Abstract
Diagnostic technologies assist clinicians and care givers in making decisions about the care of individual patients. The demands for an evidence-based approach to practice, is proving to be challenging in the field of laboratory medicine as: (i) there has been little formal requirement to demonstrate evidence of effectiveness prior to the introduction of new tests, (ii) generating evidence of effectiveness of tests depends, in most cases, on complementary actions being accomplished e.g. making the correct decision on receipt of the result, and taking the right action, (iii) the principles of evidence-based laboratory medicine highlight the fact that diagnostic tests have the potential to solve a wide range of problems, in a number of clinical settings, with the potential to generate a varied range of benefits (outcomes), and (iv) outcomes invariably accrue to parts of the health economy outside of the laboratory, which can prove challenging for health service managers.
Commissioning a new service involves (a) assessing the local health care needs – and the expected outcomes, (b) specifying the services required–including the resources, and transformational change required, (c) securing the services required – and the implementation strategy to be employed, and (d) monitoring against the contract issued, as well as evaluating the outcomes against the expectations. In this way the purchaser can ensure that diagnostic services are meeting clinical needs, and are being used appropriately to generate the best outcome for the patient and the exchequer, thereby meeting clinical and fiscal governance requirements.
Introduction
Innovation, whilst being at the foundation of the development of medicine, has proven to be a major challenge in its translation to health care, a point recognised by the Institute of Medicine in the final report by the Committee on the Quality of Health Care in America [Citation1]. Innovation itself may be sustaining or disruptive, the latter representing a markedly different – and greater – challenge than the former [Citation2], and which undoubtedly contributes to the slow speed of adoption of new technologies. Sackett and Haynes illustrated one of the key issues for diagnostic tests [Citation3] when describing an architecture for diagnostics research. Using the literature of studies on brain natriuretic peptide as an example, they pointed out that often the first step is to show that “results in affected patients differ from those in normal individuals” (a case control study). Other types of study then follow, through to the important study .......showing that “patients undergoing the diagnostic test fare better than similar untested patients”, and of which there are fewer examples in the literature. The latter study is typically conducted using a randomised controlled trial design to minimise bias, but the crucial points about this type of study are that (i) compared with the case control and other study designs a decision is made upon receipt of the result, and (ii) an outcome is measured at the end of the process – “those receiving the test fare better”; thus the whole diagnostic pathway is evaluated. Importantly, Sackett and Haynes showed that the diagnostic performance of the test was very dependent on the design of the study with the case control design significantly overestimating the performance of the test because (a) the study population did not necessarily reflect the population in which the test was going to be used (i.e. patients with heart failure compared with normal individuals as against a population suspected of having heart failure), and (b) the result was not used to make a clinical decision. So, whilst the hierarchy of research described by Sackett and Haynes might be considered to be an appropriate translational framework for the introduction of a new test, the requirement to demonstrate that ”patients undergoing the diagnostic test fare better than similar untested patients” embraces a transformational change in clinical practice that should complement the introduction of the test.
The situation may be further complicated if the test result is required rapidly e.g. the intensive care unit, or in a setting that might lead to delay due to sample transportation or inconvenience to the patient or health professional e.g. primary care or the home. All of these scenarios, where a new test is being considered, represent degrees of disruptive innovation. Point-of-care testing has been recognised as one of the key enablers of disruptive innovation in health care [Citation4]. It is interesting to note a comment made in the second report of the Economist Intelligence Unit: “The most powerful innovation in the coming decade will be structural and organisational – new ways of working, new team approaches to delivering the full cycle of care”, i.e., disruptive innovation [Citation5].
