Abstract
Today, the increase of the blood concentration of cardiac troponins is designated as surrogate for cardiac necrosis and myocardial infarction, when an appropriate clinical and/or instrumental situation is present. As cardiac troponins reflect myocyte death, biomarkers of reversible myocardial damage in the absence of necrosis are, however, still needed to detect the presence of damage even before the irreversible injury is induced and identify “vulnerable” patients before major events occur, permitting adequate treatment. Markers of plaque destabilization and/or markers of myocardial ischemia could be enormously valuable in the emergency department setting if shown to contribute additional independent diagnostic information. However, a new cardiac biomarker is of definitive clinical value only if adequate assays for its measurement are available, its predictive value is defined in the right clinical context, optimal cut-off and release kinetics are known, demonstration of the marker incremental value is clear, there is consistency of marker performance across different settings, and, more importantly, there are data on the effect on patient management and outcome and on cost-effectiveness. Despite the emergence of multiple candidates, sufficient evidence for any of these has yet been demonstrated to recommend their adoption into clinical practice.
Introduction
Biomarkers have provided important information for the clinical assessment of patients with suspected acute cardiac disease since the early 1950s and their utilization has evolved substantially over the past 30–40 years. Today, the measured increase of the blood concentration of cardiac troponins (I or T) is designated as surrogate for necrosis and myocardial infarction (MI) when an appropriate clinical and/or instrumental situation is present [Citation1]. The 2007 released document by the joint European Society of Cardiology (ESC)/American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force on the “universal” definition of MI has strengthened the role of cardiac troponins as the main criterion for MI diagnosis [Citation2]. The advent of cardiac troponins, providing high sensitivity for small injury and virtually absolute specificity for irreversible myocardial damage, now assigns a key role in detection of this disease to laboratories. Contrary to the traditional WHO definition (requiring presence of two out of three criteria, including biochemistry), an acute MI cannot be diagnosed today without the biochemical evidence of myocardial necrosis; an accurate biochemical standard has finally been established and this is represented by cardiac troponins in virtue of their exquisite biological attributes.
As cardiac troponins reflect myocyte death, biomarkers of reversible myocardial damage in the absence of necrosis are additionally needed to detect the presence of damage even before the irreversible injury is induced and identify “vulnerable” patients before major event occur, permitting adequate treatment. Indeed, an undetectable troponin is not synonymous to a lack of damage. In studies investigating the outcome of patients admitted to the emergency department (ED) with chest pain and negative troponin measurements, cardiac ischemia was detected in about one-third of subjects and the frequency of major cardiac events at six months was a non-negligible 4.8% [Citation3].
Theoretically, a multi-marker strategy, employing a patho-biologically diverse set of biomarkers, could significantly help in the assessment of patients with cardiac disease. Markers of plaque destabilization and/or markers of myocardial ischemia could be added to the existing markers of cardiac necrosis in this paradigm if shown to contribute additional independent information [Citation4]. Indeed, both industry and academia are relentlessly searching for new serum biomarkers that are released at the very beginning of the myocardial acute event.
Biomarkers for coronary plaque instability and rupture are also continuously arising (). Before considering clinical implementation, each of these biomarker candidates must be evaluated critically with respect to key analytical and clinical characteristics.
Table I. Proposed biomarkers of cardiac ischemia and coronary plaque instability and rupture.
Evaluation of biomarker performance
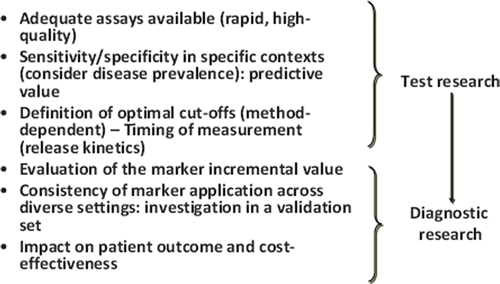
Many newly discovered biomarkers have demonstrated experimental evidence supporting their pathophysiologic role and preliminary suitability for clinical application. However, a new cardiac biomarker will be of definitive clinical value only if adequate assays for its measurement are available, its predictive value is defined in specific clinical contexts, optimal cut-off(s), which are frequently assay-dependent, and release kinetics are known. Also, demonstration of the marker incremental value should be studied, there should be consistency of marker performance across different settings, and data on the effect on patient management and outcome and on cost-effectiveness should be available ().
