Abstract
Conclusion: Inhibition of thioredoxin reductase (TrxR) may be a contributing factor in cisplatin-induced ototoxicity. Direct exposure of organ of Corti to cisplatin and oxaliplatin gives equal loss of hair cells. Objectives: Platinum-containing drugs are known to target the anti-oxidant selenoprotein TrxR in cancer cells. Two such anti-cancer, platinum-containing drugs, cisplatin and oxaliplatin, have different side effects. Only cisplatin induces hearing loss, i.e. has an ototoxic side effect that is not seen after treatment with oxaliplatin. The objective of this study was to evaluate if TrxR is a target in the cochlea. Loss of outer hair cells was also compared when cisplatin and oxaliplatin were administered directly to the organ of Corti. Methods: Organ of Corti cell culture was used for direct exposure to cisplatin and oxaliplatin. Hair cells were evaluated and the level of TrxR was assessed. Immunohistochemical staining for TrxR was performed. An animal model was used to evaluate the effect on TrxR after treatment with cisplatin and oxaliplatin in vivo. Results: Direct exposure of cochlear organotypic cultures to either cisplatin or oxaliplatin induced comparable levels of outer hair cell loss and inhibition of TrxR, demonstrating that both drugs are similarly ototoxic provided that the cochlea becomes directly exposed.
Introduction
The two anti-cancer platinum drugs cisplatin (cis- diamminedichloroplatinum) and oxaliplatin (R,R-1, 2-diaminocyclohexane platinum) are dose-limited in clinical use due to different side effects. Cisplatin displays ototoxicity in particular and the hearing loss is revealed initially at high frequencies and expands to middle frequencies with increasing cumulative doses [Citation1]. Oxaliplatin rarely displays ototoxicity. Instead, it induces peripheral neurotoxicity characterized by acute reversible paresthesia followed by chronic neuropathy [Citation2]. These strikingly different ototoxic profiles are believed to be largely explained by differences in cochlear pharmacokinetics [Citation3]. We have further investigated this hypothesis using isolated ex vivo cultures of the organ of Corti.
Molecular mechanisms of cisplatin-induced ototoxicity may involve both DNA-dependent and DNA-independent processes. Long-term exposure to cisplatin causes accumulation of platinum-DNA adducts in the inner ear [Citation4] and formation of DNA-platinum adducts also contributes to the drug's anti-neoplastic effects [Citation5]. However, DNA-independent mechanisms are also important [Citation6]. Such mechanisms may be of particular significance for cell injury in terminally differentiated cells, such as those of the cochlea, lacking active DNA replication. Indeed, triggered oxidative stress is a major reason for cisplatin-induced hair cell death [Citation7]. In organ of Corti cultures, superoxide and hydrogen peroxide [Citation7] levels increase immediately following exposure to cisplatin, while glutathione (GSH) and antioxidant enzymes, including superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase, decrease [Citation7]. These effects finally trigger lipid peroxidation [Citation8]. Furthermore, cisplatin can target the thioredoxin (Trx) enzyme system, which is of major importance for cellular anti-oxidant defense mediated by isoenzymes of thioredoxin reductase (TrxR) and Trx [Citation9]. Trx system functions are exerted by downstream Trx substrates involved in several redox pathways, including ribonucleotide reductase, peroxiredoxins, and methionine sulfoxide reductases [Citation9]. The selenoprotein TrxR keeps the active site disulfide of Trx reduced, thereby driving the entire Trx system. The ubiquitously expressed predominantly cytosolic isoenzyme TrxR1 is the major TrxR species in most mammalian cells [Citation10] and expression of TrxR1 was previously demonstrated in cochlea of mature guinea pigs [Citation3]. Of particular interest in the context of platinum drug toxicity is the highly reactive nucleophilic selenocysteine residue of TrxR1, which is readily derivatized by several electrophilic compounds, including cisplatin and oxaliplatin [Citation11–13]. However, targeting TrxR in cochlea has never been demonstrated. We thus wanted to study whether hair cell loss in cochlear cultures on direct exposure to either cisplatin or oxaliplatin could also involve inhibition of cochlear TrxR activity.
