Abstract
Preeclampsia/eclampsia remains a major cause of maternal and fetal morbidity worldwide. It also remains a leading cause of iatrogenic prematurity as delivery is currently the only way to successfully treat the disorder. The mechanisms that initiate preeclampsia in humans have been remarkably elusive, but some parts of the puzzle have begun to come together. Recently, it has been suggested that its major phenotypes, such as hypertension, proteinuria and endothelial dysfunction, are due to circulating anti-angiogenic proteins such as soluble fms-like tyrosine kinase-1 and soluble endoglin. Abnormalities in these circulating angiogenic proteins are not only present during clinical preeclampsia, but also antedate clinical symptoms by at least 5–6 weeks. The availability of automated platforms for the measurement of these angiogenic proteins has allowed clinicians to evaluate the role of these biomarkers as an aid in the diagnosis and prediction of preeclampsia. This review will highlight the recent clinical studies that have evaluated the utility of these biomarkers in preeclampsia and its related complications.
Introduction
Preeclampsia, is a leading cause of maternal and perinatal morbidity and mortality worldwide. It is a multi-systemic disease that complicates 3–8% of pregnancies, and that is characterized by new onset hypertension and proteinuria after 20 weeks of gestation [Citation1]. In the mother, the disease can progress to widespread endothelial dysfunction affecting mainly the liver, brain and kidney. In the foetus it is associated with intrauterine growth restriction and prematurity [Citation2]. As of 2010 there are still no clinically useful tests to predict the disease, and the only known cure is delivery of the placenta.
In developing countries where access to health care is limited, preeclampsia is a leading cause of maternal mortality. Of the estimated 60,000 or more deaths from preeclampsia worldwide each year, >90% of the deaths are in low and middle income countries [Citation1]. In the developed world, the burden falls on the neonate, since premature deliveries are performed to preserve the health of the mother. Although technological advances in perinatal and neonatal care have reduced infant mortality due to preterm birth, morbidity remains a serious problem. These babies are at increased risk of neurodevelopment disabilities such as cerebral palsy, mental retardation, sensory deficits and behavioural impairments [Citation3] and are also more vulnerable to metabolic disorders and cardiovascular disease later in life [Citation4–5].
Preeclampsia is not only responsible for adverse pregnancy outcomes, but also predisposes to long-term health complications entailing a major economic and familial burden in society. The ability to predict or prevent preeclampsia, and the development of a therapy that safely prolongs gestation are of critical importance and would constitute a major advance in women´s health.
Circulating angiogenic factors in the pathogenesis of preeclampsia
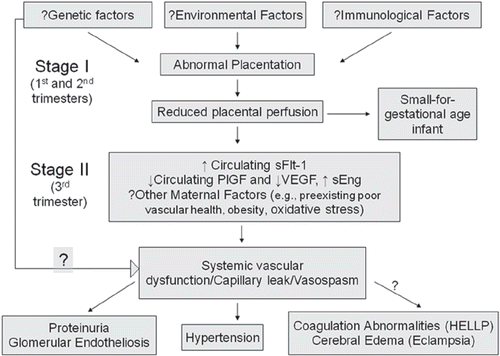
The inciting cause of preeclampsia remains obscure. However results from recent studies have established new insight into the mechanisms of several of the diseases major phenotypes [Citation6–9] (Please see for a summary of the pathogenesis of preeclampsia). The findings in these cited studies have led to an extremely plausible hypothesis that the clinical manifestations of preeclampsia result from an imbalance between circulating pro-angiogenic and anti-angiogenic factors in the maternal circulation. The two anti-angiogenic factors recently implicated are soluble vascular endothelial growth factor receptor 1 (sVEGFR1) (also referred to as soluble fms-like tyrosine kinase 1 or sFlt1) and soluble endoglin (sEng) whose levels are elevated in women with preeclampsia, while the pro-angiogenic proteins, whose circulating concentrations (free levels) are reduced in women with the disease are vascular endothelial and placental growth factors [Citation10–11].
