6]; however, with the recent withdrawal of the most utilized technology (NOGA XP electromechanical mapping system; BioSense Webster, CA, USA) from the market, there is a need to determine whether other delivery routes are also efficacious. In order to address this, we combined the data from two phase II cell therapy trials for heart failure to evaluate the efficacy of intracoronary delivery in a larger sample size.
The data presented here are a comparative re-evaluation of REGENERATE-DCM [Citation8] and REGENERATE-IHD [Citation9]. These randomized controlled clinical trials examined the combination of cytokine and autologous BMC therapy on cardiac function in patients with heart failure (dilated cardiomyopathy in REGENERATE-DCM and ischemic heart failure in REGENERATE-IHD). These trials had similar designs and end points, used the same protocol for granulocyte colony stimulating factor (G-CSF) and cell administration, and involved both the intracoronary and intramyocardial delivery of cell therapy. Although REGENERATE-DCM showed significant efficacy signals in the intracoronary arm [Citation8], the intracoronary signals in REGENERATE-IHD were not statistically significant [Citation9]. In keeping with pooled analyses [Citation10,Citation11] and trials that have used combined heart failure etiologies [Citation12,Citation13,Citation14,Citation15], this exploratory evaluation was performed to establish whether combining the intracoronary arms of both trials would lead to a clearer efficacy signal relevant for the planning of future cell therapy trials.
Methods
Study design & participants
REGENERATE-DCM and REGENERATE-IHD were randomized controlled clinical trials that examined the effect of combined G-CSF and autologous BMC therapy on cardiac function and symptoms in heart failure patients. Both studies were performed at the same institution, used the same protocol for G-CSF and cell administration, had very similar end points and were approved by the relevant ethics committees. The trial designs have been previously published [Citation16,Citation17] and are briefly outlined below.
In REGENERATE-DCM, patients with dilated cardiomyopathy (DCM) and no further treatment options were randomized to either the peripheral (control) arm or the intracoronary (IC) arm. In the peripheral arm, the patients were further randomized to placebo (saline; n = 15) or G-CSF alone (n = 15). In the IC arm, the patients were further randomized to G-CSF combined with BMCs (n = 15) or G-CSF and placebo (serum; n = 15; ) [Citation8].
BM: Bone marrow; BMC: Bone marrow-derived cell; G-CSF: Granulocyte colony-stimulating factor; IC: Intracoronary; IM: Intramyocardial, LVEF: Left ventricular ejection fraction; MACE: Major adverse cardiac events; NT-proBNP: N-terminal pro brain natriuretic peptide; NYHA: New York Heart Association Class; QoL: Quality of life scores.
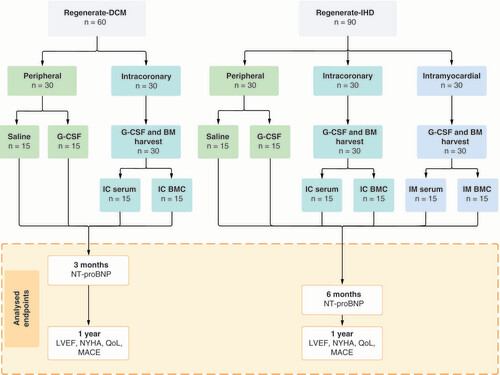
In REGENERATE-IHD, patients with ischemic heart disease (IHD) and no further treatment options were randomized to the peripheral (control) arm, IC arm or intramyocardial (IM) arm. Patients in the peripheral arm were further randomized to placebo (saline; n = 15) or G-CSF alone (n = 15). Patients in the IC arm were further randomized to G-CSF combined with BMCs (n = 15) or G-CSF and placebo (serum; n = 15). Patients in the IM arm were also further randomized to G-CSF combined with BMCs (n = 15) or G-CSF and placebo (serum; n = 15; ) [Citation9].
Both trials used the same standardized protocol for preparing and injecting the cells. In the IC and IM arms, patients underwent a bone marrow harvest after 5 days of subcutaneous G-CSF treatment. BMCs were isolated by density gradient centrifugation and were washed and resuspended in autologous serum at a final volume of 10 ml for IC delivery or 2 ml for IM injection. The cells or placebo was administered within 6 h of bone marrow harvest. The trials were open-label, but the operators were blinded to the treatment in the cell therapy arms. Data analysts were entirely masked to the group assignment in both trials.
