Abstract
Conclusions: Congenital cytomegalovirus (CMV) infection is a major cause of bilateral and unilateral sensorineural hearing loss (SNHL) in children, accounting for 9.0% of SNHL cases. The diagnostic rate using combined genetic deafness test and CMV DNA detection test was determined to be 46.4% in bilateral profound SNHL. Objectives. The present study investigated the prevalence of congenital CMV infection diagnosed retrospectively by detection of CMV DNA in dried umbilical cord specimens from children with unilateral or bilateral SNHL up to the age of 12 years. Methods: Preserved dried umbilical cords were collected from 134 children with bilateral (46 children) or unilateral (88 children) SNHL. DNA was extracted from the dried umbilical cords and CMV DNA was detected by quantitative PCR. Genetic deafness tests based on the invader assay were performed in children with bilateral SNHL. Results: CMV DNA from the dried umbilical cords was detected in 8.7% of the bilateral SNHL and 9.1% of unilateral SNHL. Deafness gene mutations were identified in 21.7% (10/46) of children with bilateral SNHL.
Keywords::
Introduction
Sensorineural hearing loss (SNHL) is one of the most common birth defects. Genetic causes of SNHL can be found in half of prelingual cases and the remaining half are ascribed to environmental or unidentified genetic factors. The most common environmental cause of SNHL is congenital cytomegalovirus (CMV) infection, with an estimated overall birth prevalence of approximately 0.3–2.4% [Citation1]. The vast majority (approximately 90%) of these infants exhibit no signs of congenital infection, which is asymptomatic at birth. Approximately 10% of infected infants are born with clinical symptoms of congenital CMV infection. SNHL reportedly occurs in 22–65% of children with symptomatic congenital CMV infections and 6–23% of children with asymptomatic infections [Citation2].
Late-onset and progressive natures are characteristic of SNHL with congenital CMV infection. The frequency of SNHL in children with asymptomatic congenital CMV infection is also uncertain. The gold standard for diagnosis of congenital CMV infection is the isolation of the virus from urine or saliva in the first 2 weeks of life. However, asymptomatic congenital CMV infection in children who develop late-onset SNHL after 2 weeks of age cannot be diagnosed on the basis of viral isolation from urine or saliva. Detection of CMV DNA in infant blood or umbilical cord using polymerase chain reaction (PCR) assays is a more feasible method to identify children with late-onset of SNHL. Blood stored as dried blood spots (DBS) on Guthrie cards and the dried umbilical cord that is generally stored at home as a memento of the birth in the Japanese culture are suitable for retrospective diagnosis of congenital CMV infection.
Congenital CMV infections and genetic defects are the two major causes of SNHL in children. For severe bilateral SNHL children, Ogawa et al. [Citation3] reported that congenital CMV infection, which was diagnosed by detection of CMV DNA in dried umbilical cord, and genetic defects (GJB2) were identified in 15% and 30% of the children, respectively. The etiology of SNHL in children including mild to moderate SNHL and unilateral SNHL is still uncertain. The purpose of the present study was to investigate the prevalence of congenital CMV infection diagnosed retrospectively by detection of CMV DNA extracted from dried umbilical cord specimens in children with unilateral or bilateral SNHL defined at an age of months or even years after birth. Genetic testing was also applied to identify the other causes of SNHL.
Material and methods
Subjects
This study evaluated 134 patients (70 males and 64 females) with bilateral (46 patients) or unilateral (88 patients) SNHL who were referred to the Department of Otolaryngology, Shinshu University School of Medicine, from May 2008 to September 2009 (). Informed consent and dried umbilical cord for the preparation of DNA specimens were collected for all of them. The ages of children who were diagnosed with SNHL ranged from 1 month to 138 months (mean age 37.7 ± 36.2 months). Children with deafness syndrome were excluded from this study by an etiologic work-up of their SNHL. Both genetic deafness testing and CMV DNA analysis were performed for children with bilateral SNHL. For children with unilateral SNHL, CMV DNA analysis and genetic test (GJB2, Mit1555) were performed.
Table I. Summary of children with bilateral and unilateral hearing loss.