Vascular endothelial growth factor (VEGF) an endothelial specific mitogen plays a key role in promoting angiogenesis. Its activities are mediated primarily by interaction with two high-affinity receptor tyrosine kinases, VEGFR1 (VEGF receptor-1 or fms-like tyrosine kinase-1 [Flt-1]) and VEGFR2 (kinase-insert domain region [KDR]/Flk1), which are selectively expressed on the vascular endothelial cell surface [Citation12]. VEGFR1 has two isoforms: a transmembranous isoform and a soluble isoform (sFlt1 or sVEGFR1). The soluble isoform arises from a truncated mRNA that lacks the cytoplasmic and transmembrane domain but contains the ligand-binding domain [Citation13]. Thus, sFlt1 can antagonize the biologic activity of circulating VEGF by binding to it and preventing VEGF's interaction with its endogenous receptors. sFlt1 also binds and antagonizes placental growth factor (PlGF), another member of the VEGF family produced predominantly in the placenta. sFlt1 is elevated during clinical preeclampsia and weeks preceding clinical symptoms [Citation10]. The elevated levels of sFlt1 have been associated with a fall in free PlGF and VEGF [Citation10]. Furthermore, elevating sFlt1 levels in pregnant rodents appears to re-create hypertension, proteinuria and glomerular endotheliosis, all hallmarks of preeclampsia [Citation7].
Soluble endoglin (sEng) another anti-angiogenic protein, is a TGF-beta 1 co-receptor [Citation14]. The role of this protein in producing preeclampsia phenotypes was evaluated based on the hypothesis that sEng may impair TGF-beta 1 binding to its cell surface receptors and decreasing endothelial nitric oxide signalling [Citation15]. sEng is placental in origin, is present in the sera of pregnant women, is elevated in preeclamptic individuals and correlates with disease severity [Citation6,Citation11]. Administration of both sFlt1 and sEng using an adenoviral expression system in rats produces a severe preeclampsia-like animal model with hypertension, proteinuria, glomerular endotheliosis, features of HELLP syndrome and small for gestational age (SGA) pups [Citation6].
An array of insults may contribute to placental damage that is proximally linked to the production of soluble pathogenic factors by this organ. Various pathways have been proposed to have key roles in inducing placental disease, including deficient heme oxygenase expression, placental hypoxia, genetic factors, autoantibodies against the angiotensin receptor, oxidative stress, inflammation, altered natural killer cell signalling and, more recently, deficient catechol-O-methyl transferase [Citation16]. Interestingly, most of these were shown to increase placental production of the anti-angiogenic factors. Still, the underlying events that induce placental disease activating the cascade of placental damage and anti-angiogenic factor production remain unknown.
The ability of angiogenic proteins to predict preeclampsia
In preeclampsia, the levels of sFlt1 are higher than normotensive controls at the time of clinical disease [Citation7–8]. In a cross-sectional nested case control study, Levine et al., compared gestational age-matched women with active preeclampsia and normal pregnancy and revealed that levels of sFlt1 were significantly higher in the former group [Citation10]. They also showed that levels of this anti-angiogenic protein were significantly elevated 5 weeks before the detection of hypertension and proteinuria. More recently other investigators have evaluated sFlt1 levels longitudinally throughout gestation in normal and preeclamptic women noting that sFlt1 levels appear elevated throughout gestation in women destined to develop preeclampsia, significant difference usually detectable 5 to 6 weeks before the disease presents [Citation17]. In addition, circulating levels of sFlt-1 were found to be elevated in conjunction with decreased free VEGF and PlGF in the bloodstream at the time of disease presentation [Citation7,Citation10,Citation18]. Serum levels of PlGF are lower in women who go on to develop preeclampsia from the first or early second trimester [Citation10,Citation19–20]. The ratio of sFlt1:PlGF has been proposed as an index of antiangiogenic activity which reflects alterations in both biomarkers and is a better predictor of preeclampsia than either measure alone [Citation21].