End points & definitions
The trials were designed with similar end points, which supports the rationale for this comparative re-evaluation. The end points analyzed here are: change in global left ventricular ejection fraction (LVEF; as assessed by cardiac magnetic resonance imaging [MRI] or computed tomography where MRI was not possible), New York Heart Association (NYHA) class, quality of life scores (as assessed by European Quality of Life-5 Dimensions Visual Analogue Scale [EQ-5D VAS] and European Quality of Life-5 Dimensions Time to Trade Off Index [EQ-5D Index]), adverse clinical events (including death, myocardial infarction and hospitalization for heart failure) and significant arrhythmias (symptomatic ventricular tachycardia or survived cardiac death) compared with baseline at 1 year. As follow-up measurements of N-terminal pro brain natriuretic peptide (NT-proBNP) concentration were made at 3 months and 1 year in REGENERATE-DCM and at 6 months in REGENERATE-IHD, the levels at 3 and 6 months compared with baseline are presented in this analysis.
Statistical analysis
For continuous outcome analysis, within-group differences between baseline and 1-year measurements were calculated and tested statistically using the paired t-test. Continuous variables were log-transformed for analyses when their distributions were considered log-normal upon assessment. For the categorical (ordinal) outcome of NYHA functional class, the percentage of patients experiencing improvement and the percentage experiencing a decrease in class by 1 year compared with baseline were calculated. The difference in NYHA functional class between baseline and 1 year was tested statistically using Bowker’s test of symmetry for paired ordinal categorical data. For the between-group analysis, a one-way analysis of variance (ANOVA) was performed with a Bonferroni correction for multiple testing. All p-values were from two-tailed tests, and p < 0.05 was considered statistically significant. All statistical analysis was performed using Stata, version 15.1 (StataCorp, TX, USA).
Results
Patients & baseline characteristics
The baseline characteristics of the patients from the two trials are summarized in Supplementary Table 1. As part of the inclusion criteria for both trials, patients needed to be on optimal medical therapy for heart failure which is reflected in Supplementary Table 1. The NYHA class at baseline was similar in both groups; all patients were NYHA class II–IV. On average, patients in the REGENERATE-IHD trial were older, more likely to be male and to have diabetes, hypercholesterolemia (and subsequently be on statins) and a family history of heart disease. The patients in REGENERATE-IHD had a lower LVEF at baseline and therefore were more likely to receive implantable cardioverter defibrillator therapy. Surprisingly, NT-proBNP concentrations were higher in the REGENERATE-DCM population (in contrast to LVEF).
Cell data
The characteristics of the administered cells are found in Supplementary Table 2, and the combined characteristics of the cells found in the peripheral blood and the injectate are listed in Supplementary Table 3 as part of routine lab practice. Mononuclear, CD34+ and endothelial progenitor cell counts were taken at day 0 (prior to G-CSF infusion) and at day 6 (following 5 days of G-CSF infusion as per protocol). The cell counts, response to G-CSF and number of cells infused were consistent across all the groups.
