Abstract
Conclusion: Daily intake of 480 mg of BNO 1016 for 15 days is an effective treatment in acute viral rhinosinusitis. Objectives: The pooled efficacy data of two similar randomized placebo-controlled clinical trials were analyzed. Safety was evaluated on the basis of the individual trials. Methods: The efficacy analysis was based on 589 patients. Treatment was performed orally with either 3 × 160 mg BNO 1016 (n = 294) or 3 × placebo (n = 295) for 15 days. In both trials patients underwent five visits to the investigational sites. Symptoms were evaluated according to the EPOS 2012 guideline. Ultrasonography was used to confirm the diagnosis at onset of treatment and the remission of symptoms at the last visit. Efficacy was evaluated by the investigator as the mean major symptom score (MSS) at the end of treatment (visit 5, day 14). Patients reported symptoms and social/emotional consequences of rhinosinusitis using a quality of life questionnaire (SNOT-20 GAV). Results: MSS improved during the treatment period by a mean of 10.02 ± 1.61 score points to 2.47 ± 2.55 for BNO 1016 and of 9.87 ± 1.52 to 3.63 ± 3.63 for placebo. Differences between treatment groups at end of therapy (1.16 ± 3.14 score points; p < 0.0001) and patient-assessed quality of life (p = 0.0015) were statistically significant in favor of BNO 1016.
Introduction
During recent years the concept rhinosinusitis has been introduced to elucidate that an inflammation occurs simultaneously in the nose and the paranasal sinuses. A rhinosinusitis usually involves one or several paranasal sinuses. It might be confirmed by ultrasonography or as an opacification in X-ray investigation. According to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS 2012) acute rhinosinusitis (ARS) is defined – besides as an inflammation of the nose and paranasal sinuses – by two or more of the following characteristic symptoms: nasal congestion or secretion combined with facial pain or pressure or loss/reduction of smell [Citation1]. Additional symptoms such as fever, fatigue or headache may occur.
Acute rhinitis is the first step in the development of rhinosinusitis. The demarcation of acute and chronic rhinosinusitis is mainly defined by length of the disease and not by specific symptoms. According to the definitions in EPOS 2012 an ARS is completely healed without remaining symptoms after 12 weeks, while in a chronic rhinosinusitis one or several symptoms still remain after that period. An acute viral rhinosinusitis lasts less than 10 days while an acute post-viral rhinosinusitis persists after 10 days. In the latter case there is often a biphasic symptom pattern with persisting or impairing symptoms by the end of the 10-day period.
ARS is predominantly caused by a number of viruses (rhinovirus, parainfluenza-1 and -2, coronavirus, and influenza viruses), which all induce an increase of proinflammatory cytokines and neutrophils [Citation2]. A similar type of reaction occurs also in a bacterial infection. Thus, an ARS can easily be misdiagnosed as a bacterial infection and is therefore treated with antibiotics, which at this stage of the disease does not improve healing.
ARS is the most common of all infectious diseases and has an enormous socio-economic impact on society besides the individual discomfort with a reduced quality of life [Citation3]. The treatment strategy is to reduce the severity of the symptoms, minimize the duration of the disease, and prevent complications as well as further development into a chronic disease.
During recent years a new treatment modality of ARS has been introduced – phytotherapeutic agents [Citation4, Citation5, Citation6]. BNO 1016 (Bionorica SE, Neumarkt, Germany) is a novel drug based on a dry extract of a fixed combination of five herbal drugs comprising Gentian root (Gentianae radix), Primula flower (Primula flos), Sorrel herb (Rumicis herba), Elder flower (Sambuci flos), and Verbena herb (Verbenae herba) in the ratio 1:3:3:3:3. This drug is a standardized high-dosage product for the treatment of ARS. Pharmacodynamic studies have demonstrated in both in vitro and animal models that BNO 1016 has antimicrobial and antiviral effects including secretolytic and anti-inflammatory activities [Citation6]. A previous phase 2b/3 study has documented positive efficacy and safety of BNO 1016 with a daily dose of 160 mg t.i.d. for 15 days [Citation7]. This was confirmed in a subsequent confirmatory phase 3 trial [Citation8].
For the present evaluation, data from the phase 2b/3 (ARhiSi-1) and the phase 3 (ARhiSi-2) trial were pooled to confirm the observed treatment effect for a bigger patient population. The analysis was based on 589 patients to compare the efficacy of 480 mg of BNO 1016 daily (3 × 160 mg) with placebo in the treatment of ARS.