Most recently Levine and colleagues published results of a nested case–control study of healthy nulliparous women, within the Calcium for Preeclampsia Prevention trial that evaluated the role of soluble endoglin and other angiogenic proteins in preeclampsia and other pregnancy complications. They analyzed three gestational windows- weeks 13–20, 21–32, and 33–42, this time measuring sEng as well as sFlt1, and PlGF [Citation11]. Included were sera from 72 women who had preterm preeclampsia (<37 weeks), 120 with term preeclampsia (≥37 weeks), 120 with gestational hypertension, 120 who delivered small for gestational age, but remained normotensive, and 120 with uncomplicated gestations (normotensive controls). sEng levels were significantly elevated between gestational weeks 17–20 in women destined to develop preterm preeclampsia, and significantly elevated between weeks 25–28 when the disorder developed at term. Of importance too, was that levels were not markedly elevated prior to disease presentation in women destined to develop gestational hypertension, nor were levels dramatically elevated in normotensive gravidas delivering small for gestational age infants. Finally a composite of sEng levels combined with the sFlt1/PlGF ratio greatly improved the odds ratio (mean OR >25) for predictability of both term and preterm preeclampsia [Citation11].
Wathen et al., measured sFlt1 in women who developed preeclampsia, others who developed intrauterine growth restriction and normotensive women with uncomplicated gestations, the samples collected during gestational weeks 12–15 and 16–20, respectively [Citation22]. They found that an elevated sFlt1 level at 16–20 weeks is associated with an increased risk of preeclampsia. Also sFlt1 concentrations decreased by 15% from first to second sample in controls but not in preeclamptic women [Citation22]. Because circulating concentrations of angiogenic factors change with gestational age, it has been proposed that sequential changes in levels of sFlt1, PlGF, and sEng could be more informative in assessing the risk for preeclampsia than are time-point measurements. Rana et al. [Citation23] and Vatten et al. [Citation24] described that sequential changes in angiogenic factors from first to second trimester differ in women destined to develop preeclampsia. A small increase in PlGF, and a high increase in sFtl1 were strong predictors of preeclampsia. The Odds Ratio (OR) was higher for sequential change than for each measurement alone. Interestingly, the combination of the lowest quartile of PlGF change and the highest quartile of sFlt1 change was associated with an OR of 35.3 (95% CI 7.6–164.2) for preterm preeclampsia, and an OR of 3.2 (95% CI 1.4–7.0) for term preeclampsia [Citation24]. Sequential changes of sEng were also predictive of preeclampsia [Citation23]. Consistent with these results, Erez et al. reported that differences in concentration of sEng, sFlt1 and PlGF between first and second trimesters were associated with increased risk of preterm preeclampsia and OR of 14.9 (95% CI 4.9–45.0), 3.9 (95% CI 1.2–12.6) and 4.3 (95% CI 1.2–15.5) respectively [Citation25]. Sequential changes were also measured at later gestational ages. The rate of rise in sFlt1 and sFlt1:PlGF ratio assessed from 22 to 36 weeks was also predictive of overall preeclampsia risk, with areas under the receiver operating characteristic curve of 92.4% (95% CI 86.3–98.5) and 93.8% (95% CI 88.2–99.4), respectively [Citation26].
Recently, Kusanovic et al. reported a remarkable performance of delta and slope of PlGF:sEng ratio, (from early pregnancy and midtrimester) with a positive LR (Likelihood ratio) of 55.6 (95% CI 36.4–55.6) and 89.6 (95% CI 56.4–89.6), respectively for predicting early onset preeclampsia. Overall, their accuracy was better than that of individual factors. Indeed, the slope PlGF:sEng ratio performed better than any other test [Citation27].
