Abstract
Conclusion. We have demonstrated differences in cortical activation with language-related visual stimuli in patients who were profoundly deafened due to genetic mutations in GJB2 and SLC26A4. The differences in cortical processing patterns between these two cases may have been influenced by the differing clinical courses and pathogenesis of hearing loss due to genetic mutations. Our results suggest the importance of hearing during early childhood for the development of a normal cortical language network. Objectives. To investigate the cortical activation with language-related visual stimuli in patients who were profoundly deafened due to genetic mutations in GJB2 and SLC26A4. Methods: The cortical activity of two adult patients with known genetic mutations (GJB2, SLC26A4) was evaluated with fluorodeoxyglucose-positron emission tomography (FDG-PET) with a visual language task and compared with that of normal-hearing controls. Results: A patient with a GJB2 mutation showed activation in the right auditory association area [BA21, BA22], and the left auditory association area [BA42] even with visual language task; in contrast, a patient with an SLC26A4 mutation showed no significant activation in the corresponding area.
Introduction
Functional brain imaging is an effective method for investigating the cortical processing of language, which has provided much evidence for the plasticity of the central auditory pathway following a profound loss of hearing [Citation1–4]. Many previous studies showed that there is a capacity of the auditory cortex for cross-modal plasticity after auditory deprivation of the brain. Cerebral glucose metabolism in the primary auditory and related cortices in individuals with prelingual deafness was shown to decrease in younger patients, but to increase as they aged and, in fact, recover fully or even exceed the normal level of activation [Citation5–7]. Children with prelingual deafness can acquire spoken language by cochlear implantation, but its efficacy decreases with age. The development of the auditory cortex is believed to depend on the patient's auditory experience within ‘critical periods’ in the early lifetime. Adults who had severe congenital hearing loss in their childhood may take advantage of hearing with cochlear implants if they had exploited residual hearing with hearing aids. It has been shown that low glucose metabolism in the temporal auditory cortex predicts a good cochlear implant outcome in prelingually deafened children, which suggests that low metabolism in the auditory cortex may indicate its potential of plasticity for spoken language acquisition [Citation7].
Meanwhile, several etiological studies suggest that at least 60% of congenital hearing loss has genetic causes. Recent advances in molecular genetics have made genetic diagnosis possible [Citation8]. The identification of the mutation responsible for hearing loss may provide some information as to cochlear damage, and help predict the time course and manifestations of hearing loss. Genetic testing can therefore be useful in decision-making regarding cochlear implantation and other necessary treatment.
Evaluation of brain function and diagnosing accurate etiology of hearing loss may be the keys to personalizing post-cochlear implantation habilitation programs and predicting the outcomes thereof.
In this study, we used 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET) to measure cortical glucose metabolism with a visual language task before cochlear implantation in profoundly deaf patients whose etiologies were identified by genetic testing.
Material and methods
Genetic diagnosis
Genetic screening was performed in two cases using an Invader assay to screen for 41 known hearing loss-related mutations [Citation9] and direct sequencing for GJB2 and SLC26A4 mutations [Citation10,Citation11].
FDG-PET scanning and image analysis
FDG-PET scanning and image analysis were performed using the method described by Fujiwara et al. [Citation12]. During the time period between the intravenous injection of 370 MBq 18 F-FDG (the dose was adjusted according to the body weight of each subject) and the PET scanning of the brain, the patients were instructed to watch a video of the face of a speaking person reading a children's book. The video lasted for 30 min, and several still illustrations taken from the book were inserted (for a few seconds each) to help the subjects to follow the story. The subjects were video-recorded to confirm that they were watching the task video. PET images were acquired with a GE ADVANCE NXi system (General Electric Medical Systems, Milwaukee, WI, USA). Spatial preprocessing and statistical analysis were performed with SPM2 (Institute of Neurology, University College of London, UK) implemented in Matlab (Mathworks, MA, USA). The cortical radioactivity of each deaf patient was compared with that of a control group of normal-hearing adults by a t test in the basic model of SPM2. The statistical significance level was set at p < 0.001 (uncorrected).
This study was approved by the Ethics Committee of Shinshu University School of Medicine and written consent was obtained from each participant.
Control group
The control group consisted of six normal-hearing right-handed adult subjects. The average (mean ± standard deviation) age of the normal-hearing subjects was 27.5 ± 3.8 years. The pure-tone average hearing levels were within 20 dB HL for all.