Many of the challenges of innovation in health care lie in the number of stakeholders, the number of processes involved, and the complexity of the relationship between stakeholders. Reid et al. [Citation6] in defining a framework for a systems re-engineering approach to health care delivery identified five key groups of stakeholders comprising (i) the patient, (ii) the care team or care providers, e.g. clinicians, nurses, pharmacists, (iii) the provider organisation e.g. hospital, clinic, health centre, (iv) the purchaser organisation, and (v) the political and regulatory environment e.g. payers, regulators. The considerations of Reid et al. were based on addressing the needs expressed by the Institute of Medicine, namely that a service should be (a) patient centred – respecting needs and values of individuals, (b) safe – avoiding harm, (c) timely – minimising delays, (d) efficient – avoiding waste, (e) equitable – offering equal quality for all, and (f) effective – being evidence-based. Whilst there is no doubt that all stakeholders will agree with these objectives it is likely that they will all have individual opinions and emphasis they will place on the individual objectives, as well as on the definition of the quality metrics to support these objectives. In addition it was recognised by Reid et al. [Citation6] that there are significant barriers to change including (i) inadequate information and information technology, (ii) policy and market barriers, (iii) organisational and managerial barriers, and (iv) educational barriers.
Laboratory medicine, translational research and the introduction of new tests
Historically tests have evolved from an understanding of the pathology of the disease and the recognition of markers associated with the pathology of the disease. Analytical methods have been developed for the measurement of the markers, and improved with respect to trueness and precision; automation and adaptation to a point-of-care technology has followed. The process of introducing a new test varies by country but can include regulatory approval and achievement of a reimbursement code; internationally, the system in the United States is probably the best understood and has been summarised recently for POCT [Citation7]. Typically however, the evidence on diagnostic tests deals more with the technical performance, with limited consideration of the impact on clinical outcomes [Citation8] – especially in the areas where there might be considered to be the greatest transformational change [Citation9,Citation10].
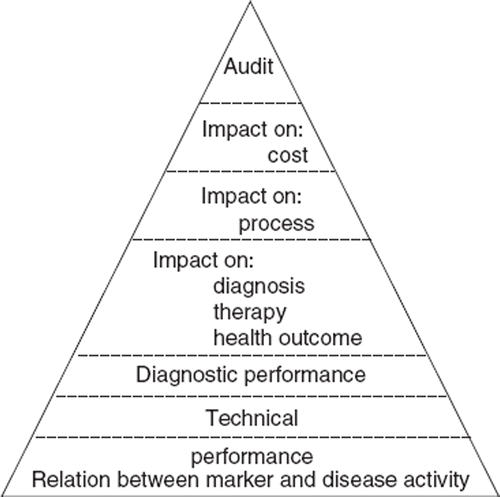
Fryback and Thornbury [Citation11] developed a six-level hierarchy of evidence of efficacy in diagnostic imaging which included (i) technical efficacy, (ii) diagnostic accuracy efficacy, (iii) diagnostic thinking efficacy, (iv) therapeutic efficacy, (v) patient outcome efficacy, and (vi) societal efficacy. This thinking was subsequently developed for in vitro diagnostics with the inclusion of operational impact (efficacy) () [Citation12]. Much of this thinking is now embodied in the principles and practice of evidence-based laboratory medicine, with the addition of two key activities – that of identifying the clinical problem (asking the right question), and applying the evidence [Citation13,Citation14].
Evidence-based laboratory medicine: translational and transformational challenges
One of the acknowledged problems in innovation is clearly identifying the need and matching it with the technology [Citation2]. Mankoff et al. [Citation15] in identifying the barriers to translational medicine pointed to the uni-directional approach to research from bench-to- bedside, with less attention paid to clinical experience. This implies that technology is not always developed against an identified need. This can be particularly problematic in the application of diagnostic tests, where the complexity of application can cover screening, diagnosis and monitoring, as well as any of these utilities being applicable in a range of clinical settings, and with different cohorts of patients. Glasziou [Citation16] made the observation that evidence-based medicine “is not about mechanisms but about outcomes”– an observation that is equally applicable to laboratory medicine, to which one might add “….not just about trueness and precision …. but about solving clinical problems, and facilitating change”! Therefore, in applying these observations to laboratory medicine, clearly the starting point for the potential use of any test has to be with identification of the problems (or need), the potential solutions and the transformational change required to deliver the outcome.