Changes in LVEF
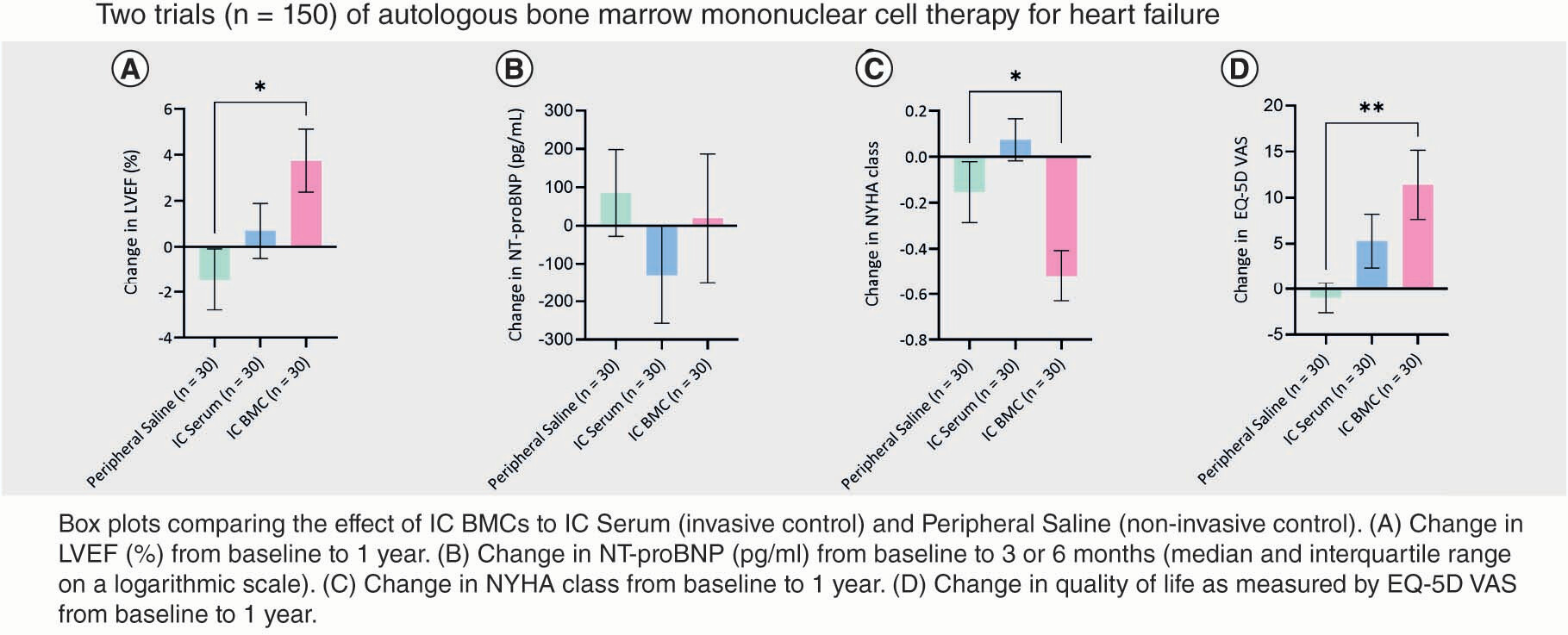
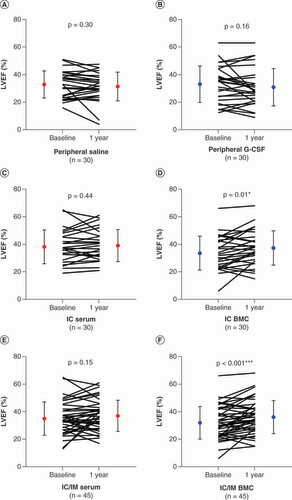
LVEF and LV volumes are presented in Supplementary Table 4. Within-group LVEF increased in all the interventional groups (IC serum, IC BMC, IC/IM serum, IC/IM BMC); however, it only reached significance in the patients that received cells (IC BMC: 3.7 ± 7.2%; p = 0.01, D; IC/IM BMC: 4.2 ± 7.4%; p < 0.001, F). In order to understand the efficacy signal for the IC group, we compared the IC group to the control group (peripheral saline), which demonstrated a significant between-group difference (5.1%; p = 0.01; A).
Solid circles indicate mean values at baseline and 1 year. Error bars indicate standard deviation. (A) Peripheral saline. (B) Peripheral G-CSF. (C) IC serum. (D) IC BMC. (E) IC/IM serum. (F) IC/IM BMC.
p-values are noted as *p < 0.05; **p < 0.01; ***p < 0.001.
BMC: Bone marrow-derived cell; G-CSF: Granulocyte colony-stimulating factor; IC: Intracoronary; IM: Intramyocardial; LVEF: Left ventricular ejection fraction.
