Abstract
We have found it an effective way of teaching symmetry in the context of stereoselectivity, to use common everyday objects with the same point groups as the substrates involved. This has helped students to distinguish between those symmetry elements which allow for stereospecificity and those which preclude it. Two symmetry elements, the simple rotation axis and the mirror plane, are needed to explain the equivalence (or its absence) between atoms or groups in the substrate while interacting with the chiral enzyme. We have used familiar objects such as blackboard erasers, scissors and purses to illustrate the point group symmetry of substrates such as ethanol, citrate, succinate and fumarate and the interaction between the EcoRI restriction enzyme and the EcoRI-DNA palindromic complex.
Recently bioscience is increasing popular with science undergraduates and graduates alike. Departmental mergers have created multidisciplinary bioscience courses where students less well equipped in chemistry have to tackle molecular aspects of the science. Since 1994 our molecular bioscience course has been the first that biological science postgraduates have to take. As the first topic of the course we offer symmetry concepts and their applications to biomolecules, especially, in explaining the stereoselectivity for atoms and groups of some apparently symmetric substrates such as ethanol, citrate, succinate and fumarate. We have found that students generally could not grasp the concepts and remained so even when shown moveable computer graphics of substrates and products. The situation became better, if only slightly, when hand-held molecular models were given to them. Thus we had to resort to using common items first for illustrating symmetry elements and the resulting point groups before using the molecular models. After this pedagogical engagement step, the subsequent applications to models of substrates became less challenging, the fact attributable to their perceiving the topic as more real or down-to-earth. It was relatively easy to convince students that natural enzymes and enantiomeric biomolecules are chiral by virtue of their being analogous to one of our hands. Therefore to show why one enantiomeric substrate is exclusively preferred to its non-identical mirror image was usually without problem. However it was more difficult to make them understand selective bond making or bond breaking in a substrate with (a) only one plane of symmetry (σ) and/or (b) a simple rotation axis(es) of symmetry (Cn). The reason for this is that parts of these biomolecules appear to be very similar or even identical to some students. Why then would there be region- and enantio-selectivity?
To engage students to the importance of stereoselectivity, the teacher can cite the notorious case of thalidomide where the S-enantiomer is teratogenic to the developing foetus whereas the R-enantiomer is not (CitationGordon and Goggin, 2003; Schoffers et al., 1996). Carvone is another example worth mentioning because the S-enantiomer smells like spearmint while the R-enantiomer smells like caraway (CitationSugawara et al., 2000). Thus in many pharmaceutical, food and cosmetic industries, enantiopure synthesis and/or enantioselective purification of materials is key. However, the mirror plane in a biomolecule is not the only symmetry element to consider in teaching this topic in molecular bioscience. The simple rotation axis and its implications is also important in understanding of the result of interaction(s) between a small biomolecule(s) with an enzyme, a receptor, DNA, etc.
To our knowledge there are excellent biochemistry textbooks (CitationMetzler, 2001; Voet and Voet, 2004) that describe exact molecular positions which atoms and/or groups are removed from or added to. However, perhaps for lack of space or because of the desire to cater to the majority of the readership, the symmetry principles are not discussed sufficiently for students to grasp the symmetry concepts and to apply them. From our teaching, we have found that for students to apply what they have learned takes more than just knowing the outcome of each reaction involving the apparently symmetric substrates. There are also monographs (CitationAlworth, 1972; Mislow, 1966; Rétey and Robinson, 1982) treating symmetry principles more extensively with many examples of regio- and enantio-selectivity including concepts of chirality and prochirality in biomolecular reactions. We propose here that these academic works should incorporate common object analogs to enable better learning.