Material and methods
Patients
For the analysis of the pooled data inclusion criteria of the ARhiSi-2 trial were applied. Adult outpatients of both sexes aged ≥ 18 and ≤ 75 years with a clinical diagnosis of ARS (ICD-10: J01.9), confirmed by ultrasonography of the maxillary sinuses for all patients, were considered for the analysis. ARS was defined as sudden onset of at least three of the main symptoms (rhinorrhea/anterior discharge, postnasal drip, nasal congestion, headache, and facial pain/pressure). At enrolment, the symptoms must have lasted for 3 days or less. All included patients must have presented an investigator-evaluated major symptom score (MSS) of ≥ 8 and ≤ 12 (of maximal 15 score points). In addition, nasal congestion must be present and a mild to moderate facial pain/pressure (score of ≥ 1 and ≤ 2). Facial pain was limited to moderate intensity to confine the enrolment to patients with a non-complicated ARS only.
Patients treated with corticosteroids or antibiotics (locally or systemically) within 4 weeks before the first visit to the investigator (‘inclusion visit’) were excluded. This also comprised subjects using medication for common cold-like symptoms, immunomodulating drugs (within 7 days before inclusion), pregnant or lactating women, and subjects with severe diseases of kidney or liver, severe somatopathic or neurological and/or psychiatric diseases.
Design of the analysis
The analysis was based on two similar prospective randomized, double-blind, placebo-controlled, parallel-group, multicenter studies performed during 2009–2010 in 37 centers (16 specialists in otorhinolaryngology, 21 specialists in internal medicine, and general practitioners) across Germany. At visit 1/day 0 outpatients suffering from ARS were included, randomized, and provided written consent. Treatment was performed orally with either 3 × 160 mg BNO 1016 or 3 × placebo for 15 days. Treatment allocation in both studies was according to the ratio 1: 1. Neither the subjects nor the investigator knew the identity of the medication, allowing treatment in a double-blind manner.
Patients documented their symptoms daily during the treatment phase. At every visit to the study center (days 3, 7, 10, and 14; visits 2, 3, 4, and 5, respectively) the investigator evaluated the five symptoms of MSS and response to treatment. Additionally during the visits the patients completed the health-related quality of life questionnaire (Sino-Nasal Outcome Test-20 German Adapted Version, SNOT-20 GAV) [Citation9].
Ultrasonography of paranasal sinuses was performed in both studies at the first visit to confirm the diagnosis. Ultrasonography at the end of the treatment (visit 5) was performed in the second study only.
The studies were approved by the German authority and received a favorable opinion from the ethics committee and were in accordance with the Declaration of Helsinki and the ICH Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95).
Efficacy measurements
Efficacy end points
All efficacy analyses were based on the pooled data of the ARhiSi-1 and ARhiSi-2 trials, see the section on Statistical analysis.
The primary end point for the pooled analysis set was the mean MSS at visit 5 (day 14, full analysis set, FAS, and per-protocol population, PP). Additionally the single symptoms of the MSS at visit 5 (day 14) were analyzed (FAS and PP). Furthermore, the SNOT-20 symptom score as total sum at visit 3 (day 7) and at visit 5 (day 14) were analyzed for FAS and PP. Additionally the responders classified by the investigator on a 4-point rating scale at visit 2 (day 3), visit 3 (day 7), visit 4 (day 10), and visit 5 (day 14) were analyzed (FAS and PP).
Assessment of symptom severity
Investigators rated the severity of each of the five symptoms of the MSS at each visit using a four-point rating scale of increasing severity (0 = none/not present, 1 = mild, 2 = moderate, 3 = severe). Pain parameters and postnasal drip were rated according to the patients’ descriptions.
Major symptom score (MSS)
The MSS combines the five most relevant symptoms of rhinosinusitis based on expert clinician recommendations (rhinorrhea/anterior discharge, postnasal drip, nasal congestion, headache, and facial pain/pressure) and has been employed as primary efficacy criterion in several clinical trials [Citation10, Citation11, Citation12]. The MSS was calculated as the sum of the five single symptom assessments.