Changes in plasma NT-proBNP concentration
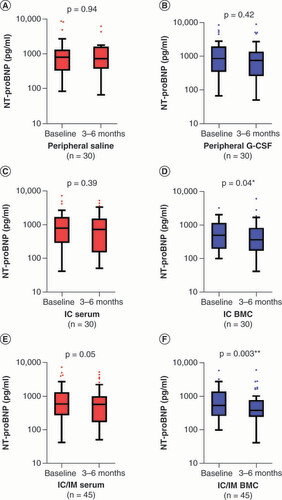
Statistical comparisons were performed with NT-proBNP values on the logarithmic scale due to a skewed distribution. Follow-up measurements of NT-proBNP levels were made at 3 months and 1 year in REGENERATE-DCM and at 6 months in REGENERATE-IHD. Given the different timepoints, NT-proBNP concentrations at 3 and 6 months are presented in this analysis. The results of the within-group comparisons only showed significant decreases in NT-proBNP in the cell-treated arms (IC BMC: median -76 pg/ml, interquartile range [IQR] -177, 0; p = 0.04, D; IC/IM BMC: median -76 pg/ml, IQR -220, 0; p = 0.003, F). In order to understand the efficacy signal for the IC group, we compared the IC group to the control group (peripheral saline), which did not demonstrate a significant between-group difference (p = 0.9; B).
Box and whisker plots showing N-terminal pro brain natriuretic peptide (pg/ml) at baseline and 3 or 6 months (median and interquartile range on a logarithmic scale). (A) Peripheral saline. (B) Peripheral G-CSF. (C) IC serum. (D) IC BMC. (E) IC/IM serum. (F) IC/IM BMC.
p-values are noted as *p < 0.05; **p < 0.01; ***p < 0.001.
BMC: Bone marrow-derived cell; G-CSF: Granulocyte colony-stimulating factor; IC: Intracoronary; IM: Intramyocardial.
Changes in NYHA class
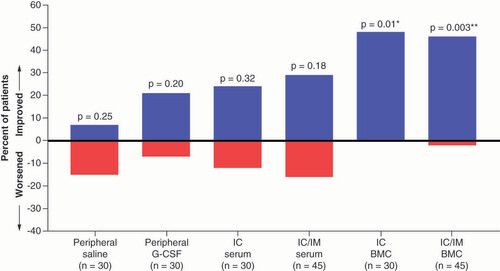
In the NYHA class analysis, the within-group comparisons only showed significant improvements in the patients treated with cells – the IC BMC and IC/IM BMC groups (percentage of patients: 48%; p = 0.01 and 46%; p = 0.003, respectively; ). In order to understand the efficacy signal for the IC group, we compared the IC group to the control group (peripheral saline), which demonstrated a significant between-group difference (p = 0.03; C).
Bars indicate the proportion of patients showing either an improvement or a worsening in New York Heart Association class at 1 year compared with baseline.
p-values are noted as *p < 0.05; **p < 0.01; ***p < 0.001.
BMC: Bone marrow-derived cells; G-CSF: Granulocyte colony-stimulating factor; IC: Intracoronary; IM: Intramyocardial.
Quality of life assessment
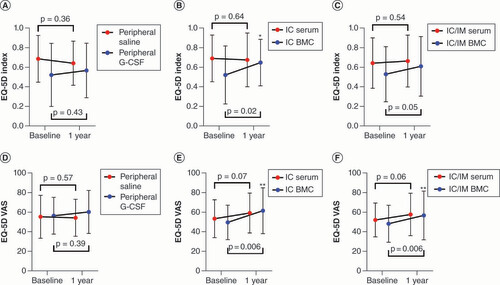
The within-group comparisons for quality of life are shown in . Only patients receiving cell therapy showed improvement in the EQ-5D Index scores (IC BMC: 0.13 ± 0.24; p = 0.02; IC/IM BMC: 0.07 ± 0.02; p = 0.05; B & C). In order to understand the efficacy signal for the IC group, we compared the IC group to the control group (peripheral saline) which demonstrated a significant between-group difference (p = 0.01; D).
(A–C) European Quality of Life-5 Dimensions Time to Trade Off Index between baseline and 1 year. (D–F) European Quality of Life-5 Dimensions Visual Analogue Scale scores between baseline and 1 year. Solid circles indicate mean values at baseline and 1 year. Error bars indicate standard deviation.
p-values are noted as *p < 0.05; **p < 0.01; ***p < 0.001.