Figure 1 The distinction between the mirror plane and the simple rotation axis (a) the right and the left fan blades are chiral and related by a mirror plane (dotted line) and the two blades are non-identical. The whole picture, the two blades plus the mirror plane, belongs to the Cs point group as citrate (see a) (b) The three fan blades are related by a simple three-fold rotation axis (▲)(perpendicular to the page at the central point) because they are equivalent
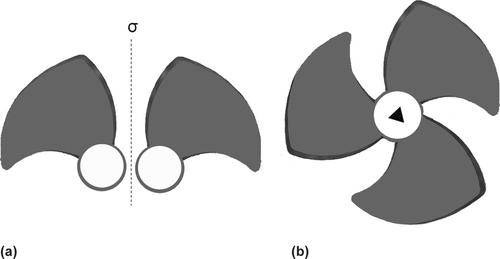
To help the students further, we ask our students to keep in mind two simple rules (a) that a mirror plane relationship between similar or even identical molecular parts makes them potentially enantiomeric (non-identical) (a) and (b) that the axis of symmetry (Cn) equates (makes identical) these related parts (b). Put in another way, although the presence of a plane of symmetry always renders the whole molecule achiral, only the simple rotation axis can equate the related atoms and groups in the eye of the enzyme. Equivalent sites of a rapidly tumbling substrate have an equal chance of being acted upon by the active site of the enzyme. For example, in ethanol () which is achiral because of the mirror plane, HB and HB1 are related by the σ making them potentially enantiomeric but a local three-fold rotation axis of symmetry equates HB, HB1 and HB2. HA and HA1 are then mirror images with no simple rotation axis of symmetry to equate them, thus the two hydrogen atoms behave differently to the enzyme alcohol dehydrogenase which converts it to acetaldehyde (CH3CHO) by removing only one particular hydrogen (CitationMetzler, 2001, p.479; CitationVoet and Voet, 2004, p.463).
Figure 2 Fisher projection of the ethanol molecule. The dotted line represents the mirror plane relating the two hydrogens (HA and HA1) bonded directly to carbon 1 as mirror images. The curved arrow indicates free rotation around the single bond acting as a local three-fold rotation axis along the C1-C2 bond equating the three hydrogen atoms (HB, HB1 and HB2) of the carbon 2. Because of the absence of a local two-fold rotation axis perpendicular to carbon 1, HA and HA1 are non-equivalent for the enzyme alcohol dehydrogenase.

In this article we recount our experiences from teaching enzymatic stereoselectivitiy for apparently symmetric substrates (with σ and/or Cn symmetry elements) using objects which are symmetry-equivalents of three substrates in the Krebs Cycle and the EcoRI-DNA palindromic complex.
I. Citric acid. The common coffee cup with one handle (a) belongs to the Cs point group because it has only one plane of symmetry and no other symmetry elements. The mirror plane makes the two similar parts non-identical (one cannot be converted into the other). Thus the chiral enzyme, aconitase (CitationLloyd et al., 1999; Metzler, 2001, p.688; CitationVoet and Voet, 2004, p.784), can tell the top half from the bottom half of the citrate substrate (see a) because the latter belongs to the C s point group also. The top side of the citrate molecule would have the 3-D arrangement of groups around the central C as —CH2COO− to —COO− to OH clockwise and the bottom anticlockwise. Thus the HO-group is moved downward to give the only isomer of isocitrate (b). A right hand holding the cup by the handle would leave an oblique thumb print on one side and never the other side. If a transparent planar liquid crystal timepiece with opaque hour and minute hands shows 10 o’clock on one side the other side will show 2 o’clock. The liquid crystal watch has only one plane of symmetry.
II. Succinic acid. The standard blackboard eraser (b) has a hard part for holding by the hand and a soft part for erasing. The object belongs to C 2v point group because it has two planes of symmetry and one simple rotation axis of symmetry formed by the two symmetry planes which are perpendicular to the broad flat part of the hard and soft surfaces. A right-handed person would leave the thumb mark on A or A1 only. Here the C2 axis equates A with A1 and B with B1. The two mirror planes make positions A(A1) and B, and positions A(A1) and B1 non-equivalent. The succinate molecule (c) thus belongs to the C 2V point group and should behave like the eraser to the thumb. Therefore for the chiral active site of the enzyme succinate dehydrogenase (CitationMetzler, 2001, p.785; CitationRétey et al., 1970; Voet and Voet, 2004, p.786) HA and HB1 are indistinguishable and HA1 and HB are indistinguishable also. Thus if HA is removed, HB1 has an equal chance of being removed also and likewise with HA1 and HB. A molecular model of succinate in fully staggered conformation belongs to C 2h point group in which HA and HB1, and HA1 and HB are also equivalent. Please be reminded that small molecules tumble very fast in solution. A comb without the handle and the letters A and B are also symmetry equivalents of the Fischer projection of succinate.