Free PlGF is freely filtered into urine and therefore has also been evaluated as a screening test for preeclampsia. Levine et al. evaluated the role of urinary PlGF levels in preeclampsia using the archived urine samples collected in the Calcium for Preeclampsia Prevention trial [Citation21]. In normal pregnancies, urinary PlGF increased during the first two trimesters, peaked at 29 to 32 weeks, and then decreased. In preeclamptic pregnancies, the pattern of urinary PlGF was similar to that of normal pregnancies before the onset of preeclampsia, but beginning at 25 to 28 weeks, and not before, levels were significantly reduced. There were particularly large differences between the controls and the cases with subsequent early onset preeclampsia. For samples collected after 21 to 31 weeks, the adjusted OR was 22.5 (95% CI 7.4–67.8). The investigators concluded that decreased urinary PlGF concentrations at midgestation are strongly associated with subsequent early development of preeclampsia [Citation21], a finding now confirmed by others [Citation28–29].
Stepan et al. performed a prospective study of sixty-three second trimester pregnant women with abnormal uterine perfusion. When combining the measurements of uterine Doppler with sFlt1 and PlGF levels in the second trimester, the sensitivity and specificity of Doppler alone to predict early onset preeclampsia increased from 67 to 83%, and from 76 to 95%, respectively. The combination of parameters performed better than any parameter alone [Citation30]. Later on, the same group also demonstrated that in pregnancies with abnormal uterine perfusions that resulted in the development of preeclampsia, second trimester levels of sEng were also increased. Combined analysis of sEng and sFlt1 in this population with abnormal uterine Doppler was able to predict early onset preeclampsia with a sensitivity of 100% and a specificity of 93.3% [Citation31].
Recently, Poon et al. evaluated 7,797 women with singleton pregnancies, during gestational weeks 11–13. This yielded very good results using an algorithm developed by logistic regression that combined the logarithms of uterine pulsatility index, mean arterial pressure, PAPP-A, serum free PlGF, body mass index and presence of nulliparity or previous preeclampsia. At a 5% false positive rate, the detection rate for early preeclampsia was 93.1% [Citation32]. The calculated positive LR was 16.5, and negative LR was 0.06 [Citation33].
Angiogenic proteins as aid in the diagnosis of preeclampsia
In addition to being useful in the prediction of preeclampsia before the onset of clinical symptoms, angiogenic factors may also prove useful in diagnosing the disease and in distinguishing it from other hypertensive disorders of pregnancy, such as gestational hypertension and chronic hypertension. Clinically, sFlt1 levels have been observed to be directly proportional to severity of proteinuria, but inversely correlated with platelet count, gestational age and neonatal birth weight adjusted for gestational age [Citation8]. In women with preeclampsia, levels of sFlt1 are higher in those with early onset (less than 37 weeks), more severe disease and SGA neonates [Citation8,Citation10]. The clinical utility of sFlt1, sEng and PlGF serum levels in differentiating among hypertensive disorders of pregnancy has also been evaluated. The sensitivity and specificity in differentiating preeclampsia from chronic hypertension were 84 and 95% for sFlt1 and 84 and 79% for sEng [Citation34]. sFlt1 and PlGF also differentiated women with superimposed preeclampsia (i.e. chronic hypertension plus preeclampsia) from those with chronic hypertension without preeclampsia [Citation35]. Circulating anti-angiogenic factors have also been used to differentiate between preeclampsia and other causes of escalating hypertension in pregnant women undergoing haemodialysis [Citation36]. More recently both sFlt1 and sEng have been associated with placental abruption, a rare complication of preeclampsia [Citation37–38]. In summary, altered angiogenic proteins are not only useful in diagnosing preeclampsia, but may also be associated with preeclampsia related adverse outcomes.