Audiologic evaluations
Audiometric evaluation was performed for each patient using auditory brainstem response (ABR) and auditory steady-state evoked response (Master 580-Navpro; Nihon Kohden Co. Ltd, Tokyo, Japan) as objective audiologic tests and behavioral audiologic tests and/or pure tone audiometry were also used. Hearing levels (average of 500, 1000, 2000, 4000 Hz) of the patients were classified into two categories on the basis of the severity of the worse ear: severe (71–90 dB) to profound (>90 dB), mild (20–40 dB) to moderate (41–70 dB). The threshold of ABR was determined as a means of hearing level in 5 of 134 children with hearing loss. The follow-up hearing assessments were performed at intervals of 6–12 months. Progressive hearing loss was defined as a decrease in hearing of 10 dB or more at one or more frequencies. Fluctuating hearing loss was defined as a decrease in hearing of >10 dB followed by an improvement of >10 dB at one or more frequencies.
Preparation of DNA samples and real-time PCR analysis and genetic testing
To analyze congenital CMV infection, we used CMV-DNA quantitative PCR (qPCR) analysis. Before qPCR analysis, the total DNA including genomic DNA and CMV DNA was extracted from preserved dried umbilical cords. Preserved dried umbilical cord samples were collected from hearing loss patients and controls. As a positive control, we used preserved umbilical cords from two patients with symptomatic congenital CMV infection, identified by CMV from urine in the first 2 weeks of life at the Department of Pediatrics, Shinshu University Hospital. As a negative control, preserved umbilical cords from five healthy children without SNHL were used. Each 5 mm section of the tissue was incubated in the lysis buffer containing proteinase K and incubated at 56°C overnight. Total DNA was extracted using a DNeasy Blood & Tissue Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. The total amount of DNA was measured by Qubit Fluorometer with Quant-iT™ dsDNA BR Assay Kit (Life Technologies-Invitrogen, Carlsbad, CA, USA). Each 10 pg total DNA was analyzed by a Step One Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using a TaqMan Universal Master Mix II (Applied Biosystems). The qPCR primers and TaqMan probe used for CMV DNA qPCR analysis are listed below: US14-1F, 5' ACGTCCACGTTAGGATGAGG 3'; US14-1R, 5' GTATGTGGCGCTTCTCTCGT 3'; US14-1 TaqMan probe, 5'-FAM-AACCTGTGCACCACAGCGCC-TAMRA-3'. To quantify the input DNA amount in each sample, qPCR with genome region was also performed, using the primers and TaqMan probe listed below. GJB2-2F, 5' ACGTCCACGTTAGGATGAGG 3'; GJB2-2, 5' GTATGTGGCGCTTCTCTCGT 3'; GJB2-2 TaqMan probe, 5'-FAM- AACCTGTGCACCACAGCGCC-TAMRA-3'. Initial preheating steps were performed for 2 min at 50°C and 10 min at 95°C. Then qPCR was performed with 43 cycles of 15 s at 95°C and 60 s at 60°C. After qPCR analysis, relative CMV concentrations of each sample were evaluated as ΔCt (delta threshold cycle), which was calculated by threshold cycle of CMV qPCR minus that of GJB2 qPCR. The invader assay described by Abe et al. [Citation4] was used for deafness genetic testing.
Ethics approval
This study was approved by the ethical committee of Shinshu University School of Medicine. Written informed consent was obtained from either the patients or their parents.
Results
shows original amplification curves of real-time PCR of the positive controls, negative controls, blank controls (samples without added DNA), and results of a typical sample (no. 12) for CMV DNA and genomic DNA (GJB2 gene). CMV DNA was amplified in two of two cases in positive controls, none of five cases in negative controls, and none of two cases in blank controls (data not shown), therefore we considered this method to be appropriate. Comparing ΔCt for each sample and positive control, CMV DNA content was 0.01–0.8 times for positive control. The present study revealed that 9.0% (12/134) of the cases of children with SNHL were attributable to congenital CMV infection. CMV DNA from preserved umbilical cords was detected in 8.7% of bilateral SNHL cases () and 9.1% of unilateral SNHL cases () in children with SNHL of unknown causes. Bilateral severe to profound SNHL, bilateral mild to moderate SNHL, unilateral severe to profound SNHL, and unilateral mild to moderate SNHL caused by congenital CMV infection were detected in 14.3% (4/28), 0% (0/18), 9.6% (7/73), and 6.7% (1/15) of the hearing-impaired children, respectively.