Quality of biomarker measurement
Cardiac biomarkers on which may be critical for clinical decisions must be assayed with highly reliable procedures. Adequate studies are needed before new methods can be implemented in the laboratory routine, and only well-documented assays should be considered for clinical use. In this respect, it is vital that all information on the assays is given, which is not always the case. Investigators are often quite quick in releasing manuscripts without collecting and including these thorough data. For example, only years after the enthusiastic studies promoting the measurement of soluble CD40 ligand concentration as biomarker for guiding antiplatelet treatment in patients with acute coronary syndrome (ACS) [Citation5,Citation6], other investigators have revealed important confounding influences of sample type and handling on its measured concentration that may invalidate previous clinical studies [Citation7,Citation8].
Today, the technology to address many analytic problems is at hand, but commitment on the part of the laboratories and their clinical customers is essential. The quality loop is not closed until the in vitro diagnostic assay is adequately verified to meet the required analytical specifications. Therefore, it is critical that, as new biomarkers are proposed, quality specifications for their measurement are developed [Citation9]. It is essential to determine the performance characteristics of proposed measurement methods in order to demonstrate that user needs are met. The responsibility of defining and implementing these issues must be a shared responsibility among laboratorians, clinicians, industry, and regulatory agencies on an international front. To date, two sets of quality specifications have been published in the field of cardiac markers, one for troponin assays and one for B-type natriuretic peptide (BNP) assays [Citation10,Citation11]. Both address analytical factors, such as calibrator characterization, antibody specificity, assay sensitivity and precision, and interferents, as well as pre-analytical factors, such as sample type and stability. Concerns that have been addressed for cardiac troponins and cardiac natriuretic peptides will need to be addressed with the same scrutiny as for all new proposed cardiac biomarkers [Citation12]. To avoid misinterpretation of a marker result, performance characteristics of the assays should be adequately described.
Biomarker qualification
Biomarkers that are indicators of atherosclerosis trait (risk) may not be useful for the detection of acute events (diagnosis). Therefore, cardiac biomarkers should be classified as diagnostic biomarkers (recognizing preclinical or clinical disease, such as plaque rupture or acute cardiac ischemia) or prognostic biomarkers (predicting risk of future cardiac events). Approaches for defining abnormal biomarker concentrations change accordingly [Citation13]. In addition to the establishment of reference intervals, which are a simple descriptive biological information without any direct clinical implication, use (and definition) of decision limits or thresholds for risk entirely depends on the biomarker value and its proposed clinical application [Citation13]. Particularly, diagnostic biomarkers, such as markers detecting plaque rupture and/or myocardial ischemia, need definition of the optimal decision limit, which represents the best concentration able to separate patients with or without the disease state to be identified.
The diagnostic appropriateness of a biomarker is usually first evaluated in terms of its sensitivity and specificity, often with the use of receiver operating characteristic (ROC) curves. However, an appropriate biomarker evaluation requires use of the Bayesian approach, integrating the pretest disease probability (disease prevalence) with biomarker accuracy (expressed in terms of sensitivity/specificity) to estimate the marker predictive value (posttest probability of disease). For a marker of acute cardiac ischemia the appropriate clinical context for its evaluation is the ED population with chest pain in which the ACS is suspected and the prevalence of unstable angina (the ischemia condition to be diagnosed) is ∼30%. Applying the test in the same setting of patients using, however, a lower disease prevalence, e.g. that of the acute MI (<10%), spuriously increases the negative predictive value of the biomarker. On the other hand, if in the studied group of patients the number of diseased individuals is artificially high, the resulting positive predictive value of the biomarker will be falsely improved.
The diagnostic window for an injury marker is the interval of time after an episode of injury during which plasma concentration of the marker is increased. Biomarkers that rapidly enter the circulation (i.e. early indicators) tend to have a diagnostic window that begin soon after the onset of the injury, whereas that of those slowly released or cleared (i.e. late indicators) generally begins later and last longer. The marker release kinetics and clearance define the optimal time for sample collection. The biomarker's diagnostic window particularly important if the time to presentation of patients in different studies varies and/or only one blood sample is collected for analysis [Citation14].