There are two key challenges if the practice of laboratory medicine is to become more evidence based. The first challenge is to improve the quality of the evidence, demonstrating that the use(s) of the test leads to better health outcomes – the translational challenge. The second challenge to ensure that the outcomes are delivered – the transformational challenge.
Service and contracting models for laboratory medicine services
The nature of the laboratory medicine service, as well as delivery and contracting models, vary across the world according to geography, and the organisation within different health economies. The two types of service are those that effectively provide a ‘results only’ service, and those that provide ‘results and interpretation’, with the latter adding tests that are considered necessary (reflex testing), as well as demand management. There are probably five main models of delivery (i) the laboratory located within the hospital setting, (ii) the laboratory that is independent of the hospital setting, (iii) the doctor's office in primary care, (iv) point-of-care testing within a hospital or primary care setting, and (v) point-of-care testing independent of the hospital and primary care setting e.g. a community pharmacy. It is possible that several of these modes of delivery exist within one health economy, and indeed may compete for services within a single health economy. An additional variation is the existence or otherwise of a mechanism for reimbursement, and the nature of that reimbursement – which is, primarily, whether tests are reimbursed according to a fee-for-service (typically direct access primary care services), or as part of a diagnostic related group (DRG) payment (typically secondary care services). Furthermore, in some countries there is no mechanism for reimbursement, with the laboratory budget invariably being included within the overall budget of the hospital where the laboratory is located. This degree of variation in delivery and contracting practice means that the transactional relationship between the purchaser and provider of laboratory services can vary quite significantly and as a consequence the approaches taken to the introduction of new services will vary. As this is intended to be a payer's perspective on the introduction of a new test, a model that is being followed will be described in such a way that readers practicing in other health economies should be able to map the framework into their own setting. In this case the payer is also the purchaser in the National Health Service – a group of Primary Care Trusts.
Commissioning laboratory medicine services
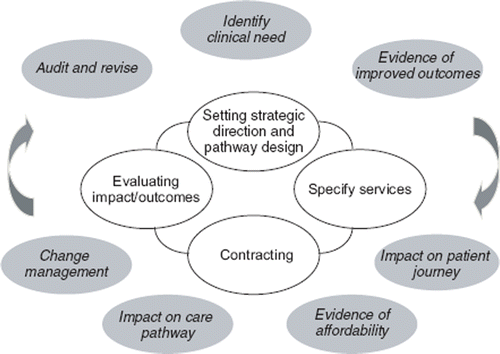
Commissioning is the process of obtaining clinical services; it is a term that is often confused with ‘contracting’ and ‘procuring’, the latter probably being the most commonly applicable term for laboratory medicine services across the world. The generic definition of ‘commissioning’ is “a means securing the services that most appropriately address the needs and wishes of the individual service user, making use of market intelligence and research, and planning accordingly [Citation17,Citation18]. There are generally considered to be four stage of commissioning (i) assessing the local health care needs – and the expected outcomes, (ii) specifying the services required – and identifying the resources required, (iii) securing the services required – and any clinical practice change and resource reallocation, and (iv) monitoring against the contract issued, and evaluating the outcomes against the expectations. An example of a commissioning cycle is shown in . It is worth noting the close parallels between the commissioning cycle and the steps involved in generating the evidence involved with demonstrating the outcomes from using a test, as well as the routine laboratory practice of clinical audit [Citation19].
Figure 2. To show the relationship between the commissioning cycle (the central panel) and the steps involved in generating the evidence to address a clinical need or policy problem (the outer, shaded panels).