Case 1
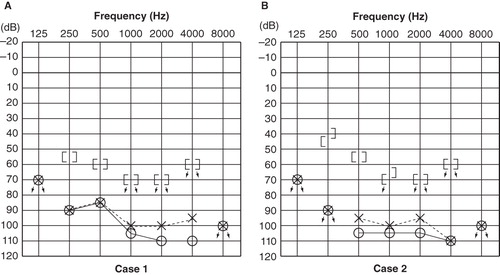
A right-handed 22-year-old female with a GJB2 mutation (235 delC homozygous) had hearing impairment that was noticed by her parents when she was 2 years old. She had used hearing aids ever since, but with insufficient hearing amplification. She used lip-reading and some sign language, and her speech was not intelligible to hearing people. Computed tomography (CT) findings of the middle and inner ear were normal. Her average pure-tone hearing levels were 102.5 dB for the right ear and 95 dB for the left ear ().
Case 2
A right-handed 26-year-old male with an SLC26A4 mutation (H723R homozygous) had hearing impairment that was noticed by his parents when he was 2 years old, from which time he had used hearing aids bilaterally. He did not use lip-reading or sign language during the acquisition age for language. He obtained spoken language with hearing aids but had progressive hearing loss, and sometimes suffered vertigo attacks. His pronunciation was clear, and his speech was almost completely intelligible. CT findings exhibited an enlarged vestibular aqueduct on each side. His average pure-tone hearing levels were 106.2 dB for the right ear and 100 dB for left ear ().
Results
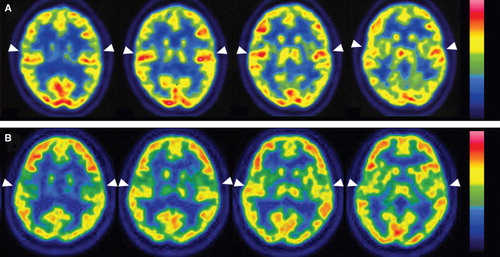
shows transaxial PET images of each participant's brain. The visual stimuli resulted in bilateral activation of the superior temporal gyrus, including Heschl's gyrus in case 1 with GJB2 mutation (, white arrowhead). In contrast, in case 2 with SLC26A4 mutation, the activation of the superior temporal gyrus was much lower than in case 1 (, white arrowhead).
Figure 2. Transaxial PET images of each participant's brain: activation (arrowheads) of the superior temporal gyrus with visual language stimuli in each case. (A) Case 1 (GJB2 mutation). The superior temporal gyri were strongly activated bilaterally. (B) Case 2 (SLC26A4 mutation). The superior temporal gyri exhibited less activation than in case 1.
shows supra-threshold clusters in each case. In case 1, activation higher than normal controls was observed in the right auditory association area [BA21, BA22], and the left auditory association area [BA42] (p < 0.001). In case 2, the right superior frontal gyrus [BA9], and the middle temporal gyrus [BA20], showed higher activation than normal controls (p < 0.001).
Figure 3. Cortical activation by language-related visual stimuli in the two profoundly deafened cases. Case 1 (GJB2 mutation) showed significant activation in the right middle temporal gyrus [BA21] (1), superior temporal gyrus [BA22] (2), and left superior temporal gyrus [BA42] (3), and left cerebellum (4), while case 2 (SLC26A4 mutation) exhibited significant activation in the right superior frontal gyrus [BA9] (1), and middle temporal gyrus [BA20] (2) (SPM2, p < 0.001, uncorrected).
![Figure 3. Cortical activation by language-related visual stimuli in the two profoundly deafened cases. Case 1 (GJB2 mutation) showed significant activation in the right middle temporal gyrus [BA21] (1), superior temporal gyrus [BA22] (2), and left superior temporal gyrus [BA42] (3), and left cerebellum (4), while case 2 (SLC26A4 mutation) exhibited significant activation in the right superior frontal gyrus [BA9] (1), and middle temporal gyrus [BA20] (2) (SPM2, p < 0.001, uncorrected).](/cms/asset/5a2f37eb-e197-48fd-b223-eec0db4d94ac/ioto_a_593719_f0003_b.jpg)
Discussion
More than half of congenital hearing loss has been estimated to be from genetic causes, and phenotypes are affected by genetic mutations. There have been no reports of the influence of phenotype on brain function associated with hearing. This is the first report on evaluation of cortical processing of language in patients with genetic mutations as a main etiology of hearing loss. The auditory association area was activated bilaterally in case 1 (GJB2 mutation), but not activated in case 2 (SLC26A4 mutation). A previous study indicated that the temporal lobe is activated during speech-reading in normal subjects [Citation13] and another study found that the temporal lobe is not activated when reading fluent speech from a talking face [Citation14]. For the present study we used a fluent speech-reading task, similar to that described by Hall et al. [Citation14]. Fujiwara et al. in a FDG-PET study using the same methods and task as the present study, showed that subjects with better spoken language skills had less temporal lobe activation [Citation12].