Material and methods
Animals
Tissue culture experiments were performed on organs of Corti isolated from 75 Sprague Dawley rat pups obtained from time-mated females at 2 (P2) or 3 (P3) days after birth (Scanbur AB, Sweden). In vivo experiments were performed using 15 female albino guinea pigs (Duncan-Hartley) at approximately 9 weeks of age, weighing 306 g ± 18 (mean ± SD), supplied from a local breeder. Animals were kept at a 12 h light/dark cycle with free access to food and water. All animal experiments were approved by the local Animal Care Committee (ethical permits N32/07, N372/08, and N320/09).
Organ of Corti cultures
Organs of Corti (organ of Corti and spiral limbus) from P2 or P3 rat cochleae were dissected into phosphate-buffered saline (PBS) and cultured in six-well plates on 0.4 µm membrane inserts (Millicell 0.4 µm; Millipore, Solna, Sweden) in advanced D-MEM (Dulbecco's Modified Eagle Medium; Invitrogen, Carlsbad, USA) supplemented with 10 µl/ml of N1 supplement (Sigma, St. Louis, USA), 5.5 µl/ml of 30% glucose, and 100 units/ml of penicillin. Five or six organs were cultured per well in medium sufficient to cover all tissue with a thin film. After 24 h, the culture medium was replaced with new medium without (control) or with cisplatin (Cisplatin Mayne, 1 mg/ml; Hospira, LakeForest, USA) or oxaliplatin (Eloxatin, 5 mg/ml; Sanofi-Aventis, Parice, France) at concentrations as indicated. After exposure for 24 h, cultures were either terminated and used for assessing enzyme activity or left in new medium without platinum drugs for another 24 h and used to estimate hair cell loss. Data presented here represent three separate experiments for each condition. The half-life (t½) of cisplatin and oxaliplatin in culture medium was determined at final concentrations of 25 µM and 10 µM, respectively, with samples taken every 30 min and analyzed as described previously [Citation3].
To estimate hair cell loss, organs were fixed in 4% paraformaldehyde for 1 h, permeabilized in 0.3% Triton X-100 in PBS for 30 min at room temperature, stained with fluorescein isothiocyanate (FITC)- conjugated phalloidin (1:1000 in PBS; Molecular Probes, Stockholm, Sweden) for 1 h, and mounted in Mowiol Antifading Agent (Calbiochem, Darmstadt, Germany). They were subsequently examined with an Olympus BX-10 microscope and images were collected along the entire length of the first turn using a ×40 objective. Outer hair cell density was estimated by counting the number of visible hair cell cuticular plates per 100 µm. Hair cell densities were calculated separately for the first and second half of the basal turn.
Immunohistochemistry
Cochleae were harvested from P2–P3 rats, fixed in 4% paraformaldehyde overnight at 4°C, and decalcified in 0.1 M EDTA for 3 days before embedding in paraffin. Antigen retrieval was performed in a Tris/EDTA buffer (pH 9; DAKO Target Retrieval Solution, Dako, Glosturp, Denmark) using the manufacturer's recommendations. Endogenous peroxidase activity was quenched by incubating the sections for 5 min in 0.3% H2O2/0.3% normal goat serum in PBS followed by a permeabilization/blocking step (1.5% normal goat serum/0.1% Triton X-100 in PBS) for 1 h at room temperature. Sections were incubated with polyclonal anti-TrxR1 antibodies (rabbit IgG, 1:1000 in blocking solution; Upstate Laboratories, New York, USA) with negative controls omitting the primary antibody. Incubation with biotinylated secondary antibody (goat anti-rabbit; VectaStain Elite ABC kit, Vector Laboratories, Burlingame, USA) for 40 min was followed by incubation with the ABC reagent and DAB-hydrogen peroxide solution according to VectaStain's protocol. Sections were dehydrated in ethanol, cleared in xylene, and mounted with Mountex (HistoLab AB, Stockholm, Sweden).
Preparing extracts for enzyme activity measurements
To determine TrxR activity in tissue extracts, the organ of Corti and lateral walls (including stria vascularis) were isolated from P2 rats (as described above). Specimens were cultured in separate wells and exposed to either normal medium (as described above) or medium containing either 20 µM cisplatin or 20 µM oxaliplatin for 24 h before subsequent harvesting and immediate processing. Tissue cultures were rinsed in PBS, transferred to lysis buffer (25 mM Tris-HCl at pH 7.5, 2.5 mM EDTA, 2.5 mM EGTA, 20 mM sodium fluoride, 1 mM sodium orthovanadate, 100 mM sodium chloride, 20 mM glycerophosphate, 10 mM disodium pyrophosohate, and 0.5% (vol/vol) Triton X-100) containing 2× Protease Inhibitor Cocktail Complete (Roche Diagnostics GmbH, Basel, Switzerland), thoroughly homogenized, and frozen at –20°C. Cell lysates were thawed and separated from the non-soluble fraction by centrifugation at 16 000 g for 10 min at 4°C.