Figure 1. An original amplification plot of real-time PCR in case no. 12 with positive CMV DNA. CMV DNA in positive control and case 12 (A) and genomic DNA (GJB2 gene) in positive control, case no. 12 and negative control (B) are amplified. These results show that our real-time PCR method is precise. Blank, sample without any added DNA.
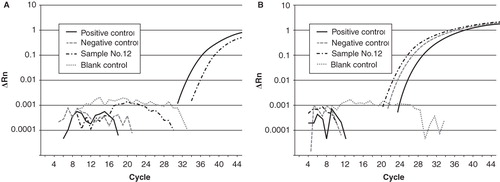
Table II. Results of CMV DNA test combined with genetic deafness testing in bilateral sensorineural hearing loss (SNHL).
Table III. Results of CMV DNA test combined with genetic deafness test in unilateral sensorineural hearing loss (SNHL).
Ten deafness gene mutations (7/10, GJB2; 3/10, SLC26A4) were identified in 10 (21.7%) of the 46 children with bilateral SNHL. If restricted to the group of children with bilateral severe to profound SNHL, the rates of deafness gene mutations and positive CMV DNA increased to 32.1% (9/28) and 14.3% (4/28), respectively ().
shows the clinical characteristics of 12 children in whom CMV DNA was identified. Of them, four children had bilateral SNHL and eight children had unilateral SNHL. All four children with bilateral SNHL had late-onset profound SNHL. The hearing fluctuation and pass at newborn hearing screening (NHS) test were confirmed in three of four children (75%). Among the 2/8 children with unilateral SNHL, pass or failure at NHS test were confirmed in two children, respectively. Only one child (12.5%) had hearing fluctuation. Inner ear anomaly was not found in any of the eight children with unilateral SNHL.
Table IV. Clinical data of children positive for CMV DNA.
Discussion
A common method for diagnosis of congenital CMV infection has been detection of CMV DNA from urine within the first 2 weeks of life and serologic testing for CMV-specific IgM antibody in serum from mother and child [Citation5]. In recent years, the detection of CMV DNA by retrospective methods has been more valuable, not only for diagnosing congenital CMV infection during later life, but also for identifying those children who are at highest risk of late-onset and progressive SNHL. There are some reports that DBS stored on Guthrie cards have been used for the retrospective diagnosis of congenital CMV infections [Citation6,Citation7]. Similarly, preserved umbilical cords have been used in Japan recently [Citation8–10]. The sensitivity varies widely depending on the DNA extraction method in the case of DBS. Some investigators reported sensitivities of 71–100% and specificities of 99–100% [Citation7,Citation11,Citation12]. In this study, the qPCR method and preserved umbilical cords were used because they were useful for more precise detection of CMV DNA.
The present study clearly showed that in a certain number of patients, hearing loss (either bilateral or unilateral) is due to CMV infection. Concerning frequencies, positive CMV DNA in children with bilateral SNHL was 8.7% (4/46). Late-onset profound SNHL (4/4: 100%) and hearing progression (3/4: 75%) were characteristic features of SNHL caused by CMV infection.
Hearing loss in children with congenital CMV infection often presents at birth, but in many instances may develop after months or even years [Citation13]. In this study, three-quarters of subjects (75%) passed the NHS. The present results are consistent with the report that children with normal hearing at 6 months of age developed hearing loss at the rate of nearly 1% per year and the cumulative risk of late-onset hearing loss was a substantial 6.9% for the population with asymptomatic congenital CMV infection [Citation13]. Children with bilateral hearing loss have speech developmental problems. Therefore retrospective diagnosis of congenital CMV infection is important to understand more about the etiology of SNHL in children. Previous reports are summarized in . The frequency of congenital CMV infection in children with bilateral SNHL varied from 3% to 36%. This large variation may be due to the various backgrounds of the subjects (number of population, severity of SNHL, etc.) or the various methods utilized (e.g. CMV-IgM, DNA from urine, DNA from DBS on Guthrie cards) [Citation6,Citation12,Citation14–18]. Meanwhile, based on the retrospective diagnostic method of using preserved dried umbilical cords, congenital CMV infection was detected in 10–12% of children with bilateral SNHL in Japan [Citation8,Citation9], but these reports were based on small numbers of subjects (10–26 cases).
