Abstract
Conclusions: The patients who received electric acoustic stimulation (EAS) cochlear implantation had relatively good vestibular function compared with the patients who did not have residual hearing. The vestibular function was well preserved after atraumatic EAS surgery. The round window approach and soft electrode are preferred to decrease the risk of impairing vestibular function. Objectives: The aim of this study was to examine the characteristic features of vestibular functions before and after implantations in patients undergoing EAS. Methods: Vestibular functions in patients who underwent EAS implantation were examined by caloric testing and vestibular evoked myogenic potential (VEMP) in 11 patients before and in 13 patients after implantation. Results: Preoperative evaluation showed that of the 11 patients, most (73%) had good vestibular function. One of 11 patients (9%) had decreased response in postoperative VEMP but all of the patients had unchanged results in postoperative caloric testing.
Keywords::
Introduction
Recently, a series of reports have shown the efficiency of electric acoustic stimulation (EAS) in patients with residual acoustic hearing in the lower frequencies [Citation1]. The development of techniques such as soft surgery when performing cochleostomy [Citation2], round window insertion [Citation3], use of atraumatic electrodes [Citation4,5], and postoperative steroid administration has enabled preservation of residual hearing after cochlear implantation (CI) surgery.
Current techniques of CI also facilitate remarkable improvement in hearing ability. However, consideration must still be given to the complications that can accompany a CI.
One possible such complication is impairment of vestibular function with resulting vertigo symptoms. The incidence of this complication as reported in the literature varies widely from 0.33% to 75% [Citation6].
Although numerous studies have reported the effects of CI on the vestibular function in deaf patients, there have been no reports examining the vestibular function in patients who had residual hearing at lower frequencies, or of the postoperative effects on vestibular function of new atraumatic concepts of electrode and surgical techniques.
We recently published a preliminary report that the round window approach (RWA) is preferable from the viewpoint of vestibular function [Citation7].
The aim of the present study was to further examine the changes in vestibular functions after implantation in patients who underwent EAS CI.
Material and methods
Patients
Thirteen patients (four males and nine females) who underwent EAS CI in our center were included in this study after obtaining informed written consent. The study was carried out with the approval of the Shinshu University Ethical Committee.
The age at implantation ranged from 30 to 60 years, and the mean age was 45.2 years. All patients fulfilled the following inclusion criteria: post-lingually acquired, bilateral sensorineural hearing loss (HL) with pure tone thresholds of <65 dB HL at the low frequencies (125, 250, and 500 Hz), of ≥80 dB HL at frequency 2 kHz, and of ≥85 dB HL at frequencies >4 kHz, and monosyllabic word recognition scores in quiet of ≤60% at 65 dB sound pressure level (SPL) in both ears in best-aided condition. Subjects were still included in this study if one of these frequencies was out of the mentioned decibel levels by only 10 dB or less.
Cochlear implantations
We performed CI with full insertion of the ME-DEL FLEXEAS® electrode (MED-EL, Innsbruck, Austria) in all patients.
All surgeries were performed by a single surgeon and the RWA was applied for electrode insertion. Systemic antibiotics and dexamethasone were administered peri- and postoperatively. Residual hearing was successfully preserved in all patients (data not shown).
Vestibular testing
The patients were examined by caloric testing and vestibular evoked myogenic potential (VEMP) before or after implantation, or both, to obtain data on semicircular canal function and otolithic function, respectively.
In VEMP testing, electromyography (EMG) was carried out using a pair of surface electrodes mounted on the upper half and the sterna head of the sternocleidomastoid (SCM) muscle. The electrographic signal was recorded using a Neuropack evoked potential recorder (Nihon Kohden Co. Ltd, Tokyo, Japan). Clicks lasting for 0.1 ms at 105 dBnHL were presented through a headphone. The stimulation rate was 5 Hz, the bandpass filter intensity was 20–2000 Hz, and analysis time was 50 ms. The responses to 200 stimuli were averaged twice. Because the amplitude of the VEMP based on the unrectified EMG is correlated with the activity of the SCM muscle during the test [Citation8], we measured the activity of the SCM muscle using the background integrated EMG response, the area under the averaged rectified EMG curve, from –20 ms to 0 ms before the sound stimulation. The correction of the amplitude was calculated as follows [Citation9]:
Corrected amplitude (ms–1) = amplitude of the averaged unrectified EMG (micro V)/background integrated EMG (micro V ms)
In caloric testing, maximum slow phase velocity (SPV) was measured by cold water irrigation (20°C, 5 ml, 20 s). We defined below 10°/s of SPV as areflexia and between 10 and 20°/s as hyporeflexia.
Statistical analysis
SPSS for Windows software (Chicago, IL, USA) was used for all analyses, and paired t test was applied when comparing differences in preoperative and postoperative vestibular functions. Statistical significance was set at p < 0.05.