In vivo administration of cisplatin and oxaliplatin
Fifteen albino guinea pigs were randomly divided into three groups. Group I (n = 5) received a single intravenous dose of cisplatin (8 mg/kg; Platinol 1 mg/ml, Bristol-Myers Squibb Pharmaceuticals, New York, USA) and group II (n = 5) received a single intravenous dose of oxaliplatin (10.6 mg/kg; Eloxatin 5 mg/ml, Sanofi-Aventis). Group III (controls, n = 5) received 1 ml saline intravenously. Drug administration was performed as described previously [Citation14].
At 24 h after drug administration, animals were sacrificed with an overdose of sodium pentobarbital. Both temporal bones were immediately removed and the organ of Corti and lateral walls were isolated from the cochlea. Left and right cochleae from the same animal were pooled in lysis buffer (described above). Samples were promptly frozen on dry ice and stored at –80°C. Thawed samples were homogenized and cell lysates were separated from the non-soluble fraction by centrifugation at 16 000 g for 10 min at 4°C.
TrxR activity assay
TrxR activity was determined using the previously described end point Trx-dependent insulin reduction method [Citation12] modified and applied to microtiter plates [Citation15].
Data analyses
In the degradation studies, areas under the chromatographic peaks of oxaliplatin or cisplatin were plotted against time and degradation half-life was calculated as ln2/kobs. The observed pseudo-first-order rate constants (kobs) for the degradation were determined by non-linear regression (Graph-Pad Prism version 3.02, San Diego, CA, USA) on the exponential decay of the relative peak areas. Hair cell densities were compared between treatments and between the two different parts of the basal turn (first half and second half) using two-way ANOVA (general linear model). The Tukey test was applied as a post hoc test using an all pair-wise multiple comparison procedure. TrxR activities were compared using one-way ANOVA or ANOVA on ranks in cases of unequal variance. All statistical procedures were performed in Sigma Plot 11.0 (Systat Software Inc., Chicago, USA).
Results and Discussion
Before treating cultured organs of Corti, the stability of cisplatin and oxaliplatin in the utilized culture medium was determined. This showed a half-life for cisplatin of 6.26 h (95% confidence interval (CI) = 5.62–7.07) and 2.21 h for oxaliplatin (95% CI = 2.11–2.31). Although the drugs were thereby cleared from the medium within a few hours, we used a protocol with an initial 24 h exposure followed by 24 h in fresh platinum drug-free medium to allow sufficient time for any apoptotic signaling events or other cell death mechanisms to be completed (if such were indeed triggered by the initial drug exposure).
Cisplatin decreased outer hair cell densities in cultured organs of Corti. This effect was pronounced in the first half of the basal turn (p < 0.001; two-way ANOVA) where outer hair cell density decreased from 39 hair cells/0.1 mm to 23, 14, and 8 on exposure to 10, 20, and 30 µM cisplatin, respectively (, filled symbols). In the second half of the basal turn, exposure to higher concentrations (20 and 30 µM) reduced cell density from 43 outer hair cells/0.1 mm to 31 and 16, respectively (, open symbols). When organ of Corti cultures were exposed to 20 and 30 µM oxaliplatin under identical conditions, outer hair cell densities also displayed significant decreases from 41 hair cells/0.1 mm to 26 and 9 hair cells/0.1 mm, respectively (). A lower oxaliplatin dose (10 µM) did not alter the outer hair cell count. Moreover, no difference between the first and second half of the basal turn was detected. Hence the outer hair cell densities are shown for the entire basal turn.