Novel biomarkers in ACS: from new to innovative
Even when a biomarker increase is associated with high positive and/or negative predictive value, its incremental value when compared with the presently available diagnostic tools should be demonstrated. The choice of comparative methods is crucial to avoid a biased result. Choosing a low performing method e.g. with a low sensitivity and/or specificity may unintentionally favour the new marker [Citation14].
It is also highly desirable that new biomarkers, initially evaluated in a training set, are fully investigated and their utility confirmed in external validation sets to assess their applicability across diverse places and in different series of individuals. After discovery of a prospective new biomarker, authors may tend to over optimistically estimate the marker's accuracy, while others can be less successful at confirming so. A typical case is represented by glycogen phosphorylase isoenzyme BB originally proposed as very early sensitive biomarker for ischemic myocardial damage [Citation15].
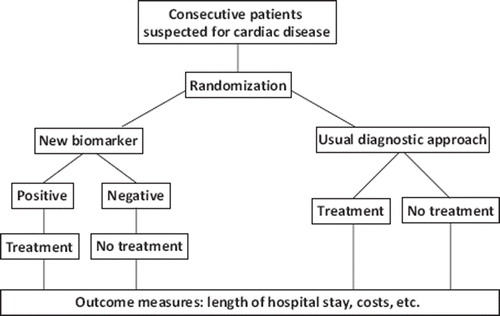
In the end, biomarkers should be evaluated with respect to their ability to improve disease management, patient outcome and cost-effectiveness in randomized clinical trials (). One example of this was the introduction of cardiac troponins for the diagnosis and treatment of patients with ACS. When compared with the traditional diagnostic approach (at that time elevated creatine kinase MB), troponin was markedly effective in altering patient management by enabling early discharge of patients, resulting in significant cost savings and increasing bed availability [Citation16].
Narrative reviews of newly proposed biomarkers for detection of atherosclerotic plaque instability and rupture and cardiac ischemia in patients with suspected ACS have been published in recent years [Citation12,Citation17]. Two examples taken from markers listed in may illustrate the challenges and issues related to the definitive clinical implementation of these biomarkers. The two selected markers, one for each group, were found in a Medline search (performed on December 1st, 2009 using the keywords “cardiac ischemia” and “plaque rupture”, in addition to the marker name) giving the greatest number of hits (93 for ischemia-modified albumin (IMA) and 21 for myeloperoxidase (MPO)). Both have US Food and Drug Administration (FDA) approval and in cardiologist journals are considered to have reached the level of clinical routine.
Ischemia-modified albumin (IMA), a marker of myocardial ischemia
The detection of ischemia prior to MI is a challenging concept. Jesse [Citation18] explored the rationale for diagnosing myocardial ischemia in advance (or even in the absence) of the occurrence of irreversible damage. As the explicit goal is to maintain microcirculatory flow to prevent even minor infarctions, only a marker that precedes necrosis and permits the prevention of its consequences can meet clinical needs. The determination of the diagnostic utility of an ischemia biomarker is, however, complicated by the lack of a suitable diagnostic standard for this condition from which to base a comparison; some surrogate approaches have been proposed, but each one has important shortcomings ().
Table II. Surrogate approaches employed in the evaluation of biomarkers for reversible myocardial ischemia.
Human albumin in blood presents a metal binding site at the N-terminus, which is particularly susceptible to degradation. Bar-Or et al. [Citation19] postulated that this N-terminus can be damaged during an ischemic event, resulting in a reduced in vivo metal binding capacity by IMA. Based on these observations, an assay was developed in which cobalt-albumin binding was proposed as a measurement of IMA. This assay was later marketed and granted regulatory (FDA) approval for clinical use in 2003 and is now available on a variety of clinical chemistry platforms [Citation20,Citation21]. The albumin-cobalt binding test is a colorimetric assay, and therefore an indirect measurement of IMA concentration expressed in arbitrary units (kU/L). The concentration is obtained by interpolating sample absorbance from a calibration curve made by a chelating agent (EDTA). Consequently, transferability and traceability of the test results are limited because of the absence of calibrators and reference materials based on the mass concentration of IMA. An immunoassay, which could improve the analytical specificity, has not yet been developed. Specific preanalytical requirements need to be followed, including performing measurements within 2 hours from blood withdrawal and avoiding sample dilutions [Citation20,Citation21]. Samples stored at 220°C, but not at 270°C [Citation22], result in a significant increase of IMA values. Heparinized plasma was found to be an unsuitable sample [Citation20] and increased endogenous lactate concentrations appear to reduce IMA concentrations.