Results
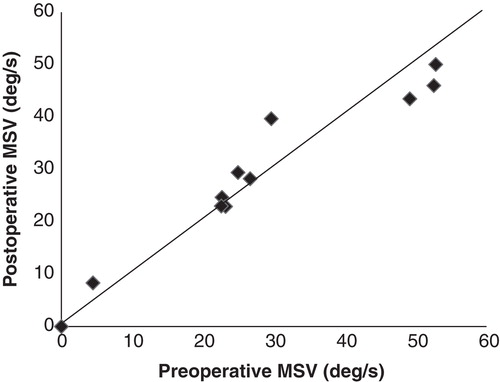
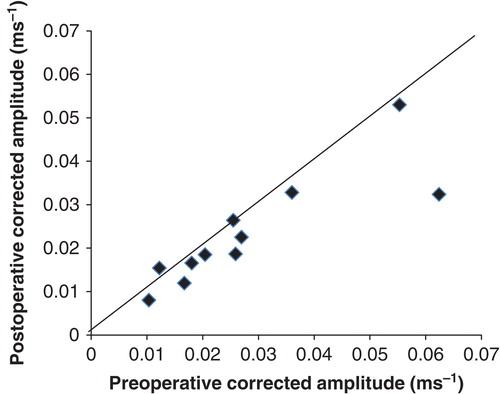
The results are summarized in .
Table I. Summary of patients' details.
Semicircular canal function
Preoperative evaluation was performed bilaterally. Three of 11 patients (27%, nos 3, 4, and 5) showed areflexia or hyporeflexia in caloric testing. Patient no. 4 had bilateral areflexia, no. 5 had implanted ear areflexia and non-implanted ear hyporeflexia, and no. 3, had hypoflexia only in the non-implanted ear.
Postoperative caloric testing was obtained after 1 month or more. All 13 patients underwent postoperative caloric testing and 11 of them were also examined before the EAS implantations. Two (nos 4 and 5) of 13 patients (15%) had abnormal postoperative caloric test results in the implanted ear, although both of them also had abnormal results before implantations. shows the caloric response before and after EAS implantations for the implanted ear. Compared with before implantations, the results after implantations were unchanged in all of the 11 patients who underwent both preoperative and postoperative testing. One patient (no. 4) had areflexia both before and after implantation. The mean SPV was 28.06°/s preoperatively (SD = 17.61) and 28.68°/s postoperatively (SD = 15.53). There were no significant differences between results before and after implantations in caloric testing (p = 0.67).
Otolithic function
When preoperative evaluation was performed, no patients showed absent response in VEMP.
Postoperative VEMP was obtained after 1 month or more. All 13 patients underwent postoperative VEMP and 11 of them were also examined before EAS implantations. No patient had absent VEMP response in the implanted ear. shows corrected VEMP amplitudes before and after EAS implantations for the implanted ear. Although one (no. 8) of the 11 patients (9%) had a decreased response in corrected VEMP amplitude, corrected VEMP amplitudes after implantations were unchanged in all but one of the patients, when compared with preoperative results. The mean corrected amplitude was 0.028 preoperatively (SD = 0.017) and 0.023 postoperatively (SD = 0.013). There were no significant differences between results before and after implantation in VEMP testing (p = 0.095).
Discussion
Previous reports showed that the frequencies of ‘preoperative' vestibular disorders in profound hearing loss patients were about 30–73% in caloric testing [Citation10–14] and about 11–65% in VEMP [Citation10–15].
In this study, we found that the ‘preoperative' frequencies of vestibular disorders in hearing loss patients with residual hearing who received EAS were 27% and 0% in caloric testing and VEMP, respectively.
This finding suggested that vestibular function of the patients who underwent EAS was relatively good compared with the patients with profound hearing loss who underwent conventional CI.
In this study, to preserve such good vestibular function, atraumatic CI surgery (RWA with flexible thin electrode) was performed. Although one patient showed a decreased VEMP result, there was no hypofunction in postoperative caloric testing when compared with preoperative results in the implanted ear.
According to previous reports, various frequencies of postoperative deterioration in vestibular function were demonstrated. Postoperative hypofunction was found in 6–58% in the caloric testing [Citation10–14,16–18], and 13–86% in VEMP [Citation10–15]. One of the reasons for such variation is probably the surgical technique applied.
Todt et al. reported that hypofunction of postoperative VEMP was seen in 50% of patients who underwent cochleostomy and 13% of those with RWA Also, abnormal postoperative caloric testing results were seen in 42.9% of the patients who underwent cochleostomy and 9.4% of those who had the RWA [Citation10].
Temporal bone studies have shown that an electrode insertion into the scala vestibuli involves damage of the osseous spiral lamina, basilar membrane, and vestibular receptors. The saccule was the most frequently damaged vestibular receptor, followed by the utricle and the semicircular canals [Citation19].
However, when the electrode was inserted into the scala tympani, no vestibular damage was found [Citation19]. Adunka et al. evaluated cochlear implant electrode insertions through the round window membrane histologically and reported that smooth implantations via round the window membrane resulted in deep, atraumatic insertions into the scala tympani [Citation20]. Unintentional lesions to the basilar membrane can be avoided by using the round window as an exact anatomic landmark that is always in direct continuity with the scala tympani [Citation20]. Previous histological and clinical studies clearly showed that the RWA is the technique that preserves the vestibular functions to the greatest extent and therefore is better than cochleostomy.