Table V. Review of previous reports.
Genetic deafness testing has become valuable for a precise diagnosis of hearing loss. The most frequent gene implicated in non-syndromic hearing loss is GJB2, which is the most prevalent gene responsible for congenital hearing loss worldwide. GJB2, SLC26A4, CDH23, and mitochondrial 12s rRNA are the major genetic causes of hearing loss in Japan [Citation19]. Genetic deafness mutations could be detected in 30% of children with congenital hearing loss [Citation4]. In the present study, deafness gene mutations were identified in 21.7% (10/46) of children with bilateral SNHL. In children with bilateral severe to profound SNHL, the frequency of deafness gene mutations and CMV DNA positive results were 32.1% (9/28) and 14.3% (4/28), respectively. The diagnostic rate was concluded to be 46.4% (13/28). If the detection test for CMV DNA could be combined with genetic deafness testing, it would enable us to find approximately 50% of causes of bilateral severe to profound hearing loss in children.
In children with unilateral SNHL, CMV DNA from preserved umbilical cords was detected in 9.1% (8/88). The frequency of congenital CMV infection has been similar in children with unilateral and bilateral SNHL. We have speculated that approximately 10% of SNHL in children is caused by congenital CMV infection. The majority of unilateral SNHL was etiologically unknown, and there were few reports showing the frequency of congenital CMV infection in children with unilateral SNHL. Although 25% (1/4) [Citation3] and 19% (8/42) [Citation12] of children with unilateral SNHL were reported to be diagnosed as having congenital CMV infection by the CMV DNA detection method, the frequency based on a large number of subjects is not available. This is the first report confirming CMV as a very important cause of unilateral SNHL. The present results also showed that CMV is a crucial cause of late-onset unilateral SNHL, and in fact, five of eight subjects passed NHS.
In conclusion, congenital CMV infection plays a major role as a cause of bilateral and unilateral SNHL in children: 9.0% of SNHL of unknown causes (bilateral SNHL, 8.7%; unilateral SNHL, 9.1%) is attributable to congenital CMV infection.
Acknowledgments
This work was supported by the Acute Profound Deafness Research Committee of the Ministry of Health, Labour and Welfare, Tokyo, Japan, and a grant from the Preventive Medical Center of Shinshu University Hospital.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17:253–76.
- Iwasaki S, Yamashita M, Maeda M, Misawa K, Mineta H. Audiological outcome of infants with congenital cytomegalovirus infection in a prospective study. Audiol Neurotol 2007;12:31–6.
- Ogawa H, Suzutani T, Baba Y, Koyano S, Nozawa N, Ishibashi K, Etiology of severe sensorineural hearing loss in children: independent impact of congenital cytomegalovirus infection and GJB2 mutations. J Infect Dis 2007;195:782–8.
- Abe S, Yamaguchi T, Usami S. Application of deafness diagnostic screening panel based on deafness mutation/gene database using invader assay. Genet Test 2007;11:333–40.
- Genser B, Truschnig-Wilders M, Stunzner D, Landini MD, Halwachs-Baumann G. Evaluation of five commercial enzyme immunoassays for the detection of human cytomegalovirus-specific IgM antibodies in the absence of a commercially available gold standard. Clin Chem Lab Med 2001;39:62–70.
- Choi KY, Schimmenti LA, Jurek AM, Sharon B, Daly K, Khan C, Detection of cytomegalovirus DNA in dried blood pots of Minnesota infants who do not pass newborn hearing screening. Pediatr Infect Dis 2009;28:1095–8.