Assessment of responders and non-responders to treatment
Overall response to treatment was assessed by the investigator at each visit using a four-point rating scale (0 = symptoms healed/cured; 1 = symptoms improved compared with visit 1; 2 = symptoms unchanged compared to visit 1, 3 = symptoms deteriorated compared with visit 1). Patients who were cured or reported improved symptoms (0 and 1 rated score) were classified as responders, whereas patients with unchanged or deteriorated symptoms (2 and 3 rated score) were classified as non-responders.
Statistical analyses
General definition of population
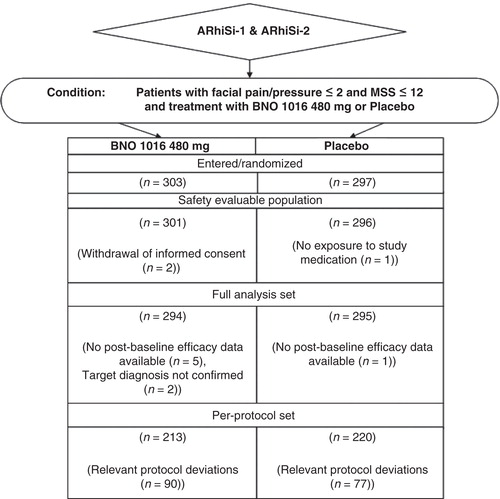
Only the treatment arms ‘placebo’ and ‘BNO 1016 480 mg’ of the ARhiSi-1 trial were included in the combined analysis: as inclusion and exclusion criteria of ARhiSi-2 were applied for the combined analysis, patients in the ARhiSi-1 study with facial pain/pressure > 2 or MSS > 12 or MSS < 8 at inclusion were excluded from the analysis sets, as this was a violation of an inclusion criterion in ARhiSi-2. The allocation of patients to the different analysis sets was done according the ‘Blinded Review Meetings’ of the respective ARhiSi-1 and ARhiSi-2 trials.
Analysis sets and handling of missing data
Efficacy analyses were performed primarily on the FAS, which comprised data for all randomized patients with acute rhinosinusitis who had received at least one dose of the study medication and at least one evaluation of efficacy. The PP comprised all randomized patients from the FAS excluding those with major protocol violations. For evaluation of safety no pooling of data was performed. Instead the safety evaluable population (SEP) of each trial was used to describe the safety results.
Here, the baseline data were used for imputation of missing values in case of early drop-out patients due to insufficient efficacy for the FAS population. Generally, in case of missing values resulting from the situation that patients recovered from the disease and discontinued the study, the last documented value of each efficacy end point was used for the imputation of the respective ‘missing’ values concerning all following visits that were not performed (last observation carried forward, LOCF).
In case the patient withdrew from the study for reasons associated with the study medication such as an unexpected worsening of disease/condition during study or lack of efficacy, the worst category was used for the global assessment of efficacy by the investigator.
In case of missing item values for the calculation of the SNOT-20 symptom score the worst item category was used if not more than two item values were missing, otherwise it was regarded as missing.
Statistical methods
All data were analyzed using the SAS version 9 statistical software. As most of the statistical tests were performed one-sided, p values ≤ 0.025 indicate statistical significance.
If not indicated otherwise, deviations are indicated as standard error of the mean (SEM).
All efficacy analyses were performed with the pooled data set (see Statistical analyses). In the analysis of the pooled data the primary end point of ARhiSi-2 was evaluated using the analysis of covariance (ANCOVA).
A difference of one score point in MSS between the treatment groups was prospectively (in analogy to ARhiSi-2) judged to be clinically relevant.
All secondary end points were analyzed exploratively. Categorical variables were tested by the chi-squared test. Continuous data were analyzed by ANCOVA similarly to the primary end point or by the Cochran–Mantel–Haenszel test. Baseline values were compared between treatment groups and tested by the Mann–Whitney–Wilcoxon test (continuous variables) or chi-squared test (categorical test).
Results
Patient allocation
The patient allocation is shown in . A total of 303 patients were allocated to the BNO 1016 treatment group whereas 297 were allocated to the placebo group in the pooled data set. Also, 589 of these 600 randomized patients were considered for the FAS, 294 in the BNO 1016 group (97%) and 295 in the placebo group (98.2%).
The criteria for the PP were fulfilled by 213 (70.3%) patients in the BNO 1016 group and 220 (74.1%) patients in the placebo group.