Figure 1. Outer hair cell densities in the basal turn of the organ of Corti following 24 h in culture with cisplatin or oxaliplatin. (A) Exposure to cisplatin (10, 20, and 30 µM). Filled symbols indicate the first half of the basal turn. Outer hair cell density in cultures exposed to 10, 20, and 30 µM cisplatin decreased significantly when compared with control (p < 0.001 [control vs 30 µM, control vs 20 µM]; p = 0.03 [control vs 10 µM]). Open symbols indicate the second half of the basal turn. Outer hair cell density was significantly affected by exposure to 20 and 30 µM cisplatin (p < 0.001 [control vs 30 µM]; p = 0.04 [control vs 20 µM]). (B) As exposure to oxaliplatin made no difference in outer hair cell densities between the two halves of the basal turn, data for the entire basal turn are shown for each concentration (10, 20, and 30 µM). Exposure to 20 and 30 µM oxaliplatin reduced outer hair cell density significantly (p < 0.001 [control vs 30 µM]; p = 0.002 [control vs 20 µM]) and the three experimental conditions differed significantly from each other (p < 0.001 [10 µM vs 30 µM, 20 µM vs 30 µM]; p = 0.002 [10 µM vs 20 µM]).
![Figure 1. Outer hair cell densities in the basal turn of the organ of Corti following 24 h in culture with cisplatin or oxaliplatin. (A) Exposure to cisplatin (10, 20, and 30 µM). Filled symbols indicate the first half of the basal turn. Outer hair cell density in cultures exposed to 10, 20, and 30 µM cisplatin decreased significantly when compared with control (p < 0.001 [control vs 30 µM, control vs 20 µM]; p = 0.03 [control vs 10 µM]). Open symbols indicate the second half of the basal turn. Outer hair cell density was significantly affected by exposure to 20 and 30 µM cisplatin (p < 0.001 [control vs 30 µM]; p = 0.04 [control vs 20 µM]). (B) As exposure to oxaliplatin made no difference in outer hair cell densities between the two halves of the basal turn, data for the entire basal turn are shown for each concentration (10, 20, and 30 µM). Exposure to 20 and 30 µM oxaliplatin reduced outer hair cell density significantly (p < 0.001 [control vs 30 µM]; p = 0.002 [control vs 20 µM]) and the three experimental conditions differed significantly from each other (p < 0.001 [10 µM vs 30 µM, 20 µM vs 30 µM]; p = 0.002 [10 µM vs 20 µM]).](/cms/asset/b4d22be1-1ab3-4210-89de-92cde28660db/ioto_a_879740_f0001_b.jpg)
Overall, cisplatin and oxaliplatin displayed similar toxicity profiles for organs of Corti in culture. This finding gives further insight into the dissimilarities in ototoxic effect induced by these two platinum-containing drugs. Our results strongly support the finding that the absence of oxaliplatin ototoxicity in a clinical setting is not due to reduced toxicity per se, but is mainly explained by its lower concentrations in the inner ear [Citation3]. This may reflect that active transport across the blood–labyrinth barriers described for cisplatin [Citation16] may possibly not occur for oxaliplatin. Such an effect is not valid in the organ of Corti culture system used in this study and that is today well established for toxicity studies with cochlear hair cells [Citation7]. Our data on the extent of hair cell loss following cisplatin exposure agree with results of previous studies [Citation7]. To our knowledge, however, the direct toxicity of oxaliplatin has not previously been studied using organ of Corti cultures.
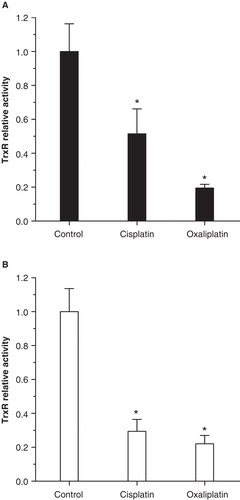
Having discovered that both cisplatin and oxaliplatin are toxic to organ of Corti ex vivo, we wanted to determine whether TrxR1 is expressed in these organs and whether both treatments can inhibit its enzymatic activity. Immunohistochemical staining of non-treated P2 rat control cochlea revealed strong immunoreactivity for TrxR1 in stria vascularis, organ of Corti, and the spiral ganglion (). In the organ of Corti, positive staining for the enzyme was seen in the apical parts of both inner and outer hair cells, as well as in parts of the developing spiral limbus (arrows in ). The stria vascularis exhibited intense staining. We also found that 24 h exposure of organ of Corti cultures to cisplatin significantly reduced TrxR activity to 52% of controls, whereas oxaliplatin reduced activity even further to only 20% (). In extracts of the lateral wall, TrxR activity was reduced to 29% and 22% upon exposure to cisplatin and oxaliplatin, respectively (). Thus, the two platinum compounds (both ototoxic in culture) also inhibited TrxR activity in