Qualification of IMA: proof of concept studies
The precise mechanism of IMA generation is still unknown. It has been suggested that IMA may be a marker of oxidative stress as conditions associated with raised IMA may be associated with other markers of oxidative stress [Citation23]. Results from in vitro studies suggest that the generation of reactive oxygen species (ROS) such as hydroxyl radicals can modify the N-terminal region of albumin to origin IMA [Citation24]. Induction of oxidative stress with increased oxygen free-radical production, however, is not necessarily associated with myocardial ischemia.
Significant changes in albumin cobalt binding have been documented to occur minutes after balloon angioplasty considered to represent an in vivo model to induce myocardial ischemia (“supply” ischemia by a peri-procedural reduction of coronary blood flow) [Citation25]. This has been considered to prove the merit of IMA for the diagnosis of myocardial ischemia. However, protocols were not designed to distinguish reversible ischemia from the presence of small areas of myocardial necrosis. Furthermore, because the balloon deflation gives rise to unavoidable reperfusion injury, free radicals generated by oxidative stress, and not ischemia itself, may be of major importance in increasing IMA in this condition. Finally, these studies were carried out without measuring the albumin concentrations simultaneously which may significantly affect IMA results after percutaneous coronary intervention, transforming significant differences in IMA values to insignificant changes [Citation26]. Van der Zee et al. [Citation27] performed IMA measurements in subjects who were undergoing symptom-limited exercise perfusion scintigraphy (used for detection of myocardial ischemia) and showed that albumin concentration was the only independent predictor of IMA concentrations. Importantly, IMA concentrations were significantly lower at maximum exercise than at baseline, without any difference in patients with and without ischemia. These results were confirmed by Sbarouni et al. [Citation28], who also demonstrated post-exercise decrease in IMA concentrations, and this reduction was similar in both patients with positive and negative exercise stress-test responses, suggesting that the observed changes in IMA concentrations may not reflect myocardial ischemia. Significant caution is advised in the interpretation of IMA data using different surrogate approaches for myocardial ischemia. “Demand” ischemia during a stress test may be different in either physical or pharmacological stress and may not be as severe as the “supply” ischemia that occurs during angioplasty or in ACS setting [Citation29].
Initial clinical studies were typically performed with various study designs in small number of subjects. In these studies there were significant problems with sample stability and the employed assay method was still in its pre-release version, which has later been reformulated. The first clinical study evaluating IMA diagnostic performance using correct enrolment criteria (consecutive patients admitted to an ED with manifestations suggestive of acute myocardial ischemia) showed a poor diagnostic accuracy of IMA in discriminating between ischemic and non-ischemic patients (area under the ROC curve (AUC), 0.63) [Citation30]. However, when the test was applied in a similar training set, but only in subjects with non-diagnostic electrocardiogram (ECG) and negative troponin in whom the highest benefit of an ischemia test is expected, the discrimination ability of IMA seemed to improve (AUC, 0.78) [Citation31]. Major limitations in the latter study (e.g. use of a high troponin cutpoint and an unusually high prevalence of ACS) did not, however, permit to definitively qualify the marker. Importantly, since more than 80% of patients who present to the ED with features of possible ischemic cardiac chest pain had an IMA concentration above the manufacturer's suggested cut-off value (>85 kU/L), this markedly lowered specificity rates with frequent false positives that may limit the practical value of the test [Citation32]. Indeed, increased IMA values have been observed in patients with cancer, infections, end-stage renal disease, liver disease, stroke, peripheral vascular disease, as well as strenuous physical exercise [Citation23]. More recently, some authors have tried to improve the IMA specificity by increasing the decision limit [Citation33]. When an IMA concentration of about 117 kU/L was considered positive, in a group of 76 sequential patients who presented to the ED with chest pain and negative troponin results (ischemic ACS prevalence, 19.7%), the negative predictive value of the test was 85.6% and the positive predictive value was 37% [Citation33]. Release kinetics of IMA and its diagnostic time window have never been described in ongoing ischemic ACS without evidence of irreversible myocardial damage.
