Abstract
Effective treatment of rheumatoid arthritis (RA) has been hampered by the heterogeneity of the disease. Although early intervention can result in disease remission, it requires early diagnosis – and current diagnostic tests are not sufficiently accurate or sensitive in the early stages of RA. As a result, RA is typically diagnosed only once damage to the joints has already begun, a time at which the window for optimal treatment may have been missed. Furthermore, a significant proportion of RA patients do not respond to any given therapeutic. Research efforts are increasingly focused on discovery of biomarkers that enable early diagnosis and stratification of RA, and thus the implementation of timely, targeted therapy. Biomarkers have the potential to transform the management of RA by enabling not only early diagnosis, but also assessment and prediction of disease severity, selection of therapy, and monitoring of response to therapy. In this mini review, we discuss the development of molecular biomarkers for RA.
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory joint disease of autoimmune etiology, affecting approximately 0.5% of the population. Effective treatment of RA has been impeded by a paucity of accurate diagnostic and prognostic tests, owing in part to the heterogeneity of the disease. When medical attention for arthritis symptoms is first sought, the most common diagnosis is undifferentiated arthritis, i.e., arthritis that cannot be classified as a specific disease. Patients with undifferentiated arthritis are followed over time and are not formally diagnosed with RA unless their arthritis persists for at least six weeks and they meet the current diagnostic criteria of RA [Citation1]. RA patients are then administered therapies on a trial-and-error basis, with dose escalation, introduction of additional therapies, and change of therapy instigated if the patient does not respond satisfactorily or experiences adverse side effects. Clearly, the management of RA is currently far from optimal. It is hoped that the recent focus on biomarker discovery and development will unearth indicators of disease subtypes that can improve the diagnosis and prognosis of RA, and thus enable the implementation of targeted therapy.
Biomarkers and targeted therapy: Lessons from other diseases
Targeted therapy is needed because the uniqueness of an individual's genetic make-up and the heterogeneity of most diseases mean that empirically prescribed medicine is not efficacious or safe in all patients. Targeted therapy is often referred to as personalized medicine, or the tailoring of therapeutic interventions to the individual patient; however, with a few exceptions—such as the treatment of a patient's cancer with a vaccine based on molecules derived from that same patient's tumor [Citation2] – this is not an accurate description. Typically, targeted therapy entails matching a patient with a specific therapeutic that is most likely to be effective and safe based on similarities between the patient's ‘disease signature’ and that of a cohort that has historically responded well to the therapeutic. The tools of this trade are the biomarkers, characteristics of an individual that can be objectively measured and serve as indicators of organ activity, pathogenic processes, or response to treatment. The idea is that, through the analysis of biomarkers in patient populations, a disease can be stratified into distinct subsets that exhibit differential outcomes and responses to specific therapeutics. Thus, “stratified medicine” has been proposed as a more accurate term for the majority of the current endeavors towards targeted therapy [Citation3].
Biomarkers come in many guises: they can be clinical, histological, or imaging parameters, as well as specific molecules or molecular patterns. Molecular biomarkers include genomic, proteomic, and lipidomic biomarkers. They can reflect changes that occur early in the disease process or in the response to therapy and are thus considered one of the most valuable type of biomarker—both for discovery of disease mechanisms and therapeutic targets and for clinical decision making. In select cases, a single biomarker will suffice. For instance, possession of the BCR-ABL genotype indicates that a patient with chronic myeloid leukemia is likely to respond to treatment with imatinib mesylate [Citation4], while overexpression of the HER2 gene dictates whether a patient with breast cancer is likely to respond to trastuzumab [Citation5]. In these examples, the biomarker is a molecule that is directly related to the pathogenesis of the disease and is targeted by the therapeutic; thus it provides a high level of predictive utility. Nevertheless, there is growing consensus that a panel of multiple biomarkers— reflecting a so-called molecular signature—will perform better than a single biomarker in many situations, a concept that has been borne out by findings in a range of diseases, including autoimmune disease, cancer, and cardiac disease [Citation6–8]. Indeed, tests now entering the clinic are often based on profiling of multiple biomarkers, a prime example being a prognostic test based on a 70-gene expression signature that recently obtained approval from the US Food and Drug Administration (FDA) for clinical decision making in the treatment of breast cancer (MammaPrint; Agendia, Huntington, CA, US) [Citation9].