cultured organ of Corti and lateral walls. To our knowledge, this is the first study demonstrating inhibition of TrxR activity in inner ear tissue following exposure to platinum drugs, although TrxR is a known cisplatin target in cancer cells [Citation17]. Targeting of this enzyme in the organ of Corti may suggest that its inhibition could play a role in cisplatin ototoxicity. As mentioned previously, cisplatin triggers extensive oxidative stress in hair cells [Citation7,18]. With the GSH and Trx systems being the only two major reductive systems in cells [Citation9], inhibiting TrxR could clearly contribute to cisplatin ototoxicity. A lower measured activity of TrxR upon treatment could in theory be due to either a reduction of the amount of TrxR enzyme due to protein degradation or, perhaps more likely as judged from earlier studies (see above), the intracellular formation of inhibited TrxR protein species. In both scenarios inhibition of TrxR activity would be the result, thereby subsequently diminishing the capacity of all thioredoxin-dependent antioxidant defense mechanisms. In addition, derivatized forms of TrxR1 produced by targeting its selenocysteine residue by platinum-containing drugs may trigger cell death through a pro-oxidant gain of function as SecTRAPs (Selenium compromised thioredoxin reductase-derived apoptotic proteins) [Citation15]. The importance of TrxR as a molecular target in cisplatin ototoxicity is thus clearly plausible and should be further studied.
Figure 2. Immunohistochemical staining showed positive immunoreactivity for thioredoxin reductase (TrxR) in P2 rat cochlea. (A) Positive staining was seen in the apical part of inner and outer hair cells (arrowheads), in the developing stria vascularis, and in the cells of Claudius (arrows). (B) Positive staining in the spiral ganglion. (C) Negative control was obtained by omitting primary antibody. Scale bars = 50 µm in A and B, 100 µm in C.
Figure 3. Both cisplatin and oxaliplatin inhibit thioredoxin reductase (TrxR) enzyme activity in cochlear organotypic cultures. Relative activity (± SD) of TrxR was measured in cultures of organ of Corti (A) and lateral walls (B) cultured for 24 h with 20 µM cisplatin, 20 µM oxaliplatin or normal medium (control). Enzyme activity was significantly inhibited in cultures treated with either cisplatin or oxaliplatin, but there was no difference between the two treatments. Asterisks indicate statistically significant difference from control: ANOVA on ranks (A); p < 0.05, or one-way ANOVA (B); p < 0.001.
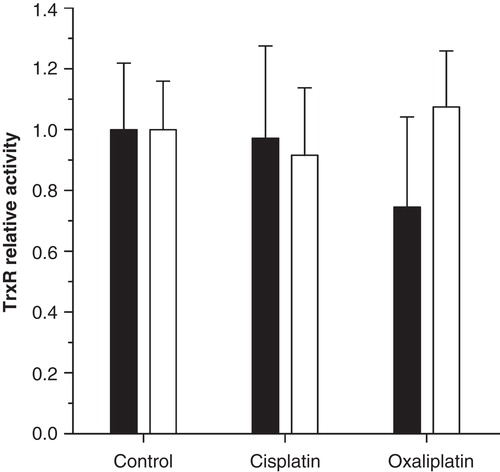
In attempts to assess if cochlear TrxR activity is affected also in vivo, guinea pigs were treated with single bolus doses of equimolar concentrations of cisplatin (8 mg/kg) or oxaliplatin (10.6 mg/kg). These doses of cisplatin and oxaliplatin used in the guinea pig are extreme amounts of the drugs compared with what is given in clinical practice. Cisplatin given intravenously at this dose reduces the hearing threshold [Citation19], whereas oxaliplatin does not [Citation3]. With this set-up and timing, however, we could not detect any effect on total TrxR activity using inner ear tissue harvested 24 h after intravenous administration (). There may be several reasons for failure to demonstrate TrxR inhibition with this set-up. Clearly, the in vivo situation is more complex than organ cultures, rendering the window of measurement important. It has been shown that 8 mg/kg cisplatin induces hair cell loss in guinea pigs 3–4 days after drug administration [Citation20], i.e. at time points later than those in the present study. Furthermore, inhibition of TrxR1 typically triggers synthesis of additional TrxR1 due to Nrf2 activation. These mechanisms can complicate assessment of cochlear TrxR1 inhibition in vivo. In fact, only intravenous doses of cisplatin exceeding LD50 induce changes of electrophysiological hearing thresholds and loss of hair cells as soon as 24 h after administration [Citation20]. A major difference between the in vivo and in vitro situations employed in this experiment is that inner ear transport of cisplatin occurs at much lower concentrations after administration via the intravenous route in the whole guinea pig model [Citation3]. It is thus plausible that the perilymphatic peak concentrations of cisplatin applied here were insufficient to inhibit TrxR activity in the organ of Corti, while still not ruling out the enzyme as a cochlear target in cisplatin ototoxicity.