BMCs: Bone marrow-derived cell; EQ-5D Index: European Quality of Life-5 Dimensions Time to Trade Off Health Index; EQ-5D VAS: European Quality of Life-5 Dimensions Visual Analogue Scale; G-CSF: Granulocyte colony-stimulating factor; IC: Intracoronary; IM: Intramyocardial.
(A) Change in LVEF (%) from baseline to 1 year. (B) Change in N-terminal pro brain natriuretic peptide (pg/ml) from baseline to 3 or 6 months (median and interquartile range on a logarithmic scale). (C) Change in New York Heart Association class from baseline to 1 year. (D) Change in quality of life as measured by European Quality of Life-5 Dimensions Time to Trade Off Index from baseline to 1 year. (E) Change in quality of life as measured by European Quality of Life-5 Dimensions Visual Analogue Scale from baseline to 1 year.
p-vales are noted as *p < 0.05; **p < 0.01; ***p < 0.001 (ANOVA with Bonferroni correction).
BMC: Bone marrow-derived cell; EQ-5D Index: European Quality of Life-5 Dimensions Time to Trade Off Health Index; EQ-5D VAS: European Quality of Life-5 Dimensions Visual Analogue Scale; IC: Intracoronary; LVEF: Left ventricular ejection fraction; NT-proBNP: N-terminal pro brain natriuretic peptide; NYHA: New York Heart Association.
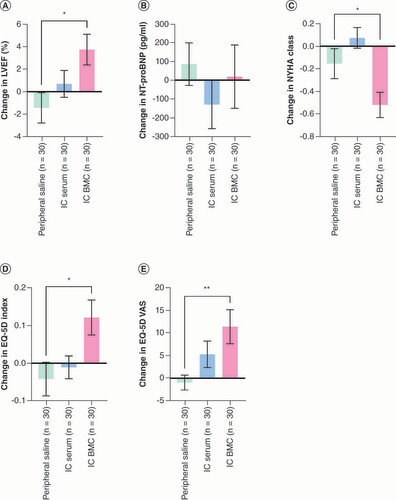
Similarly, for the EQ-5D VAS score, only the cell-treated patients showed improvement (IC BMC: 12 ± 19; p = 0.006; IC/IM BMC: 8 ± 18; p = 0.006; E & ). In order to understand the efficacy signal for the IC group, we compared the IC group to the control group (peripheral saline) which demonstrated a significant between-group difference (p = 0.008; E).
Safety
Adverse event rates are listed in . Although it did not reach statistical significance (p = 0.7), the adverse event rate was lower in the cell-treated patients (IC BMC: 6.7%; IM BMC: 6.7%) compared with the noninvasive controls (peripheral saline: 10%; peripheral G-CSF: 10%). Furthermore, in the invasive arms, the event rate was lower in the cell-treated patients compared with the control (serum) arms (IM/IC BMC: 6.7% vs IM/IC serum: 20%). Overall, there were no adverse procedural events and a very low rate of hospitalization for heart failure and cardiac death. Of the deaths, there was one cardiac death in the peripheral G-CSF group due to myocardial infarction at 219 days, two noncardiac deaths in the IC BMC group (from noncardiac surgical procedure complication at 468 days and pneumonia at 206 days) and one noncardiac death in the IM serum group from pneumonia at 364 days.
Table 1. Adverse clinical events at 1 year.
Discussion
This comparative re-evaluation has demonstrated a new efficacy signal for combined autologous cell and cytokine therapy when given via the IC route, which was not previously demonstrated in REGENERATE-IHD (it had only shown a positive benefit with IM injection). This comparative re-evaluation demonstrated a significant improvement in LVEF (IC BMC: 3.7%; p = 0.01), a decline in NT-proBNP (IC BMC: median -76 pg/ml; IQR: -177, 0; p = 0.04), a decrease in NYHA class (IC BMC percentage of patients: 48%; p = 0.01) and an improvement in quality of life scores (EQ-5D VAS: IC BMC 12 ± 19, p = 0.006; EQ-5D Index: IC BMC 0.13 ± 0.24, p = 0.02) in heart failure patients treated with an IC infusion of BMCs alongside cytokine therapy. In order to confirm these within-group findings, we compared the IC group with the control group (peripheral saline). This demonstrated significant improvements in LVEF (5.1%; p = 0.01), NYHA class (p = 0.03) and quality of life scores (EQ-5D Index: p = 0.01; EQ-5D VAS: p = 0.008).