Seeking biomarkers for rheumatoid arthritis
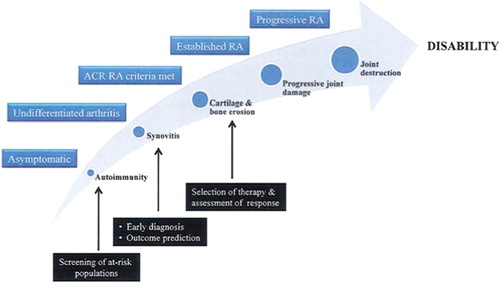
As discussed, biomarkers are integral to disease stratification and hence targeted therapy. They have the potential to transform the management of RA by enabling (i) early diagnosis, (ii) assessment and prediction of disease severity, (iii) selection of therapy, and (iv) monitoring of response to therapy (Figure).
Early diagnosis—prevention or early intervention
Findings from the BeSt trial indicate that early therapeutic intervention can result in disease remission in a substantial proportion of RA patients [Citation10]. In practice, however, diagnosis, and hence therapeutic intervention, is frequently delayed, resulting in increased tissue damage. Even “early’ diagnosis is currently made once erosion of cartilage and bone has already begun, a time at which the window for optimal treatment may have been missed. Biomarkers that allow earlier diagnosis of RA in patients who present with undifferentiated arthritis are needed. The most significant progress in the diagnosis of RA over the last decade has been the development of assays for the detection of autoantibodies against cyclic citrullinated peptides (anti-CCP antibodies) [Citation11]. Anti-CCP antibodies may be involved in the pathogenesis of RA [Citation12] and, unlike the traditional RA biomarker rheumatoid factor (RF), are highly specific to RA [Citation13]. However, the diagnostic sensitivity of anti-CCP antibody positivity in cohorts of early synovitis has been reported to range between 40% and 71% [Citation14–16]. This may partly be because approximately 30% of RA patients never develop anti-CCP antibodies [Citation17]. Thus, the search for biomarkers that provide greater sensitivity and specificity in the diagnosis of early RA continues.
Even better than early intervention is prevention – but to prevent one must be able to predict. Although an increased risk of developing RA is indicated by several factors, including combinations of genetic and environmental factors [Citation18], there are no tests that identify which at-risk patients will indeed develop clinical RA. Biomarkers that can predict the onset of clinical RA during the asymptomatic phase are actively being sought. Evidence to date suggests that the presence of anti-CCP antibodies might serve as a predictor of RA onset. In retrospective analyses of blood samples collected from individuals before they developed RA, anti-CCP antibodies were found to be present up to 9 years before the onset of clinical RA and to be of value in predicting the onset of clinical RA [Citation19,Citation20]. Although estimated to be 5–16 % in the general population [Citation19,Citation20], the positive predictive value (PPV) of anti-CCP antibodies is likely to be much higher in at-risk populations. Nielen et al. [Citation19] estimate that the presence of anti-CCP antibodies in an individual with ≤2 first-degree relatives with RA would indicate a 69.4% risk of developing RA within 5 years. These retrospective findings are encouraging but need to be validated in prospective longitudinal studies. The recent establishment of prospective cohort studies, e.g., SERA (Studies of the Etiology of Rheumatoid Arthritis) [Citation21], enrolling first-degree relatives of probands with RA is anticipated to facilitate both investigation into the natural history of RA and assessment of the predictive value of anti-CCP antibodies and other biomarkers. That development of anti-CCP antibodies can precede the onset of clinical RA by several years, coupled with the cost and toxicities associated with therapeutic intervention, highlights the need for accurate biomarkers that provide temporal information indicating when an asymptomatic individual will develop RA.