Efficacy results
Study duration and treatment compliance
The median participation in the study was 29 days for both groups, with a range of 3–57 days with BNO 1016 and 3–86 days with placebo. Compliance with treatment, according to tablet count, was 99.7% in the BNO 1016 group and 100.2% in the placebo group.
Major symptom score (MSS)
The baseline (mean MSS) at enrolment was without statistical differences between the two treatment groups (). MSS improved gradually in both groups during the 15-day treatment period by a mean of 10.02 ± 1.61 to 2.47 ± 2.55 for BNO 1016 and 9.87 ± 1.52 to 3.63 ± 3.63 with placebo (, ). The difference between treatment groups at visit 5 was statistically significant in favor of the BNO 1016 product (FAS, p < 0.0001).
Table I. Major symptom score (MSS) from visit 1 (day 1) to visit 5 (day 14): FAS and PP.
Figure 2. ARhiSi combined analysis of ARhiSi-1 and ARhiSi-2: mean MSSINV ± 1.96*SEM from day 0 to day 14 (FAS, full analysis set; n = 589). MSS, major symptom score; V, visit.
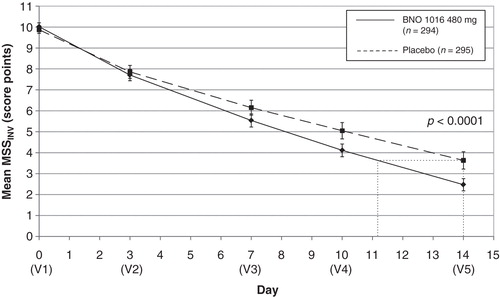
An obvious difference in MSS between the two groups was already evident at visit 4 (day 10), indicating a faster recovery for the BNO 1016 group, showing a difference of 0.94 score points with mean scoring values 4.11 versus 5.05. At visit 5 (day 14) the values were 2.47 ± 2.55 (BNO 1016) and 3.63 ± 3.63 (placebo), respectively – a difference of 1.16 ± 3.14 score points. This translates into an almost 3 day faster recovery with BNO 1016 (day 11 and day 14, respectively). The difference between treatment groups at the end of therapy for the PP analysis set accounted for 1.70 ± 3.13 score points (p < 0.0001). This equates to a 4 day faster recovery with BNO 1016 at the end of therapy (day 10 and day 14, respectively).
Single symptoms of MSS at visit 5 (day 14) are shown in . In the FAS each individual symptom shows a statistical significance in favor of BNO 1016 (p < 0.0001).
Table II. Single symptoms of major symptom score (MSS) at visit 5 (day 14): FAS and PP.
Response to treatment
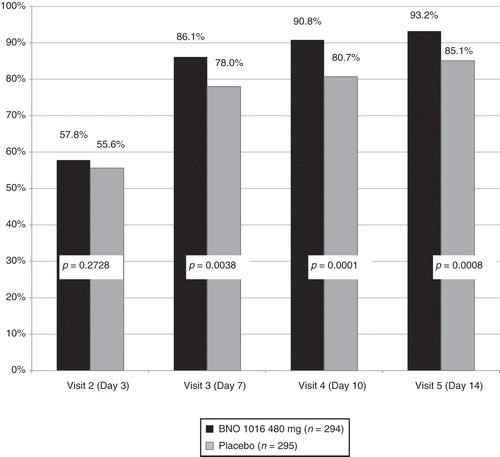
Absolute and relative treatment results are summarized in . A statistically significant improvement (for both FAS and PP) in favor of BNO 1016 was seen already on day 7 (visit 3). shows combined analysis of ARhiSi-1 and ARhiSi-2: response (healed or improved) to treatment from visit 2 (day 3) to visit 5 (day 14).
Table III. Response (healed or improved) to treatment from visit 2 (day 3) to visit 5 (day 14): FAS and PP.
Quality of life measures
SNOT-20 total score results are shown in . A highly significant difference in favor of BNO 1016 was evident at the end of treatment (p = 0.0015).
Table IV. SNOT-20: total score from visit 3 (day 7) to visit 5 (day 14) (FAS).