To summarize these reports, the patients with hearing aids with better spoken language skills have less temporal lobe activation with a visual language task. Otherwise, Nishimura et al. [Citation15] reported a sign language activation of the bilateral auditory association areas in a congenitally deafened subject. However, detailed clinical data for the subject – including his hearing levels, time course of hearing loss, and the cause of deafness – were not described. The different visual language activation patterns in the auditory cortices revealed in the current two profoundly deafened subjects with different genetic etiologies and hearing loss progressions may, thus, add further knowledge of the cross-modal plasticity brought about in the superior temporal association areas by lack of hearing.
The differences in cortical processing patterns between cases 1 and 2 – who both had hearing loss of cochlear origin – may have been influenced by the differing clinical courses of hearing loss. GJB2 is currently known to be the most prevalent gene responsible for congenital hearing loss worldwide. Patients with severe phenotypes who have GJB2 mutations are good candidates for implantation, because their hearing loss is of cochlear origin and non-progressive [Citation16,Citation17]. SLC26A4 is known as a commonly found gene and is associated with enlarged vestibular aquaduct [Citation11]. This phenotype includes congenital and progressive hearing loss, usually associated with vertigo [Citation18]. In most cases hearing remains in low frequencies, enabling the understanding of spoken language with hearing aids. Cochlear implantation has resulted in remarkable improvements in auditory skills and speech perception for patients with profound hearing loss associated with SLC26A4 mutations as well as GJB2.
Comparing case 1 (GJB2 mutation) with case 2 (SLC26A4 mutation), the crucial importance of the use of hearing aids during childhood up to age 6 years for acquisition of better hearing is evident. In case 1, even though she was able to hear sound with the use of hearing aids, she was unable to recognize enough speech language due to insufficient hearing amplification during the critical periods in her childhood. She therefore used lip-reading and some sign language in addition to hearing aids. Increased metabolism was observed by FDG-PET in the auditory association area, where no significant activation was found in the normal-hearing controls. In contrast, in case 2, a 26-year-old patient with an SLC26A4 mutation, there was no significant activation in the corresponding area. He obtained rather hearing ability and spoken language by hearing aids with residual hearing at lower frequencies during his childhood. His hearing was supposed to be better than case 1, because 1) he did not use lip-reading or sign language during the acquisition age for language from anamnestic evaluation; 2) his pronunciation was clear, indicating better hearing (at least 40–50 dB) during the acquisition age for language; 3) from an etiological point of view, patients with SLC26A4 mutation usually have mild to moderate hearing loss during childhood and this shows a progressive nature [Citation18]. He had progressive hearing loss in the natural history as a phenotype of SLC26A4 mutation. The difference in activation patterns in the cases with GJB2 and SLC26A4 mutations was clearly demonstrated by statistical processing with SPM, as well as in the PET scans. These results suggest the importance of hearing during early childhood for the development of a normal cortical language network, and that reorganization had occurred in the auditory cortex of the patient with a GJB2 mutation; i.e. processing visual aspects of language in the superior temporal gyri. This implies that cross-modal plasticity as a consequence of the lack of hearing during the critical period for spoken language acquisition in early childhood was influenced by the time course of hearing loss characterized by genetic mutations.
Previous studies have suggested that auditory areas presented high accumulation of FDG with deafness of early onset, and plastic changes in auditory cortices were strongly affected by the duration of auditory deprivation [Citation1,Citation5,Citation6,Citation19,Citation20]. Since low activation of the auditory cortices with visual stimuli suggests the subject's lesser dependence on visual communication methods and substantial residual plasticity in his auditory cortices, case 2 with an SLC26A4 mutation may be determined to be an appropriate candidate for cochlear implantation.
Accurate diagnosis of hearing loss and early cochlear implantation are important for successful spoken language development. The approach using PET could help those involved in the habilitation and education of prelingually deafened children to decide upon the suitable mode of communication for each individual.