Disease assessment and prognosis
The disease course in RA can range from mild and self-limiting to severe and progressive. Biological therapeutics are expensive and can have life-threatening side effects; therefore, biomarkers predictive of progression to severe RA would be useful for alerting clinicians to the need for early initiation of aggressive treatment regimens. Although it is a reliable predictor of further joint destruction in established RA, the presence of cartilage and bone erosion is not a useful prognostic biomarker in early RA, when erosions have yet to appear. Instead, the molecular harbingers of erosion must be discovered. The presence of serum RF, the presence of anti-CCP antibodies, elevations in markers of bone and cartilage turnover, and elevations in acute phase reactants have all been proposed to predict a more destructive course of RA [22-25]. Nevertheless, the diagnostic sensitivity and specificity of these biomarkers are insufficient to guide therapeutic decision making. A panel of multiple biomarkers is anticipated to be more accurate and informative than a single biomarker in assessing the prognosis of RA, by enabling detailed stratification of the disease. Gene-expression profiles in synovial fibroblasts have been shown to define molecularly distinct subsets of RA [Citation26]; however, such profiling is based on an invasive procedure and is thus not feasible or justifiable in routine clinical practice. By analyzing serum samples, we demonstrated that a biomarker signature comprising elevated levels of autoantibodies could stratify early-stage RA into distinct molecular subsets that were differentially associated with clinical parameters predictive of disease severity [Citation27]. Biomarkers need to be further developed and refined to attain sensitivity and specificity sufficient for the accurate identification of individuals who will develop erosive RA, and hence the implementation of appropriate intervention.
Selection of optimal therapy
The advent of biologic therapeutics, including several different anti-TNF agents, an antibody against CD20, an IL-1 receptor antagonist, and CLTA4-Ig, has led to significant advances in the treatment of RA. However, selection of therapies is still conducted on a trial-and-error basis, and less than 50% of RA patients exhibit greater than 50% improvement in their arthritis in response to any one biologic therapeutic [Citation28–30]. Administration of a therapeutic to which a patient is not in fact responsive allows the disease to progress and places undue demand on the cost-restricted healthcare system. Therefore, biomarkers that predict response to therapy, and thereby guide the selection of appropriate therapy, are warranted.
Changes in levels of specific inflammatory mediators early during treatment have been assessed as predictors of response to treatment after 3 months [Citation31]. Although such molecular changes may be useful as pharmacodynamic biomarkers for the monitoring of response to therapy, biomarkers at baseline (i.e., before treatment is commenced) are ultimately needed for the prediction of response to therapy. RF and anti-CCP-antibody status at baseline is associated with response to anti-TNF agents, but it accounts for only a small proportion of the variance in response and cannot predict the response [Citation32,Citation33]. Gene-expression profiles in white blood cells [Citation34] or whole blood [Citation35], and cytokine levels in whole blood [Citation36], have been shown to predict response to anti-TNF agents in single cohorts. While informative, molecular signatures identified in single cohorts in some cases cannot be replicated in independent cohorts [Citation37]. Using blood samples from three ethnically distinct cohorts, we identified a biomarker signature comprising 13 autoantibodies and 11 cytokines that could distinguish between RA patients who will respond to anti-TNF treatment and those who will not [Citation38]. The PPV of this first-generation signature ranged from 58% in the Japanese cohort to 71% in the North American cohort, but could potentially be improved by incorporation of additional proteomic, genetic, clinical or imaging-based biomarkers. Because both autoantibodies and cytokines play a key role in the pathogenesis of RA [Citation12,Citation39], biomarker profiles that include these parameters are anticipated to enable stratification of disease.
In addition to biomarkers predictive of drug response, biomarkers predictive of drug toxicity are being developed, in the hope of improving the safety of RA therapy. For instance, genotypes of folate-dependent enzymes have been proposed to be of value in predicting which RA patients will develop adverse side effects following treatment with the antifolate agent methotrexate [Citation40]. Biomarkers predictive of toxicity due to biologic agents have yet to be reported.