Safety results
Serious adverse events (SAEs) were not reported in either the ARhiSi-1 or the ARhiSi-2 trial. In ARhiSi-1 a total of 42 adverse events (AEs) occurred in 33 patients of the SEP (safety evaluable population, n = 450): 33 AEs in 26/300 patients (8.7%) under treatment with BNO 1016 (2 treatment groups with BNO 1016 in a daily dose of 240 mg or 480 mg) and 9 AEs in 7/150 patients (4.7%) under treatment with placebo. In ARhiSi-2 a total of 53 AEs occurred in 46 patients of the SEP (n = 385): 21 AEs in 19/194 patients (9.8%) under treatment with BNO 1016 480 mg and 32 AEs in 27/191 patients (14.1%) under treatment with placebo. The majority of AEs reported under BNO 1016 were of mild to moderate intensity.
Discussion
This analysis shows that oral administration of 480 mg (3 × 160 mg) of the herbal drug BNO 1016 is effective in the treatment of acute viral rhinosinusitis. This new treatment concept – in what is usually a self-limiting disease – gives faster recovery from symptoms, gives a higher rate of complete recovery as compared with placebo, and improves quality of life in patients. Results for FAS were exceeded by the PP analysis set throughout the study.
Until now there has been limited knowledge of the beneficial effects of herbal medicines in the treatment of ARS. Pharmacodynamic studies on BNO 1011 have demonstrated both antiviral activity [Citation13] and stimulation of beat frequency in human respiratory epithelia in vitro by activation of forskolin-stimulated chloride secretion [Citation14]. BNO 1011 is a dry extract without excipients whereas for BNO 1016 excipients are added for technical reasons, for example, to enable the extract to be pressed into tablets.
The clinical evidence for BNO 101 (Sinupret) (with the same constituents as BNO 1016 but in a lower dose) in the treatment of sinusitis has recently been reviewed, showing a favorable effect [Citation15]. This herbal medicinal product exerts significant oral anti-inflammatory effects by a reduction of cyclooxygenase (COX)-2 expression and prostaglandin (PG)E(2) formation [Citation16]. Thus, the mode of action rationalizes its therapeutic use in the treatment of sinusitis and other viral/microbial nasal infections that are associated with inflammation. Experimental studies on Sinupret show a reduction of bacterial growth after only 4 days [Citation6]. Other herbals – although chemically less defined than Sinupret – seem similarly to have some anti-inflammatory effect, for example, in chronic sinusitis [Citation17].
Although intranasal corticosteroids (alone or in combination with antibiotics) are generally recommended for ARS there seems to be a need for further documentation of their clinical use [Citation18]. A recent meta-analysis of the efficacy of the intranasal corticosteroid mometasone for the treatment of ARS showed a ?????? (number needed to treat) of 11 for improving or resolving symptoms [Citation19]. For BNO 1016 an number needed to treat of 10 was calculated for the pooled data set. Thus, herbal drug BNO 1016 seems at least equally beneficial – or even more beneficial – than some locally applied corticosteroids in the treatment of ARS.
The use of MSS in our analysis is in accordance with the EPOS 2012 recommendations combining the five most relevant symptoms of ARS and is often used as a standard for primary efficacy criteria in clinical studies [Citation1]. Furthermore, ultrasonography confirmed the treatment effect [Citation8]. Our analysis together with the ARhiSi-2 study on the clinical efficacy of BNO 1016 [Citation8] are the first well-controlled investigations (to assess the effect of a fixed dose combination of herbs) fulfilling all the current quality standards to be prospective, double blind, randomized, and placebo-controlled.
There is no gold standard treatment for ARS. Antibiotics are not indicated in the treatment of uncomplicated ARS. The great number of different viruses causing ARS has made it difficult to manufacture an effective vaccine. The socio-economic costs of this disease are tremendously high, requiring health-care resources, and lead to loss of productivity [Citation3]. On average, every adult becomes affected by upper respiratory disease two to five times per year. In this perspective every treatment modality shortening the length of the disease and improving the quality of life of individual patients is beneficial both for society and for the individual.
As no SAEs occurred with BNO 1016 and as the frequency and intensity of AEs are similar for BNO 1016 and placebo, it can be concluded that BNO 1016 has a similar safety profile to placebo. Accordingly, BNO 1016 has a favorable benefit/risk ratio.
In conclusion, this analysis confirms the results of the confirmatory phase 3 trial with BNO 1016. The analysis has demonstrated that daily intake of 480 mg BNO 1016 for 2 weeks is a safe and effective treatment option in uncomplicated ARS. The drug provides a faster and clinically relevant remission of symptoms and improves quality of life as compared with placebo.