Figure 3 (a) Cup with one handle showing the only symmetry element (the mirror plane) making it belonging to the Cs point group (b) Eraser with 2 intersecting planes of symmetry and the C2 (c) The official Union Jack ensign with the broad white band at top left, the central filled oval shows the position of the perpendicular C2, a mirror plane is in the plane of the page (d) A version of the Star of David, the filled oval showing the position of the rotation axis perpendicular to the page
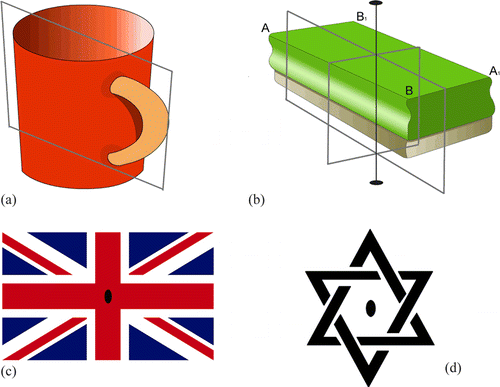
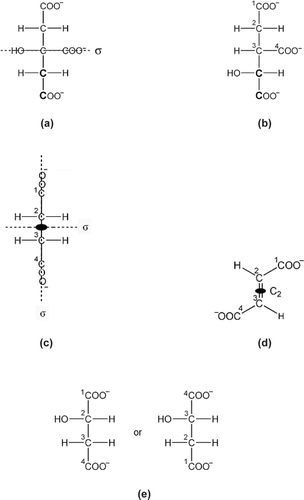
III. Fumaric acid. The British Union Jack ensign (c) and a version of the Star of David (d), embedded in a sheet of transparent plastic, belong to C 2h point group having the plane of symmetry along the central plane of the plastic sheets and the 2-fold simple rotation axis perpendicular to the plane. Thus rotating the plastic sheets 180° on the table top gives identical pictures. The flip sides are the non-identical mirror images of the top sides and become identical upon rotating 180° around the C2 axis. Fumarate (d) is a flat molecule with the 2 carboxylic groups trans across the double bond, thus it belongs to C 2h point group. We can conclude therefore that the top parts as seen here are not equivalent to the bottom parts, these two parts are non-superimposable mirror images and should behave differently at the enzyme fumarate hydratase active site (CitationMetzler, 2001, pp.683-684; CitationMohrig et al., 1995; Voet and Voet, 2004, p.788). However the atoms and groups related by the C2 axis, be it at the top or bottom, are equivalent. If the bottom carbon 2 can be hydroxylated to give Ls-malate (e), the bottom carbon 3 can also be equally hydroxylated to give the other possible Ls-malate (e). To show the front of the Union Jack ensign with the right hand one touches the top right hand corner having the red slanting stripe farther from the top right blue triangle, whereas to show the top of the flip side the right hand has to hold the top side with the slanting red line nearer the top right blue triangle.
Figure 4 (a) Citrate showing the only symmetry element, the σ plane perpendicular to the page (b) Isocitrate (c) Succinate with two intersecting mirror planes (σ) and a central C2, all perpendicular to the page (d) Fumarate has one plane of symmetry in the page and a C2 perpendicular to it (e) Two possible, but identical, Ls-malate products from fumarate of (d)
It is not immediately obvious to most of our students that the official ensign of the British Union Jack (c) has a front and a back that are different. And upon rotating 180° on the page the ensign looks the same as before for both the front and back. The students in our molecular bioscience course find it even more difficult to see such subtle differences in parts of some substrate molecules with one or more symmetry elements. Thus they fail to grasp the regio- and enantio-selectivity particularly for those apparently symmetric substrates in enzyme-catalyzed reactions.