Genomic vs proteomic biomarkers
Both genomic and proteomic biomarkers are under investigation as potential tools for clinical decision making in RA. There are pros and cons associated with both. Genetic biomarkers are incontrovertibly valuable indicators of susceptibility to the development of RA. Most prominent of such genetic biomarkers is the HLA-DR shared epitope, which, in combination with cigarette smoking, is known to be a risk factor for the development of anti-CCP-antibody-positive RA [Citation18]. Additional genetic biomarkers of RA susceptibility continue to be uncovered by ongoing genome-wide association studies [Citation41]. The value of genetic biomarkers in prognosis of disease outcome or of response to therapy is less clear [Citation33,Citation42]. Gene-expression profiles, on the other hand, may serve as prognostic biomarkers in RA [Citation26,Citation34–35], though preliminary findings remain to be validated. Nevertheless, biomarker discovery based on genotyping or transcriptional profiling has certain limitations. Changes in genetic sequence do not always translate to changes in gene expression or protein activity, and the concordance between levels of gene transcripts and levels of the proteins they encode is low. Furthermore, profiling of gene transcripts does not take into account posttranslational modifications, which can play an important role in disease pathogenesis. This is particularly true of autoimmune diseases such as RA, in which autoantibody production and the formation of neoepitopes through posttranslational modification play pivotal roles in disease pathogenesis [Citation43]. For these reasons, many researchers are now focusing their efforts on the discovery of proteomic biomarkers, though proteomic-biomarker discovery has its share of limitations, including biased screening and technical challenges [Citation37].
Stratified medicine: A win-win situation
Diagnostic and prognostic biomarkers have the potential to benefit patients immensely, by enabling precise and timely diagnosis of disease and selection of the most appropriate therapy. But the patients are not the only ones set to benefit—stratified medicine is appealing to the clinical trialists and drug developers, too. By enriching cohorts in clinical trials with patients likely to respond to a therapeutic, prognostic biomarkers can reduce the trial size required to determine therapeutic efficacy [Citation3]. Moreover, they could potentially allow older drugs that were deemed unsafe or ineffective to be reassessed in discreet patient subsets. Pharmacodynamic biomarkers can be used to monitor the efficacy and safety of an investigational drug and can accelerate early-stage clinical trials in which one of the major objectives is to define the range of active drug doses. While efforts aimed at biomarker discovery are burgeoning, the use of biomarkers in clinical trials and clinical practice is in its infancy. Replication and validation of biomarkers in prospective clinical trials will be of paramount importance, as will proof of their impact on management of disease and utilization of health-care resources. We anticipate that, as additional therapeutics and enabling technologies become available, stratified medicine based on biomarkers will increasingly take a leading role in the practice of medicine.
Acknowledgements
This work was supported by NIH NIAMS RC1 AR058713, NIAMS R01 AR-054822, and Veterans Affairs Health Care System funding to W.H.R.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arnett FC, Edworthy SM, Bloch DA, . The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–24.
- Belli F, Testori A, Rivoltini L, . Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol 2002;20:4169–80.
- Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov 2007;6:287–93.
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov 2002;1: 493–502.
- Nahta R, Esteva FJ. HER-2-targeted therapy: lessons learned and future directions. Clin Cancer Res 2003;9:5078–84.
- LaGasse JM, Brantley MS, Leech NJ, . Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care 2002;25:505–11.
- Mor G, Visintin I, Lai Y, . Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A 2005;102:7677–82.
- Zethelius B, Berglund L, Sundström J, . Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358:2107–16.
- Glas AM, Floore A, Delahaye LJ, . Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics 2006;7:278.
- Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Breedveld FC, Dijkmans BA. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol 2006;24:S-77–82.
- van Schaardenburg D, Dijkmans BA. Clinical approaches to early inflammatory arthritis. Nat Rev Rheumatol 2009;5: 627–33.
- Kuhn KA, Kulik L, Tomooka B, . Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest 2006;116:961–73.