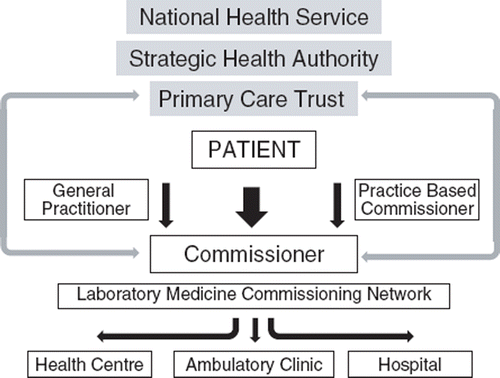
Health reform in England over the past few years has moved to a clearer separation between the purchaser and provider of services, as well as toward a more transactional based approach to service provision [Citation20]. The service is underpinned by a process of commissioning at a local level which (i) ensures that services are delivered to meet local needs, and (ii) allows for choice and competition in the delivery of services [Citation17]. The UK Department of Health's perspective is that commissioning is ‘the means by which we secure the best value for patients and taxpayers, meaning (i) the best possible health outcomes, (ii) the best possible healthcare, (iii) within the resources made available by the tax payer’ [Citation21, Citation22].Whilst commissioning was initially designed for the provision of clinical services, an independent review of laboratory medicine services in England [Citation23,Citation24] recommended that “commissioners take full account of the potential contribution which pathology (laboratory medicine) services can make in improving the effectiveness and responsiveness of the wider health care system”. The vision for the laboratory medicine service developed in the review was of an end-to-end service, addressing the whole process between need and outcome. Thus the Cumbria and Lancashire Primary Care Trusts (the local National Health Service) set up the Cumbria and Lancashire Pathology Commissioning Network. sets out the relationship between the commissioner, the purchaser and the provider. Set out below is a case study on how this network has commissioned a natriuretic peptide service.
Figure 3. An outline of the relationship between the commiss ioners, purchasers and providers. The commissioners in the primary care trust will work with general practitioners (primary care physicians) to identify the clinical needs of the local population (possibly with practice based commissioners), and then determine where the care will be delivered. The pathology commissioning network will work with the commissioners to determine the specification of the laboratory medicine (pathology) service required to meet the need identified. The shaded boxes denote the elements of the organisation involved in funding the provision of health care.
Assess local needs and priorities. Typically the statement of need may come from a strategic planning exercise that looks at the current performance of health services, identifying needs and priorities for change and additional investment. This may be followed by a prioritisation exercise, including the use of health technologies [Citation25]. The template for developing many of the clinical services is based on the Map of Medicine pathway [Citation26]. In this case study the local Cardiac and Stroke Network (serving the needs of the five constituent Primary Care Trusts – the purchasers) using the Map of Medicine in the development of heart failure services, requested that a natriuretic peptide service be set up for use in primary care following a strategic review of local needs, a review of the literature, and a pilot study with a natriuretic peptide service offered to primary care physicians in one locality. The core evidence lay in a health technology assessment which concluded that if patients presenting with a suspicion of the presence of heart failure have a history of a myocardial infarction, evidence of lung crepitations, or oedema, then they should be referred directly for echocardiography; otherwise to perform a natriuretic peptide test and refer for echocardiography if the level is increased above specified cut-off values [Citation27].
Thus the clinical question or policy problem was to determine if the use of a natriuretic peptide measurement could be used to rule out the presence of heart failure in symptomatic patients presenting with suspected heart failure. The expected outcomes would include a reduction in the number of patients referred for echocardiography, a reduction in the time to diagnosis, reduction in number of patients presenting to the Emergency Department with undiagnosed heart failure, and possibly earlier recognition of heart failure in patients presenting with minor symptoms. At this point it is also possible to consider the use of modelling to identify potential costs and benefits [Citation28]. A subsequent strategy document published by the Cardiac and Stroke Network identified that consideration should be given in the future to (i) screening those patients considered at risk of developing heart failure e.g. patients with hypertension, diabetes and those on certain chemotherapies, and (ii) using natriuretic peptide measurement for monitoring therapy in patients with heart failure. This is effectively adding a ‘diagnostic pathway’ to map onto the clinical pathway developed in the Map of Medicine approach, and clearly represented additional and separate clinical questions which required separate review of the evidence, along – possibly – with further research.