Figure 5 (a) EcoRI enzyme (scissors) complexing with its DNA double helical palindrome (GAATTC) (b) Coin holder (purse). Both objects have only one symmetry element, the C2.
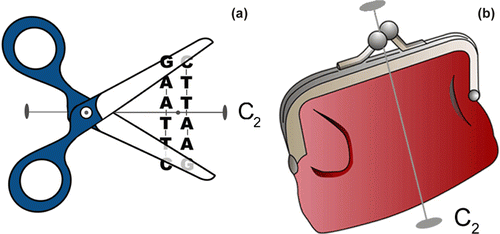
IV. EcoRI-DNA Palindromic Complex. The apparently “impartial” scissors in a are not ambidextrous, i.e., they do not suit all people equally: the pair shown in the a favours right-handers: we have proven this many times over. The right hand of a person holding these scissors has a diastereomeric (not enantiomeric) relationship with the left hand holding another pair of scissors of the same type. The enantiomeric relationship will arise with an enantiomeric pair of scissors, ones suitable for left handers, held in the left hand. This type of scissors having only one 2-fold simple rotation axis belongs to C 2 point group and objects belonging to this group are chiral. The EcoRI palindrome of the B form DNA double helix (the substrate) has one C2 axis between the two adjacent TA base pairs of different strands which run antiparallel. Base sequence is read from 5’-end to 3’-end. The EcoRI restriction enzyme itself is a homodimer and also has only one C2 axis (CitationMcClarin et al., 1986; Metzler, 2001, pp.652-653; CitationVoet and Voet, 2004, pp.102-103). Thus the two types of macromolecules fit very well and when assembled still display weak-bond interactions with a common 2-fold rotation symmetry throughout the palindromic region of the protein-DNA complex. Our analogous common objects in a represent the DNA palindrome by a palindromic zipper having the right-handed scissors with the cutting edges posed at equivalent positions between G and A. An enantiomeric conformation of the restriction enzyme would not be able to cut the DNA at the correct bonds on both DNA strands. As a reminder, the enantiomeric equivalent of the above complex has to be a left-hand DNA double helix with L-deoxyriboses and with the restriction enzyme having a mirror-image conformation and D-amino acids! A coin purse of the type shown in b also favours right-handers. Incidentally, one proposed mechanism of action for the S-enantiomer of thalidomide is that it can intercalate into the DNA double helix whereas the R-enantiomer cannot (CitationStephens et al., 2000).
Learning Outcomes
Since the introduction of the everyday items in addition to molecular models students have shown greater interest, more understanding and also better retention of concepts of enzymatic stereoselective reactions. However, we cannot claim complete success. It is expected that our graduates should be able to apply what they have learned to other examples, however there have been students in each class that can barely cope with examination questions different from or parallel to the examples taught. More active and collaborative learning by students themselves, e.g. searching for common items with strictly specified point groups as the given biomolecules, has helped a little more. By having to brainstorm and actively look for items with the specified symmetries, students have become more agile at applying symmetry elements to biomolecules. In fact the authors have benefited from their quests.
There is another minor problem, viz. configuration. Some students stubbornly cannot understand configuration in the Fischer projection and cannot relate it to the 3-D one in the molecular model. So we give them Dreiding or Dreiding-like pieces of molecular models to assemble molecules while looking at the Fischer projections. To ensure that they master both types of molecular representation they also have to write the Fischer projections from assembled molecules. The more adjacent chiral centres a molecule has the more challenging.