- van Venrooij WJ, Hazes, JM. . Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med 2002;60:383–8.
- Goldbach-Mansky R, Lee J, McCoy A, . Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res 2000;2:236–43.
- Nielen MM, van der Horst AR, van Schaardenburg D, . Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann Rheum Dis 2005;64:1199–204.
- Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 2002; 46:357–65.
- Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis 2003;62:870–4.
- Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004; 50:3085–92.
- Nielen MM, van Schaardenburg D, Reesink HW, . Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6.
- Rantapaa-Dahlqvist S, de Jong BA, Berglin E, . Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9.
- Kolfenbach JR, Deane KD, Derber LA, . A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum 2009;61:1735–42.
- van der Heijde DM, van Riel PL, van Leeuwen MA, van‘t Hof MA, van Rijswijk MH, van de Putte LB. Prognostic factors for radiographic damage and physical disability in early rheumatoid arthritis. A prospective follow-up study of 147 patients. Br J Rheumatol 1992;31:519–25.
- Forslind K, Ahlmen M, Eberhardt K, Hafström I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis 2004;63: 1090–5.
- Garnero P, Landewe R, Boers M, . Association of baseline levels of markers of bone and cartilage degradation with long-term progression of joint damage in patients with early rheumatoid arthritis: the COBRA study. Arthritis Rheum 2002;46:2847–56.
- Combe B, Landewe R, Lukas C, . EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34–45.
- Galligan CL, Baig E, Bykerk V, Keystone EC, Fish EN. Distinctive gene expression signatures in rheumatoid arthritis synovial tissue fibroblast cells: correlates with disease activity. Genes Immun 2007;8:480–91.
- Hueber W, Kidd BA, Tomooka BH, . Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum 2005;52:2645–55.
- Edwards JC, Szczepanski L, Szechinski J, . Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81.
- Genovese MC, Becker JC, Schiff M, . Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23.
- Moreland LW, Baumgartner SW, Schiff MH, . Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997;337:141–7.
- Buch MH, Seto Y, Bingham SJ, . C-reactive protein as a predictor of infliximab treatment outcome in patients with rheumatoid arthritis: defining subtypes of nonresponse and subsequent response to etanercept. Arthritis Rheum 2005;52:42–8.
- Braun-Moscovici Y, Markovits D, Zinder O, . Anti- cyclic citrullinated protein antibodies as a predictor of response to anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis. J Rheumatol 2006;33: 497–500.
- Potter C, Hyrich KL, Tracey A, . Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis 2009;68:69–74.
- Lequerre T, Gauthier-Jauneau AC, Bansard C, . Gene profiling in white blood cells predicts infliximab responsiveness in rheumatoid arthritis. Arthritis Res Ther 2006;8: R105.
- Julia A, Erra A, Palacio C, . An eight-gene blood expression profile predicts the response to infliximab in rheumatoid arthritis. PLoS One 2009;4:e7556.
- Fabre S, Dupuy AM, Dossat N, . Protein biochip array technology for cytokine profiling predicts etanercept responsiveness in rheumatoid arthritis. Clin Exp Immunol 2008; 153:188–95.
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol 2006;24:971–83.
- Hueber W, Tomooka BH, Batliwalla F, . Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res Ther 2009;11:R76.
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008;118: 3537–45.
- Weisman MH, Furst DE, Park GS, . Risk genotypes in folate-dependent enzymes and their association with methotrexate-related side effects in rheumatoid arthritis. Arthritis Rheum 2006;54:607–12.
- Plenge RM. Recent progress in rheumatoid arthritis genetics: one step towards improved patient care. Curr Opin Rheumatol 2009;21:262–71.
- Ranganathan P. Pharmacogenomics of tumor necrosis factor antagonists in rheumatoid arthritis. Pharmacogenomics 2005;6:481–90.
- Klareskog L, Widhe M, Hermansson M, Ronnelid J. Antibodies to citrullinated proteins in arthritis: pathology and promise. Curr Opin Rheumatol 2008;20: 300–5.