This new efficacy signal for IC delivery is important given the recent withdrawal of one of the main technologies used to deliver IM therapies (NOGA XP electromechanical mapping system, BioSense Webster, CA, USA). Although other IM injection systems are available, they do not have the same targeting capability and have not been used much in clinical trials. IM delivery also carries pragmatic and logistical issues inherent with the use of such a specialized technology (specialized equipment, training, additional costs and extra time in the catheterization laboratory). Accordingly, from a practical perspective, further investigation of the IC route as an alternative is important. This administration route may well prove similarly efficacious and, on a positive note, is far more accessible (it is cheaper, no specialist training is required and it uses equipment found in most cardiac catheterization laboratories) [Citation6].
This comparative re-evaluation is important as it suggests there is an efficacy signal for IC infusion. This finding is similar to that of Vrtovec et al., who compared a transendocardial delivery to an IC delivery of CD34+ cells in 40 DCM patients. The study demonstrated that both delivery methods led to an improvement in LVEF, NT-proBNP and exercise capacity (although the IM route more so) [Citation18]. Like the REGENERATE trials, the small sample size may suggest that a larger patient cohort is needed to demonstrate significant positive effects for the IC route. The authors commented that, despite the higher cell retention rates demonstrated in the IM route (a threefold increase), the magnitude of change in clinical parameters was somewhat lower than expected. Similarly, in the latest Cochrane review of cell therapy for ischemic heart failure (38 trials and 1907 participants), a comparison between IM and IC delivery routes showed no difference in mortality, NYHA class, Canadian Cardiovascular Society Angina Score (CCS) and exercise performance [Citation19]. Another meta-analysis (13 trials and 956 participants), which evaluated the effect of mesenchymal stem cell therapy on ejection fraction after acute myocardial infarction, also did not show any difference between IM and IC delivery [Citation20].
In order to further situate these findings within the existing literature, we constructed an overview of delivery route efficacy based on the clinical parameters used in this re-evaluation (Supplementary Table 5). The overview included accessible randomized controlled trials (RCTs) of cell therapy for DCM and IHD, and similarly showed that there is no clear preferential delivery route based on efficacy.
With regards to safety data, this combined evaluation revealed a lower number of adverse events in patients treated with cells (by either the IC or the IM method) compared to any other group (including the noninvasive controls; ). Compared with the EMPEROR study [Citation21], the patients in the two REGENERATE trials were more advanced in heart failure symptoms (according to NYHA class) and, therefore, the mortality figures are favorable (13.4% all-cause mortality in patients receiving empagliflozin in the treatment arm of EMPEROR vs 4.4% in the IC/IM BMC group in the REGENERATE trials). Overall, there were very few adverse events in the trials which means that further analysis between the groups is difficult. Nonetheless, it is reassuring to demonstrate that the adverse event rate in the invasive cell therapy arms was certainly no higher than in the other invasive and noninvasive arms. This is further reinforced by the overview of cell therapy RCTs for heart failure (Supplementary Table 5), which did not indicate any safety concerns.
The combined evaluation of these two heart failure trials has amplified the effect of cell therapy seen on various clinical parameters rather than diluting it. Specifically, this re-evaluation reinforced the association between cell-based therapy and improvements in LVEF (IC/IM BMC: 4.2%; p < 0.001), NT-proBNP (IC/IM BMC: median -76 pg/ml; IQR: -220, 0; p = 0.003), NYHA class (IC/IM BMC percentage of patients: 46%; p = 0.003) and quality of life scores (IC/IM BMC: 8 ± 18; p = 0.006). This evaluation supports the findings of meta-analyses of cell therapy for heart failure, which have similarly shown benefits to LVEF, NYHA class, exercise tolerance, quality of life, rehospitalization for heart failure and mortality [Citation5,Citation11,Citation19].