Recently, three studies described the use of automated assays for measuring angiogenic factors [Citation35,Citation39–40]. Currently, assessment relies on detection by manual ELISA kits, which are suitable for research purposes but not for the widespread use in the clinical setting. Automated tests will allow a fast and easy-to-implement assessment of angiogenic factors in the everyday clinical routine context. These large prospective studies showed that sFlt1/PlGF ratio was very useful in differentiating preeclampsia and in particular preterm preeclampsia from normotensive and hypertensive subjects with clinical sensitivities and specificities >95% [Citation35,Citation39].
Conclusions
Preeclampsia remains a major cause of maternal and fetal morbidity. The substantial loss of life, as well as serious sequalia of preeclampsia could be eliminated if we could predict, prevent, and better manage the disease. The role of angiogenic factors in the pathogenesis of preeclampsia, as well as evidence that measurement of these factors showed changes that predicted the occurrence of the disorder, especially severe disease, weeks prior to clinical manifestation is a major advance in maternal foetal medicine. A number of clinical studies have demonstrated a potential role for use of these angiogenic biomarkers for aid in the diagnosis and prediction of preeclampsia. We now need clinical trials that the use of these assays will change obstetrician's management decisions, improve health outcomes and/or reduce costs to the health care system. Uncovering the mechanisms of altered angiogenic factors in preeclampsia may also provide insights into novel preventive and therapeutic options.
Disclosures
S.A.K is listed as a co-inventor on multiple patents filed by the Beth Israel Deaconess Medical Center for the use of angiogenic proteins for the diagnosis and therapy of preeclampsia that have been licensed to multiple companies. S.A.K. is a consultant to Johnson & Johnson, Beckman Coulter, Roche and Abbott Diagnostics.
Acknowledgements
S.A.K. is an investigator of the Howard Hughes Medical Institute. The Gulbenkian Programme for Advanced Medical Education is sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde e Fundação para a Ciência e Tecnologia, Portugal.
References
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99.
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008; 371:261–9.
- Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, . Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–86.
- Irving RJ, Belton NR, Elton RA, Walker BR. Adult cardiovascular risk factors in premature babies. Lancet. 2000;355: 2135–6.
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, . Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, . Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111:649–58.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, . Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–7; discussion 7–50.
- Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–91.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, . Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, . Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355: 992–1005.
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71.
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–9.
- Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, . Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–30.
- Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, . A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–92.
- Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat Med. 2008;14:810–2.
- Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, . Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702–3.
- Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003;101:1266–74.
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, . First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–5.
- Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–72.
- Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, . Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85.
- Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, . Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91:180–4.
- Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, . Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–42.
- Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239, e1–6.
- Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, . The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–87.
- Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244, e1–8.
- Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, . A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38.
- Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int. 2006;69:621–4.
- Buhimschi CS, Norwitz ER, Funai E, Richman S, Guller S, Lockwood CJ, . Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005 Mar;192(3):734–41.
- Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49:818–24.
- Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175, e1–6.
- Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009 May;53(5):812–8.
- Levine RJ, Lindheimer MD. First-trimester prediction of early preeclampsia: a possibility at last! Hypertension. 2009;53:747–8.
- Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:28, e1–6.
- Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, . Automated assays for sVEGFR1 and PlGF as an aid in the diagnosis of preterm preeclampsia: a prospective clinical study. Am J Obstet Gynecol. 2010;202:40, e1–7.
- Shan HY, Rana S, Epstein FH, Stillman IE, Karumanchi SA, Williams ME. Use of circulating antiangiogenic factors to differentiate other hypertensive disorders from preeclampsia in a pregnant woman on dialysis. Am J Kidney Dis. 2008;51:1029–32.
- Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, . Circulating angiogenic factors and placental abruption. Obstet Gynecol. 2006;108:338–44.
- Signore C, Mills JL, Qian C, Yu KF, Rana S, Karumanchi SA, . Circulating soluble endoglin and placental abruption. Prenat Diagn. 2008;28:852–8.
- Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, . An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161, e1–e11.
- Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, . Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res. 2010 Epubmed EISSN: 1348–4214.