Specification of service required. Consultation with the local primary care physicians, as the purchasers of the service, identified that the majority required a 12 hour turnaround time for the results. However in one locality, a more rural setting where patient access was more problematic, there was a plan to set up one-stop breathlessness clinics, in which case a point-of-care testing service would be required. It was suggested that this service could be supported by the laboratory services with respect to equipment procurement, education and training of operators, quality control and problem solving. Assessment of the number of echocardiograms requested per annum and the annual incidence data for heart failure was used to determine the likely workload both for the laboratory and point-of-care services. All of this information together with the data on expected outcomes enabled the production of the business case, and the service specification. At the same time a set of key performance indicators (quality metrics) were developed that were to be used to monitor the implementation of the service, covering aspects of both the operation of the laboratory service and the impact on the clinical service (the care pathway).
Securing services. The service specification was then used to secure the service from an accredited provider at a defined level of quality and productivity. In health economies where there is contestability of services with competition between providers the specification can be used as the core of the tendering process. Alternatively if there is already a local provider then the specification forms the basis for the negotiation with the local laboratory service provider. At this point a tariff for the provision of the service will be agreed – if there is not a national tariff structure.
Monitoring performance and evaluating outcomes. After a period of implementation, when all primary care physicians should be trained in the use of the new service – including being made aware of the change in clinical practice that is expected, monitoring of the use of the natriuretic peptide service should begin covering the specified key performance indicators, as well as feedback from both users and providers of the service. This information can then be transmitted back to the Primary Care Trusts (the purchasers). If there is any major deviation from the workload projection or expected outcomes then a cause should be sought and corrective action taken if required.
Conclusions
The core of the commissioning process is that a clinical need is identified which can be met by the use of a diagnostic test. Good quality evidence should be identified to support the use of the test, and from this it should be possible to identify the cost of providing the test, and an assessment of the benefits that would accrue from introducing the test. It is important from the payer's perspective to recognise that whilst the cost would primarily be incurred in the laboratory medicine budget, all of the benefits – including savings – are to be found in other budget lines within the local health economy, including a reduction in the number echocardiograms requested. This is likely to lead to a reduction in the income derived by the acute hospital from providing the echocardiograms. There may also be additional hospital visits saved from more accurate diagnosis of heart failure and a reduction in the emergency admissions of patients with previously undiagnosed heart failure. The implication of commissioning more services in primary care has been recognised [Citation29], including a loss of business in secondary care, as well as specialists being asked to come and provide more services in primary care. It is possible to see this in the longer term vision of the cardiac network with respect to the use of natriuretic peptide measurements to screen for early signs of disease, and then to help manage the disease when it has been diagnosed. This is structural and organisational innovation (transformational change) being facilitated by the appropriate commissioning of a laboratory medicine service.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Institute of Medicine, Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. National Academy Press, Washington DC, 2001. 337.
- Christensen CM, Bohmer R, Kenagy J. Will disruptive innovations cure health care? Harv Bus Rev. 2000;78:102–12.
- Sackett DL, Haynes RB. The architecture of diagnostic research. BMJ 2002;324:539–41.
- Christensen CM, Grossman JH, Hwang J. The innovator's prescription. A disruptive solution for health care. New York, McGraw Hill, 2009. 441.
- Economist Intelligence Unit. Doctor Innovation. Shaking up the Health System. Economist Intelligence Unit, London, New York, Hong Kong; 2009.
- . National Academy of Engineering and Institute of MedicineReid PP, Compton WD, Grossman JH, Fanjiang G. Building a better delivery system. National Academy Press, Washington; 2005. 262.