Acknowledgments
The authors thank Bionorica SE, Neumarkt, Germany, for financial support and the investigators at the study centers for recruiting and monitoring the patients in compliance with the study protocol and Good Clinical Practice. The authors are also grateful to the patients for their participation and compliance with the study protocol. The project and data management was conducted by the independent contract research organization Pharmalog Institute for Clinical Research, Munich, Germany.
Declaration of interest: M. Mondigler is employed by Bionorica SE. P. Stierna and C. Bachert act as scientific consultants for Bionorica SE. H. Stammer is Managing Director of the CRO in charge of operations of the clinical trials. R. Jund was coordinating investigator of the multicenter clinical trials according to §40 German Drug Law.
References
- WJFokkens, VJLund, JMullol, CBachert, IAlobid, FBaroody, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. Rhinology 2012:1–229.
- AMasood, IMaoumoulidis, JPanesar. Acute rhinosinusitis in adults: an update on current management. Postgrad Med 2007;83:402–8.
- JHellgren, ACervin. Nordling, S, Bergman A, Cardell L-O. Allergic rhinitis and the common cold – high costs to society. Allergy 2010;65:776–83.
- RGuo, PHCanter, EErnst. Herbal medicines for the treatment of rhinosinusitis: a systematic review. Otolaryngol Head Neck Surg 2006;135:496–506.
- JReden, DEl-Hifnawi, TZahnert, THummel. The effect of herbal combination of primrose, gentian root, vervain, elder flowers, and sorrel on olfactory function in patients with a sinonasal olfactory dysfunction. Rhinology 2011;49:342–6.
- CIsmail. [Pharmacology of Sinupret. Recent results on the rational for the Sinupret compound.]. HNO 2005;53:S38–42; in German.
- CBachert, MMondigler, HSteindl, HStammer, PStierna, HEskötter, et al. Multicentre, randomised, double-blind, placebo-controlled, parallel-group dose-finding study of herbal medicine (dry extract) BNO 1016 in acute rhinosinusitis (ARhiSi-1). 84th Annual Meeting of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery 2013.
- RJund, MMondigler, HSteindl, HStammer, PStierna, CBachert. Clinical efficacy of a dry extract of five herbal drugs in acute viral rhinosinusitis. Rhinology 2012;50:417–26.
- IBaumann, GBlumenstock, HDe Maddalena, JFPiccirillo, PKPlinkert. Quality of life in patients with chronic sinusitis: validation of the Sino-Nasal Outcome Test-20 German Applied Version. HNO 2007;55:42–7.
- CBachert, ASchapowal, PFunk, MKieser. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: a randomized, double-blind, placebo-controlled trial. Rhinology 2009;47:51–8.
- EOMeltzer, CBachert, HStaudinger. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol 2005;116:1289–95.
- DARevicki, MKMargolis, CLThompson, EOMeltzer, DWSandor, JWShaw. Major symptom score utility index for patients with acute rhinosinusitis. Am J Rhinol Allergy 2011;25:99–106.
- BGlatthar- Saalmüller, UTauchhaus, SRode, JHaunschild, ASaalmüller. Antiviral activity of two preparations of the herbal medicinal product Sinupret™ against viruses causing respiratory infections. Phytomedicine 2011;19:1–7.
- JLKreindler, BChen, YKreitman, JKofonow, KMAdams, NACohen, et al. 1011 stimulates chloride transport and ciliary beat frequency in human respiratory epithelial cultures. Am J Rhinol Allergy 2012;26:439–43.
- JMeltzer, RSaller, ASchapowal, RBrignoli, BNO-Systematic review of clinical data with101 (Sinupret) in the treatment of sinusitis. Forsch Komplementmed 2006;13:78–87.
- ARossi, FDehm, CKiesselbach, JHaunschild, LSautebin, OWerz. The novel Sinupret dry extracts exhibit anti-inflammatory effectiveness in vivo. Fitoterapia 2012;83:715–20.
- JSLee, ISKim, JHKim, DHKim, CYYun. Suppressive effects of Houttuynia cordata Thunb (Saururaceae) extract on Th2 immune response. J Ethnopharmacol 2008;117:34–40.
- EOMeltzer, CBachert, HStaudinger. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol 2005;116:1289–95.
- AZalmanovici Trestioreanu, JYaphe. Intranasal steroids for acute sinusitis (review). The Cochrane Library 2010, Issue 7.