Myeloperoxidase (MPO) a proposed markers of coronary plaque instability and rupture
A growing understanding of the importance of atherosclerotic plaque rupture in the pathogenesis of coronary events has led to the identification of an expanding array of markers of plaque instability. Indeed, it now appears that plaque rupture, rather than the degree of stenosis, precipitates coronary thrombosis resulting in cardiac ischemic events.
MPO, a member of the heme peroxidase superfamily, is a tetrameric hemoprotein consisting of a pair of heavy (57 kDa) and light (15 kDa) chains. It is stored in azurophilic granules of polymorphonuclear neutrophils and monocytes-macrophages and, when released in a state of inflammation, catalyzes the conversion of chloride anion and hydrogen peroxide to hypochlorite, a metal ion-independent chlorinating oxidant, which possesses potent microbicidal activity, thus having a role in host defense against pathogens. MPO is released into the extracellular fluid and general circulation during inflammatory conditions. MPO-dependent halides, tyrosyl radicals, and ROS may generate proatherogenic oxidized LDL and thereby promote subsequent foam cell formation. This combination of detrimental effects has culminated in the concept that MPO may be an active mediator in atherosclerotic cardiovascular disease [Citation34]. MPO could be also involved in the development of endothelial dysfunction, because MPO uses the atheroprotective endothelial-derived nitric oxide as a substrate thus reducing its bioavailability.
Qualification of MPO: Proof of concept studies
MPO may have a causative role in coronary plaque destabilization through its ability to activate latent metalloproteinases (MMPs). Infiltrating macrophages and neutrophils participate in the transformation of stable coronary artery plaques to unstable lesions with a thin fibrous cap through secretion of MMPs and MPO, which degrade the collagen layer that protects atheromas from erosion or abrupt rupture [Citation35]. As a result, plaques that have been highly infiltrated with macrophages have a thin fibrous cap and are vulnerable to erosion or rupture, precipitating late-stage atherosclerosis into acute cardiac events.
There have been a few clinical studies examining the diagnostic role of MPO in ED patients with ACS symptoms but negative troponin. The first report assessing MPO diagnostic performance was published by Brennan et al. in 2003 [Citation36]. Although the AUC was lacking, the ROC curve for the prediction of troponin negative ACS clearly showed a poor diagnostic ability of MPO. The use of a too high cut-off for troponin positivity (troponin T >0.10 μg/L) and an unusually high prevalence of ACS (>50%) further complicated interpretation of data. The inability of MPO in distinguishing between unstable angina and non-ACS patients was confirmed in two cross-sectional studies, in which, paradoxically, the latter group displayed higher MPO concentrations [Citation37,Citation38]. Indeed, an increased MPO concentration is not likely to be specific to cardiac diseases, as activation of neutrophils and macrophages can occur in any infectious, inflammatory, or infiltrative disease process. In contrast, a prospective trial, analyzing the behaviour of various markers in the tracking of chest pain, found that MPO had the earliest elevation and, in patients with presentation between 0 and 3 h after symptom onset, demonstrated an AUC of 0.77 when unstable angina was compared with noncardiac pain patients [Citation39]. Available studies indicate, however, that measurement of MPO concentrations in patients presenting with acute chest pain provides poor diagnostically relevant information.
Quality of MPO measurement
MPO activity concentration has been measured in blood and tissues by assays using hydrogen peroxide and o-dianisidine dihydrochloride as substrates. More recently, mass assays based on sandwich ELISA methods have been developed and made commercially available. One of these assays has been approved by the FDA in 2005 for use in conjunction with clinical history, ECG and other cardiac biomarkers to evaluate patients presenting with chest pain at risk of major adverse cardiac events. This assay has been licensed to three other companies and made suitable for automatic platforms or point-of-care instruments. It should be noted that different assays using different MPO antigens for calibration may generate different results. An additional issue that may hamper evaluation of MPO related clinical studies is the type of specimen used. Spuriously higher MPO concentrations are found in serum owing to the leakage of enzyme from leukocytes during coagulation. MPO is also continuously released from white blood cells in the heparinized blood with a spurious increase in MPO concentrations when left standing at room temperature. Therefore samples collected in EDTA must be preferred [Citation40]. Unfortunately, the majority of the above quoted clinical reports do not include information on the sample type used [Citation36,Citation37,Citation39]. Samples from individuals positive for anti-MPO antineutrophil cytoplasmic autoantibodies (ANCAs) can show spuriously decreased MPO concentrations [Citation41].