Figure 4. Cochlear thioredoxin reductase (TrxR) enzyme activity following in vivo administration of platinum compounds. Relative activity (± SD) of TrxR measured 24 h after intravenous injection of equimolar concentrations of cisplatin, oxaliplatin or saline (control) in mature guinea pigs. No effect was detected on TrxR activity in the organ of Corti (filled bars) or in the lateral wall (open bars).
We conclude that both cisplatin and oxaliplatin are toxic to outer hair cells when organs of Corti become directly exposed to these drugs, and that TrxR becomes inhibited in parallel with signs of toxicity. We suggest that this toxicity is likely to involve inhibition of TrxR activity, while the importance of TrxR targeting in platinum drug ototoxicity in vivo requires further scrutiny. We propose that it cannot be excluded that during clinical treatment regimens with high doses of cisplatin, TrxR may be a mediator of cisplatin ototoxicity.
Acknowledgments
We thank Hinrich Staecker for help with the organ of Corti culture system and Anette Fransson for isolating guinea pig tissues. This work was supported by KI Cancer, the Swedish Cancer Society, AFA Insurance, Tysta Skolan, and KI Stiftelser.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol 2007;25:1190–5.
- Pasetto LM, D’Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol 2006;59:159–68.
- Hellberg V, Wallin I, Eriksson S, Hernlund E, Jerremalm E, Berndtsson M, et al. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst 2009;101:37–47.
- van Ruijven MW, de Groot JC, Hendriksen F, Smoorenburg GF. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hear Res 2005;203:112–21.
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005;4:307–20.
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem 2003;278:9100–6.
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol 1997;18:559–71.
- Kim SJ, Park C, Han AL, Youn MJ, Lee JH, Kim Y, et al. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear Res 2009;251:70–82.
- Gromer S, Urig S, Becker K. The thioredoxin system – from science to clinic. Med Res Rev 2004;24:40–89.
- Sun QA, Zappacosta F, Factor VM, Wirth PJ, Hatfield DL, Gladyshev VN. Heterogeneity within animal thioredoxin reductases. Evidence for alternative first exon splicing. J Biol Chem 2001;276:3106–14.
- Witte AB, Anestal K, Jerremalm E, Ehrsson H, Arner ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med 2005;39:696–703.
- Arner ES, Nakamura H, Sasada T, Yodoi J, Holmgren A, Spyrou G. Analysis of the inhibition of mammalian thioredoxin, thioredoxin reductase, and glutaredoxin by cis-diamminedichloroplatinum (II) and its major metabolite, the glutathione-platinum complex. Free Radic Biol Med 2001;31:1170–8.
- Becker K, Herold-Mende C, Park JJ, Lowe G, Schirmer RH. Human thioredoxin reductase is efficiently inhibited by (2,2':6',2' '-terpyridine)platinum(II) complexes. Possible implications for a novel antitumor strategy. J Med Chem 2001;44:2784–92.
- Skjonsberg A, Bucinskaite V, Laurell G, Ulfendahl M. Augmented ototoxic effect of cisplatin in heterozygotes of the German waltzing guinea pig. Audiol Neurootol 2008;13:97–104.
- Anestål K, Prast-Nielsen S, Cenas N, Arnér ES. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS One 2008;3(4):e1846.
- Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol 2010;176:1169–80.
- Urig S, Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol 2006;16:452–65.
- Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am J Otol 2000;21:513–20.
- Ekborn A, Lindberg A, Laurell G, Wallin I, Eksborg S, Ehrsson H. Ototoxicity, nephrotoxicity and pharmacokinetics of cisplatin and its monohydrated complex in the guinea pig. Cancer Chemother Pharmacol 2003;51:36–42.
- Laurell G, Bagger-Sjoback D. Degeneration of the organ of Corti following intravenous administration of cisplatin. Acta Otolaryngol 1991;111:891–8.