Despite meta-analyses demonstrating an overall beneficial effect of cell-based therapy for heart failure, individual clinical trials have shown mixed results. However, cell therapy trials that have used adjunctive cytokine therapy (e.g., G-CSF) have shown more consistently promising results [Citation6,Citation22,Citation23,Citation24,Citation25,Citation26,Citation27,Citation28]. The data presented in this comparative re-evaluation further support the concept that a combination of cytokine and cell-based therapies can more reliably lead to an improvement in cardiac function, symptoms and clinical outcomes in heart failure patients.
Therefore, despite over 15 years of research into cell therapy for heart failure, it is still not clear which is the best method of cell delivery. However, given the withdrawal of the main technology used for IM administration, and the concurrent complexities associated with this delivery route, this comparative re-evaluation and overview of the field raises important points to consider when planning future trials of cell therapy. The data presented here suggest that the IC route is associated with beneficial effects on heart function and symptoms; and, although it may require a larger sample size to show efficacy, it is a far more accessible approach to cell therapy.
Limitations
This comparative re-evaluation was not powered to detect changes in clinical outcome as data were collected from small phase II trials powered around intermediate efficacy measures. Therefore, the small sample size has the potential to create a clinical selection bias (however, this could either benefit or disadvantage the results). Thus, the results presented here should be considered purely exploratory and hypothesis-generating. Moreover, as these trials were designed to test whether BMC therapy in conjunction with G-CSF provided additional benefits, it is not certain whether cell therapy alone would produce similar effects (although, as discussed, other trials without cytokine therapy have been inconclusive). Furthermore, the follow-up timepoints of NT-proBNP were inconsistent between the two trials (3 months and 1 year in REGENERATE-DCM and 6 months in REGENERATE-IHD). Although we could not analyze data on the differences in drug treatments during the follow-up between the trial cohorts, all patients had to be fully optimized before being recruited onto the trials and the follow-up only lasted 1 year; therefore, it is not expected that significant changes to medications occurred.
Conclusion
This re-evaluation of the REGENERATE-DCM and REGENERATE-IHD trials suggests that the IC method of cell delivery combined with cytokine therapy in patients with heart failure is an efficacious route. Although it may require a larger sample size to show efficacy, this signal, alongside the logistical simplicity of this route, should be taken into consideration in the planning of future cell therapy trials.
This analysis reinforces the benefits of autologous cell therapy for heart failure patients.
This analysis established a new efficacy signal for intracoronary cell delivery for heart failure patients.
Overall, randomized controlled trials do not show clear superiority of any delivery route on patient outcomes.
The intracoronary route is a practical and widely accessible method for cell delivery.
Author contributions
DS Sim: analysis of data, original draft of the work; DA Jones: interpretation of data, revision of work; C Davies: acquisition of data, revision of work; D Locca: acquisition of data, revision of work; J Veerapen: acquisition of data, revision of work; A Reid: interpretation of data, revision of work; T Godec: analysis of data, revision of work; J Martin: interpretation of data, revision of work; A Mathur: conception and design of work, acquisition and interpretation of data, revision of work. All authors: final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical conduct of research
The authors have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human investigations. In addition, informed consent has been obtained from the participants involved.
Supplemental Document
Download MS Word (63.3 KB)Acknowledgments
The authors thank all the staff at the London Chest catheterization laboratories, The Heart Cells Foundation, Barts and the London Charity and Chugai Pharma. The work at UCLH was supported by the UCLH Biomedical Research Centre.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/rme-2022-0138
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- MacIntyreK , CapewellS , StewartSet al.Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation102(10), 1126–1131 (2000).
- MosterdA , CostB , HoesAWet al.The prognosis of heart failure in the general population: the Rotterdam study. Eur. Heart J.22(15), 1318–1327 (2001).
- WangY , XuF , MaJet al.Effect of stem cell transplantation on patients with ischemic heart failure: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther.10(1), 125 (2019).
- JayarajJS , JanapalaRN , QaseemAet al.Efficacy and safety of stem cell therapy in advanced heart failure patients: a systematic review with a meta-analysis of recent trials between 2017 and 2019. Cureus11(9), e5585 (2019).
- FisherSA , DoreeC , MathurAet al.Meta-analysis of cell therapy trials for patients with heart failure. Circ. Res.116(8), 1361–1377 (2015).