- de Vries JC, Claas EC, Kroes AC, Vossen AC. Evaluation of DNA extraction methods for dried blood spots in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2009;46:S37–42.
- Ogawa H, Baba Y, Suzutani T, Inoue N, Fukushima E, Omori K. Congenital cytomegalovirus infection diagnosed by polymerase chain reaction with the use of preserved umbilical cord in sensorineural hearing loss children. Laryngoscope 2006;116:1991–4.
- Tagawa M, Tanaka H, Moriuchi M, Moriuchi H. Retrospective diagnosis of congenital cytomegalovirus infection at a school for the deaf by using preserved dried umbilical cord. J Pediatr 2009;155:749–51.
- Koyano S, Inoue N, Nagamori T, Yan H, Asanuma H, Yagyu K, Dried umbilical cords in the retrospective diagnosis of congenital cytomegalovirus infection as a cause of developmental delays. Clin Infect Dis 2009;48:e93–5.
- Atkinson C, Walter S, Sharland M, Tookey P, Luck S, Peckham C, Use of stored dried blood spots for retrospective diagnosis of congenital CMV. J Med Virol 2009;81:1394–8.
- Barbi M, Binda S, Caroppo S, Ambrosetti U, Corbetta C, Sergi P. A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J 2003;22:39–42.
- Rosenthal LS, Fowler KB, Boppana SB, Britt WJ, Pass RF, Schmid DS, Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J 2009;28:515–20.
- Jakubikova J, Kabatova Z, Pavlovcinova G, Profant M. Newborn hearing screening and strategy for early detection of hearing loss in infants. Int J Pediatr Otorhinolaryngol 2009;73:609–12.
- Samileh N, Ahmad S, Mohammad F, Framarz M, Azardokht T, Jomeht E. Role of cytomegalovirus in sensorineural hearing loss of children: a case-control study Tehran, Iran. Int J Pediatr Otorhinolaryngol 2008;72:203–8.
- Stehel EK, Shoup AG, Owen KE, Jackson GL, Sendelbach DM, Boney LF, Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics 2008;121:970–5.
- Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 2008;41:57–62.
- Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. Hearing loss in children with congenital cytomegalovirus infection in relation to the maternal trimester in which the maternal primary infection occurred. Pediatrics 2008;122:e1123–7.
- Usami S, Wagatsuma M, Fukuoka H, Suzuki H, Tsukada K, Nishio S, The responsible genes in Japanese deafness patients and clinical application using Invader assay. Acta Otolaryngol 2008;128:446–54.
- Walter S, Atkinson C, Sharland M, Rice P, Raglan E, Emery VC, Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed 2008;93:280–5.
- Mizuno T, Sugiura S, Kimura H, Ando Y, Sone M, Nishiyama Y, Detection of cytomegalovirus DNA in preserved umbilical cords from patients with sensorineural hearing loss. Eur Arch Otorhinolaringol 2009;266:351–5.
- Boudewyns A, Declau F, Smets K, Ursi D, Eyskens F, Van den Ende J, Cytomegalovirus DNA detection in Guthrie cards: role in the diagnostic work-up of childhood hearing loss. Otol Neurotol 2009;30:943–9.
- Choi KY, Schimmenti LA, Jurek AM, Sharon B, Daly K, Khan C, Detection of cytomegalovirus DNA in dried blood spots of Minnesota infants who do not pass newborn hearing screening. Pediatr Infect Dis J 2009;28:1095–8.
- Tagawa M, Tanaka H, Moriuchi M, Moriuchi H. Retrospective diagnosis of congenital cytomegalovirus infection at a school for the deaf by using preserved dried umbilical cord. J Pediatr 2009;155:749–51.
- Kimani JW, Buchman CA, Booker JK, Huang BY, Castillo M, Powell CM, et al. Sensorineural hearing loss in a pediatric population: association of congenital cytomegalovirus infection with intracranial abnormalities. Arch Otolaryngol Head Neck Surg 2010;136:999–1004.
- Adachi N, Ito K, Sakata H, Yamasoba T. Etiology and one-year follow-up results of hearing loss indentified by screening of newborn hearing in Japan. Otolaryngol Head Neck Surg 2010;143:97–100.