- National Institute of Biomedical Imaging and Bioengineering/National Heart, Lung and Blood Institute/National Science Foundation Workshop Faculty (eds Price CP and Kricka LJ). Improving healthcare accessibility through point-of-care technologies. Clin Chem 2007;53:1665–75.
- Balk EM, Ioannidis JP, Salem D, Chew PW, Lau J. Accuracy of biomarkers to diagnose acute cardiac ischemia in the emergency department: a meta-analysis. Ann Emerg Med. 2001;37:478–94.
- Hobbs FD, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, . A review of near patient testing in primary care. Health Technol Assess 1997;1(5):1–230.
- National Academy for Clinical Biochemistry. Laboratory Medicine Practice Guidelines Evidence-Based Practice for Point-of-Care Testing (Nichols JH). Accessed at http://www.aacc.org/members/nacb/LMPG/OnlineGuide/PublishedGuidelines/poct/Pages/poctpdf.aspxon November 17th 2009.
- Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991;11:88–94.
- Price CP. Evidence-based laboratory medicine: Supporting decision-making. Clin Chem 2000;46:1041–50.
- Price CP, Christenson RH. Evidence-based laboratory Medicine: principles, practice and outcomes. Second Edition. Washington DC, AACC Press, 2007. 545.
- Price CP, Lozar Glenn J, Christenson RH. Applying evidence-based laboratory medicine: A step-by-step guide. Washington DC, AACC Press, 2009. 260.
- Mankoff SP, Brander C, Ferrone S, Marincola FM. Lost in translation: Obstacles to translational medicine. J Translational Med 2004;2:14.
- Glasziou P, Del Mar C, Salisbury J. Evidence-based practice workbook. Second edition. Blackwell Publishing, Oxford 2007. 202.
- Woodin, J. Healthcare commissioning and contracting. Walshe K, Smith J. Healthcare management. Open University Press, Maidenhead, 2006. 525.
- Institute of Commissioning Professionals. What is commissioning? Accessed at http://www.iocp.co.uk/ on November 17th 2009.
- Price CP, Jones RG. The challenges in commissioning laboratory medicine (pathology) services. Journal of Management and Marketing in Healthcare. 2008;1:166–78.
- Mannion R. General practitioner commissioning in the English National Health Service: Continuity, change, and future challenges. Int J Health Serv. 2008;38:717–30.
- Department of Health. Health reform in England: Update and commissioning framework; annex the commissioning framework. 2006. Accessed at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4137230.pdf on November 17th 2009.
- Department of Health. Commissioning a patient-led NHS. 2005. Accessed at http://www.dh.gov.uk/en/Publicationstatistics/Publications/PublicationsPolicyAndGuidance/DH_4116716 on November 17th 2009.
- Lord Carter of Coles. Report of the review of NHS pathology services in England 2006. Accessed at http://www.pathologists.org.uk/publications-page/Carter%20Report-The%20Report.pdf on November 17th 2009.
- Lord Carter of Coles. Report of the second phase of the review of NHS pathology services in England 2008. Accessed at http://www.dh.gov.uk/en/Healthcare/Pathology/DH_075531 on November 17th 2009.
- Wilson E, Sussex J, Macleod C, Fordham R. Prioritizing health technologies in a primary care trust. J Health Serv Res Policy. 2007;12:80–5.
- NHS Choices, Your Health, Your Choices. Map of Medicine: Heart Failure – suspected. Accessed at http://healthguides.mapofmedicine.com/choices/map/heart_failure1.html on November 17th 2009.
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, . Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009;13(32):1–232.
- Airoldi M, Bevan G, Morton A, Oliveira M, Smith J. Requisite models for strategic commissioning: the example of type 1 diabetes. Health Care Manag Sci. 2008;11: 89–110.
- Charlton R. Practice-based commissioning: implications for secondary care. Clin Med. 2008;8:61–4.