- ReidA , MathurA. Cell-based regenerative therapy. In: The PCR-EAPCI Textbook.WijnsW, SerruysPW, VahanianA, EeckhoutE, DePalma R, van SambeekM ( Eds). Europa Digital and Publishing, Toulouse, France (2021).
- StrauerBE , SteinhoffG. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J. Am. Coll. Cardiol.58(11), 1095–1104 (2011).
- HamshereS , ArnousS , ChoudhuryTet al.Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: The REGENERATEDCM clinical trial. Eur. Heart J.36(44), 3061–3069 (2015).
- ChoudhuryT , MozidA , HamshereSet al.An exploratory randomized control study of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with ischaemic cardiomyopathy: the REGENERATE-IHD clinical trial. Eur. J. Heart. Fail.19(1), 138–147 (2017).
- HenryT , LosordoD , TraverseJet al.Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J.39(23), 2208–2216 (2018).
- TripathiA , KhanMS , KhanARet al.Cell therapy for nonischemic dilated cardiomyopathy: a systematic review and meta-analysis of randomized controlled trials. Stem Cells Transl. Med.10(10), 1394–1405 (2021).
- ZhaoXF , XuY , ZhuZYet al.Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet. Mol. Res.14(2), 3010–3017 (2015)
- ArgüeroR , Careaga-ReynaG , Castaño-GuerraRet al.Cellular autotransplantation for ischemic and idiopathic dilated cardiomyopathy. preliminary report. Arch. Med. Res.37(8), 1010–1014 (2006).
- HenryTD , TraverseJH , HammonBLet al.Safety and efficacy of ixmyelocel-T an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ. Res.115(8), 730–737 (2014).
- BolliR , PerinEC , WillersonJTet al.Allogeneic mesenchymal cell therapy in anthracycline-induced cardiomyopathy heart failure patients: the CCTRN SENECA trial. JACC CardioOncology2(4), 581–595 (2020).
- ArnousS , MozidA , MathurA. The bone marrow derived adult stem cells for dilated cardiomyopathy (REGENERATE-DCM) trial: study design. Regen. Med.6(4), 525–533 (2011).
- YeoC , MathurA. Autologous bone marrow-derived stem cells for ischemic heart failure: REGENERATE-IHD trial. Regen. Med.4(1), 119–127 (2009).
- VrtovecB , PoglajenG , LezaicLet al.Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation128(Suppl. 11), S42–S49 (2013).
- FisherSA , DoreeC , MathurAet al.Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev.12(12), CD007888 (2016).
- AttarA , BahmanzadeganJahromi F , KavousiSet al.Mesenchymal stem cell transplantation after acute myocardial infarction: a meta-analysis of clinical trials. Stem Cell Res. Ther.12(1), 600 (2021).
- PackerM , KumbhaniDJ , BhattDL. Empagliflozin outcome trial in patients with chronic heart failure and a reduced ejection fraction – EMPEROR-Reduced. N. Engl. J. Med.383(15), 1413–1424 (2020).
- VrtovecB , PoglajenG , LezaicLet al.Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ. Res.112(1), 165–173 (2013).
- GuX , XieY , GuJet al.Repeated intracoronary infusion of peripheral blood stem cells with G-CSF in patients with refractory ischemic heart failure: a pilot study. Circ. J.75(4), 955–963 (2011).
- PoglajenG , SeverM , CukjatiMet al.Effects of transendocardial CD34+ cell transplantation in patients with ischemic cardiomyopathy. Circ. Cardiovasc. Interv.7(4), 552–559 (2014).
- VrtovecB , PoglajenG , SeverMet al.Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J. Card. Fail.17(4), 272–281 (2011).
- LezaicL , SocanA , PoglajenGet al.Intracoronary transplantation of CD34+ cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. J. Card. Fail.21(2), 145–152 (2015).
- VrtovecB , PoglajenG , SeverMet al.Effects of repetitive transendocardial CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circ. Res.123(3), 389–396 (2018).
- BocchiEA , BacalF , GuimarãesGet al.Granulocyte-colony stimulating factor or granulocyte-colony stimulating factor associated to stem cell intracoronary infusion effects in non ischemic refractory heart failure. Int. J. Cardiol.138(1), 94–97 (2010).