Both of the patients received cochlear implantation after PET examination. Further follow-up of these cases may indicate that efficacy of the combination of genetic diagnosis and functional brain imaging helps to predict long-term outcomes of cochlear implantation. Examination of more cases is necessary to define the relationship of the varying cortical activation patterns with each genetic mutation.
Acknowledgments
We thank A.C. Apple-Mathews for help in preparing the manuscript. We also thank Masanori Sakaguchi MD and radiologic technologists, Kouichi Anraku and Hiroyuki Fujimoto, for excellent technical assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Okazawa H, Naito Y, Yonekura Y, Sadato N, Hirano S, Nishizawa S, Cochlear implant efficiency in pre- and postlingually deaf subjects. A study with H2(15)O and PET. Brain 1996;119:1297–306.
- Naito Y, Hirano S, Honjo I, Okazawa H, Ishizu K, Takahashi H, Sound-induced activation of auditory cortices in cochlear implant users with post- and prelingual deafness demonstrated by positron emission tomography. Acta Otolaryngol 1997;117:490–6.
- Naito Y, Tateya I, Fujiki N, Hirano S, Ishizu K, Nagahama Y, Increased cortical activation during hearing of speech in cochlear implant users. Hear Res 2000;143:139–46.
- Tateya I, Naito Y, Hirano S, Kojima H, Inoue M, Kaneko K, Inner ear hearing loss modulates ipsilateral temporal lobe activation by monaural speech stimuli. Neuroreport 2003;14:763–7.
- Catala´n-Ahumada M, Deggouj N, De Volder A, Melin J, Michel C, Veraart C. High metabolic activity demonstrated by positron emission tomography in human auditory cortex in case of deafness of early onset. Brain Res 1993;623:287–92.
- Deggouj N, Devolder A, Catalan M, Melin J, Michel C, Gersdorff M, Positron emission tomography in deaf patients at rest. Adv Otorhinolaryngol 1995;50:31–7.
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Cross-modal plasticity and cochlear implants. Nature 2001;409:149–50.
- Usami S, Wagatsuma M, Fukuoka H, Suzuki H, Tsukada K, Nishio S, The responsible genes in Japanese deafness patients and clinical application using Invader assay. Acta Otolaryngol 2008;128:446–54.
- Abe S, Yamaguchi T, Usami S. Application of deafness diagnostic screening panel based on deafness mutation/gene database using Invader Assay. Genetic Testing 2007;11:333–40.
- Abe S, Usami S, Shinkawa H, Kelley PM, Kimberling WJ. Prevalent connexin 26 gene (GJB2) mutations in Japanese. J Med Genet 2000;37:41–3.
- Usami S, Abe S, Weston MD, Shinkawa H, Van Camp G, Kimberling WJ. Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet 1999;104:188–92.
- Fujiwara K, Naito Y, Senda M, Mori T, Manabe T, Shinohara S, Brain metabolism of children with profound deafness: a visual language activation study by 18 F-fluorodeoxyglucose positron emission tomography. Acta Otolaryngol 2008;128:393–7.
- Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SC, McGuire PK, Activation of auditory cortex during silent lipreading. Science 1997;276:593–6.
- Hall DA, Fussell C, Summerfield AQ. Reading fiuent speech from talking faces: typical brain networks and individual differences. J Cogn Neurosci 2005;17:939–53.
- Nishimura H, Hashikwa K, Doi K, Iwaki T, Watanabe Y, Kusuoka H, Sign language ‘heard’ in the auditory cortex. Nature 1999;397:116.
- Fukushima K, Sugata K, Kasai N, Fukuda S, Nagayasu R, Toida N, Better speech performance in cochlear implant patients with GJB2-related deafness. Int J Pediatr Otorhinolaryngol 2002;62:151–7.
- Tsukada K, Nishio S, Usami S-i. A large cohort study of GJB2 mutations in Japanese hearing loss patients. Clin Genet 2010;78:464–70.
- Suzuki H, Oshima A, Tsukamoto K, Abe S, Kumakawa K, Nagai K, Clinical characteristics and genotype–phenotype correlation of hearing loss patients with SLC26A4 mutations. Acta Otolaryngol 2007;127:1292–7.
- Nishimura H, Doi K, Iwaki T, Hashikawa K, Oku N, Teratani T, Neural plasticity detected in short- and long-term cochlear implant users using PET. Neuroreport 2000;11:811–15.
- Giraud AL, Truy E, Frackowiak R. Imaging plasticity in cochlear implant patients. Audiol Neurootol 2001;6:381–93.