In the present study, the FLEXEAS electrode was used for all of the patients. The cross-sectional diameter of the electrode is smaller than a conventional electrode, varying from 0.33 by 0.49 mm at the apex and to 0.8 mm at the basal, and a major feature of the device is its superior flexibility. Histology and dissection of human temporal bones performed by Adunka et al. confirmed the atraumatic character of this device [Citation20]. Insertion forces with the conventional array and FLEX array were measured in an acrylic model of the scala tympani, demonstrating that insertion force could be reduced significantly by more than 40% with the FLEXEAS electrode [Citation4]. As in our previous study [Citation7], such a smaller diameter and more flexible electrode might enable less damage to not only the cochlear tissue, but also the vestibular organs.
In conclusion, patients undergoing EAS implantation have good vestibular function compared with the vestibular function of the patients with profound hearing loss. It is important to preserve not only residual hearing but also the vestibular function of the implanted ears, using atraumatic surgical techniques. The RWA with soft electrode is preferable to decrease the risk of damage to vestibular function.
Acknowledgments
We thank A.C. Apple-Mathews for help in preparing the manuscript. This study was supported by a Health and Labour Sciences Research Grant for Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labour and Welfare of Japan (S.U.) and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (S.U.).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- von Ilberg CA, Baumann U, Kiefer J, Tillein J, Adunka OF. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurootol 2011;16:1–30.
- Lehnhardt E, Laszig R. 1994. Specific surgical aspects of cochlear implant soft surgery. In Hochmair-Desoyer IJ, Hochmair ES, editors. Advances in cochlear implants. Vienna: Manz. p. 228–9.
- Skarzynski H, Lorens A, Piotrowska A, Anderson I. Preservation of low frequency hearing in partial deafness CI (PDCI) using the round window surgical approach. Acta Otolaryngol 2007;127:41–8.
- Adunka O, Kiefer J, Unkelbach MH, Lehnert T, Gstoettner W. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope 2004;114:1237–41.
- Baumgartner WD, Jappel A, Morera C, Gstöttner W, Müller J, Kiefer J, et al. Outcomes in adults implanted with the FLEXsoft electrode. Acta Otolaryngol 2007;127:579–86.
- Buchman CA, Joy J, Hodges A, Telischi FF, Balkany TJ, Vestibular effects of CI. Laryngoscope 2004;114:1–22.
- Usami S, Moteki H, Suzuki N, Fukuoka H, Miyagawa M, Nishio SY, et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol 2011;131:405–12.
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 1994;57:190–7.
- Shojaku H, Takemori S, Kobayashi K, Watanabe Y. Clinical usefulness of glycerol vestibular-evoked myogenic potentials: preliminary report. Acta Otolaryngol Suppl 2001;545:65–8.
- Todt I, Basta D, Ernst A. Does the surgical approach in cochlear implantation influence the occurrence of postoperative vertigo? Otolaryngol Head Neck Surg 2008;138:8–12.
- Melvin TA, Della Santina CC, Carey JP, Migliaccio AA. The effects of cochlear implantation on vestibular function. Otol Neurotol 2009;30:87–94.
- Krause E, Louza JP, Wechtenbruch J, Gürkov R. Influence of cochlear implantation on peripheral vestibular receptor function. Otolaryngol Head Neck Surg 2010;142:809–13.
- Krause E, Wechtenbruch J, Rader T, Gürkov R. Influence of cochlear implantation on sacculus function. Otolaryngol Head Neck Surg 2009;140:108–13.
- Wagner JH, Basta D, Wagner F, Seidl RO, Ernst A, Todt I. Vestibular and taste disorders after bilateral cochlear implantation. Eur Arch Otorhinolaryngol 2010;267:1849–54.
- Licameli G, Zhou G, Kenna MA. Disturbance of vestibular function attributable to cochlear implantation in children. Laryngoscope 2009;119:740–5.
- Krause E, Louza JP, Hempel JM, Wechtenbruch J, Rader T, Gürkov R. Effect of cochlear implantation on horizontal semicircular canal function. Eur Arch Otorhinolaryngol 2009;266:811–17.
- Fina M, Skinner M, Goebel JA, Piccirillo JF, Neely JG, Black O. Vestibular dysfunction after cochlear implantation. Otol Neurotol 2003;24:234–42.
- Enticott JC, Tari S, Koh SM, Dowell RC, O'Leary SJ. Cochlear implant and vestibular function. Otol Neurotol 2006;27:824–30.
- Tien HC, Linthicum FH Jr. Histopathologic changes in the vestibule after cochlear implantation. Otolaryngol Head Neck Surg 2002;127:260–4.
- Adunka O, Unkelbach MH, Mack M, Hambek M, Gstoettner W, Kiefer J. Cochlear implantation via the round window membrane minimizes damage to cochlear structures: a histologically controlled insertion study. Acta Otolaryngol 2004;124:807–12.