Finally, to warn teachers and students alike not to be so taken by the above examples of high specificity to lose sight of less specific enzyme reactions, we wish to point out that although at one extreme some enzymes are highly regioselective, e.g. glucose dehydrogenase is so selective that only the β-anomer and not the ±-anomer of D-glucose is oxidized to δ-gluconolactone (CitationWohlfahrt et al., 1999), at the other extreme there are haloperoxidases which do not show regio- nor enantio-selectivity to certain types of organic substrate at all (CitationButler and Carter-Franklin, 2004). Thus one has to consider substrate binding and the catalytic mechanism more closely before discussing spectificity. Finally, apart from just enzyme-substrate stereospecificity we should look at other interesting examples of specificity e.g., interactions between drug and target molecule and also hormone and receptor. The example of thyroxine (tetraiodothyronine) showing atropisomeric specificity in binding to its receptor is of great significance for the design of this small hormone’s analogs (CitationDuggan and Craik, 1997; Wagner et al., 1995). As regards the Union Jack ensign in c, the one that is tied to its flag pole by the right side (normal situation) can be easily distinguishable from that which is attached by its left side (distress sign). Here the two cases have a diastereomeric relationship.
References
- AlworthW.L. (1972) Stereochemistry and its application in biochemistry. The relation between substrate symmetry and biological stereospecificity. New York, John Wiley & Son
- ButlerA. and Carter-FranklinJ.C. (2004) The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Natural Product Reports, 21, 180-188
- DugganB.M. and CraikD.J. (1997) Conformational dynamics of thyroid hormones by variable temperature nuclear magnetic resonance: The role of side chain rotations and cisoid/transoid interconversions. Journal of Medical Chemistry, 40 (14), 2259-2265
- GordonJ.N. and GogginP.M. (2003) Thalidomide and its derivatives: Emerging from the wilderness. Postgraduate Medical Journal, 79, 127-132
- LloydS.J., LaubleH., PrasadG.S. and StoutC.D. (1999) The mechanism of aconitase: 1.8å resolution crystal structure of the S642A: citrate complex. Protein Science, 8, 2655-2662
- McClarinJ.A., FredericC.A., WangB.C., GreeneP., BoyerH.W., GrabelJ. and RosenbergJ.M. (1986) Structure of the DNA-Eco RI endonuclease recognition complex at 3A resolution. Science, 234, 1526-1541
- MetzlerD.E. (2001) Biochemistry. (2nd edn.) The chemical reactions of living cells. San Diego, Harcourt Academic Press
- MislowK. (1966) Introduction to stereochemistry. W.A. Benjamin
- MohrigJ.R., MoerkeK.A., CloutierD.L. LaneB.D., PersonE.C. and OnaschT.B. (1995) Importance of historical contingency in the stereochemistry of hydratase-dehydratase enzymes. Science, 269, 527-529
- RéteyJ. and RobinsonJ.A. (1982) Stereospecificity in organic chemistry and enzymology. Weinheim, VCH
- RéteyJ., SeiblJ. and ArigontD. (1970) Stereochemical studies of the exchange and abstraction of succinate hydrogen on succinate dehydrogenase. European Journal of Biochemistry, 14 (2), 232-242
- SchoffersE., GolebiowskiA. and JohnsonC.R. (1996) Enantioselective synthesis through enzymatic asymmetrization. Tetrahedron, 52 (11), 3769-3826
- SugawaraY., HaraC., AokiT., SugimotoN. and MasujimaT. (2000) Odor distinctiveness between enantiomers of linalool: Difference in perception and responses elicited by sensory test and forehead surface potential wave measurement. Chemical Senses, 25, 77-84
- StephensT.D., BundeC.J.W. and FillmoreB.J. (2000) Mechanism of action in thalidomide teratogenesis. Biochemical Pharmacology, 59, 1489-1499
- VoetD. and VoetJ.G. (2004) Biochemistry (4th Edn). New York, John Wiley & Son
- WagnerR.L., AprilettiJ.W., McGrathM.E., WestB.L., BaxterJ.D. and FletterickR.J. (1995) A structural role for hormone in the thyroid hormone receptor. Nature, 378, 690-697
- WohlfahrtG., WittS., HandleJ., SchomburgD., KaliszH.M. and HechtH. (1999) 1.8 and 1.9 Ǻ resolution structures of Penicillium amagasakiense and Aspergillus niger glucose oxidase as a basis for modeling substrate complexes. Acta Crystallographica, D55, 969-977