Conclusions
Despite the emergence of multiple candidate biomarkers for plaque vulnerability and myocardial ischemic damage, none of these has sufficient evidence to recommend widespread adoption into clinical practice. New markers of ACS may be enormously valuable in the ED setting, but crossing the boundary from research to the clinical application is quite challenging. All in all, we require a better validation before they are ready for prime time use.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Panteghini M. The basics of cardiac marker interpretation. Biochim Clin 2007;31:9–12.
- Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation 2007;116:2634–53.
- Sanchis J, Bodí V, Llácer A, Núñez J, Consuegra L, Bosch MJ, Bertomeu V, Ruiz V, Chorro FJ. Risk stratification of patients with acute chest pain and normal troponin concentrations. Heart 2005;91:1013–8.
- Panteghini M. Acute coronary syndrome biomarkers. RIMeL/IJLaM 2005;1:30–6.
- Heeschen C, Dimmeler S, Hamm CW, van der Brand MJ, Boersma E, Zeiher AM, Simoons ML. Soluble CD40 ligand in acute coronary syndrome. N Engl J Med 2003;348: 1104–11.
- Varo N, de Lemos JA, Libby P, Morrow DA, Murphy SA, Nuzzo R, Gibson CM, Cannon CP, Braunwald E, Schönbeck U. Soluble CD40L. Risk prediction after acute coronary syndromes. Circulation 2003;108:1049–52.
- Halldórsdóttir AM, Stoker J, Porche-Sorbet R, Eby CS. Soluble CD40 ligand measurement inaccuracies attributable to specimen type, processing time, and ELISA method. Clin Chem 2005;51:1054–7.
- Ivandic BT, Spanuth E, Haase D, Lestin HG, Katus HA. Increased plasma concentrations of soluble CD40 ligand in acute coronary syndrome depend on in vitro platelet activation. Clin Chem 2007;53:1231–4.
- Panteghini M. The importance of analytical quality specifications for biomarker assays currently used in acute cardiac care. Acute Card Care 2006;8:133–8.
- Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AH. Quality specifications for cardiac troponin assays. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clin Chem Lab Med 2001;39: 174–8.
- Apple FS, Panteghini M, Ravkilde J, Mair J, Wu AHB, Tate J, . Quality specification for B-type natriuretic peptide assays. Clin Chem 2005;51:486–93.
- Apple FS, Wu AHB, Mair J, Ravkilde J, Panteghini M, Tate J, . Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem 2005;51:810–24.
- Vasan RS. Biomarkers of cardiovascular disease. Molecular basis and practical considerations. Circulation 2006;113: 2335–62.
- Jaffe AS, Katus H. Acute coronary syndrome biomarkers. The need for more adequate reporting. Circulation 2004; 110:104–6.
- Mair J. Glycogen phosphorylase isoenzyme BB to diagnose ischaemic myocardial damage. Clin Chim Acta 1998;272: 79–86.
- Zarich S, Bradley K, Seymour J, Ghali W, Traboulsi A, Mayall ID, Bernstein L. Impact of troponin T determinations on hospital resource utilization and costs in the evaluation of patients with suspected myocardial ischemia. Am J Cardiol 2001;88:732–6.
- Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 2007;27:15–26.
- Jesse RL. Rationale for the early clinical application of markers of ischemia in patients with suspected acute coronary syndromes.Cardiac Markers, 2nd. Wu AHB. Humana Press, Totowa, NJ, 2003:245–57.
- Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-A preliminary report. J Emerg Med 2000;19:311–5.
- Gidenne S, Ceppa F, Fontan E, Perrier F, Burnat P. Analytical performance of the albumin cobalt binding (ACB®) test on the Cobas MIRA® Plus analyzer. Clin Chem Lab Med 2004;42:455–61.
- Maguire OC, O'Sullivan J, Ryan J, Cunningham SK. Evaluation of the albumin cobalt binding (ACB®) assay for measurement of ischaemia-modified albumin (IMA®) on the Beckman Coulter LX-20. Ann Clin Biochem 2006;43: 494–9.
- Seamonds B. The effect of sample storage conditions and time to assay performance on the measurement of ischemia modified albumin. Clin Chem 2009;55(suppl):A54.
- Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet 2009;24:333–41.
- Roy D, Quiles J, Gaze DC, Collinson P Kaski JC, Baxter GF. Role of reactive oxygen species on the formation ofr the novel diagnostic marker ischaemia modified albumin. Heart 2006;92:113–4.
- Sinha MK, Gaze DC, Tippins JR, Collinson PO, Kaski JC. Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation 2003; 107:2403–5.
- Demir H, Topkaya BC, Erbay AR, Dogan vM, Yucel D. Ischaemia-modified albumin elevation after percutaneous coronary intervention reflects albumin concentration rather than ischaemia. Ann Clin Biochem 2009;46:327–31.
- Van der Zee PM, Verberne HJ, van Straalen JP, Sanders GTB, Van Eck-Smit BLF, de Winter RJ, Fischer JC. Ischemia-modified albumin measurements in symptom-limited exercise myocardial perfusion scintigraphy reflect serum albumin concentrations but not myocardial ischemia. Clin Chem 2005;51:1744–6.
- Sbarouni E, Georgiadou P, Theodorakis GN, Kremastinos DT. Ischemia-modified albumin in relation to exercise stress testing. J Am Coll Cardiol 2006;48:2482–4.
- Kurz K, Voelker R, Zdunek D, Wergeland R, Hess G, Ivendic B, Katus H, Giannitsis E. Effect of stress-induced reversible ischemia on serum concentrations of ischemia-modified albumin, natriuretic peptides and placental growth factor. Clin Res Cardiol 2007;96:152–9.
- Anbwaruddin S, Jannuzzi Jr JL, Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005;123:140–5.
- Roy D, Quiles J, Aldama G, Sinha M, Avanzas P, Arroyo-Espliguero R, Gaze D, Collinson P, Kaski JC. Ischemia modified albumin for the assessment of patients presenting to the emergency department with acute chest pain but normal or non-diagnostic 12-lead electrocardiograms and negative cardiac troponin T. Int J Cardiol 2004;97:297–301.
- Keating L, Benger JR, Beetham R, Bateman S, Veysey S, Kendall J, Pullinger R. The PRIMA Study: presentation ischaemia-modified albumin in the emergency department. Emerg Med J 2006;23:764–8.
- Talwalkar SS, Bon-Homme M, Miller JJ, Elin RJ. Ischemia modified albumin, a marker of acute ischemic events: a pilot study. Ann Clin Lab Sci 2008;38:132–7.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005;25:1102–11.
- Tavora FR, Ripple M, Li L, Burke AP. Monocytes and neutrophils expressing myeloperoxidase occur in fibrous caps and thrombi in unstable coronary plaques. BMC Cardiovasc Disord 2009;9:27.
- Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349:1595–604.
- Esporcatte R, Cramer Veiga Rey H, Dias Rangel FO, Mourilhe Rocha R, de Mendonça Filho HTF, Rocha Dohmann HF, Albanesi Filho FM. Predictive value of myeloperoxidase to identify high risk patients admitted to the hospital with acute chest pain. Arq Bras Cardiol 2007;89:341–7.
- Eggers KM, Dellborg M, Johnston N, Oldgren J, Swahn E, Venge P, Lindahl B. Myeloperoxidase is not useful for the early assessment of patients with chest pain. Clin Biochem 2010;43:240–5.
- Blankenberg S, Schnabel R, Lowenstein CJ, Caidahl K, Muenzel TF, Lubos E, Lackner KJ, Hollander JE. Diagnostic value of multimarker testing including myeloperoxidase in patients with acute coronary syndrome. Results from a Multicentre Biomarker Study. Circulation. 2006;114 (Suppl II):II–418.
- Scheffer PG, van der Zwan LP, Schindhelm RK, Vermue HPA, Teerlink T. Myeloperoxidase concentrations in EDTA-plasma of healthy subjects are discordant with concentrations in heparin-plasma and serum. Clin Biochem 2009;42:1490–2.
- Datwyler SA, Hsu SC, Matias MS, Pacenti DP, Shih J. Potential interference by antineutrophil cytoplasmic autoantibodies in myeloperoxidase immunoassays. Clin Chem 2008; 54:2084–6.