Abstract
Risk stratification for cardiovascular diseases (CVD) remains suboptimal even after the introduction of global risk assessment by various scores. This has prompted the search for additional biomarkers which might help to improve risk stratification. Basically, there are blood biomarkers representing various pathophysiological pathways of atherosclerosis, markers of subclinical disease, and potentially genetic markers. Since inflammatory processes accompany all stages of atherosclerosis, measurement of plasma/serum concentrations of circulating inflammatory biomarkers has received great attention. Such biomarkers can be measured systemically by sensitive assays and elevated concentrations in the circulation have been shown to be associated with future CVD events. Thus, they might add to the predictive value of the atherogenic lipoprotein phenotype to further improve CVD risk assessment. In addition, several non-invasive imaging techniques are available for which also a predictive value for CVD could be established, in particular measurement of the intima-media thickness of the carotid artery using high resolution ultrasound and measurements of coronary calcium by coronary computed tomography. However, for most of these biomarkers the clinical utility has not yet been firmly established. This applies even more to an integrated approach combining blood biomarkers and markers of subclinical disease. Thus, more data, preferably from serial measurements in large populations taking also into account new candidates from “omics” technology are needed to gain further insight in the potential clinical usefulness of an integrated approach.
Introduction
Over the past 50 years the traditional risk factor concept for cardiovascular diseases (CVD) has been firmly established. While for many years various national and international societies involved in the prevention and treatment of CVD have focused on single risk factors like hypertension and hypercholesterolemia, current guidelines by the AHA/ACC [Citation1] and the ESC [Citation2] recommend a global risk assessment based on traditional risk factors to evaluate the absolute risk of an individual for CVD. However, that these scores do have limitations based on the small number of risk factors included, e.g. not considering some of the most important ones namely diabetes, obesity, and a family history of coronary heart disease (CHD). Based on such incomplete risk assessment, subjects are divided into three groups: Those at low risk, at intermediate risk and at high risk. Using the Framingham risk score (FRS), high risk would be present in all those with a 10-year risk > 20% for a major CHD event, intermediate risk equals 6% or 10% to 20% 10-year risk, and all individuals with a risk below 6% or 10% over ten years would be at low risk. Whereas guidelines are specific on what to do with those at high risk, namely recommending lifestyle changes and potentially prescription of a statin, there is still uncertainty about those at intermediate risk. For low risk subjects, no intervention is indicated and they will be re-invited for risk assessment after another 5 years. It has to be noted here that the 20% risk over 10 years cut-off advocated by NCEP III is an arbitrary one and, based on the distribution of myocardial infarctions (MI) in the population it is fairly evident that the vast amount of acute events comes from those at intermediate risk because they represent the largest group in the population.
Such evidence has prompted the search for novel markers of cardiovascular risk. Due to the complex pathophysiology of atherosclerosis, markers representing inflammatory pathways, coagulation, platelet aggregation, lipoproteins or lipid related mechanisms, genetic predisposition or subclinical disease have been investigated regarding their potential to aid in improved risk prediction. Based on what has been mentioned above, subjects at intermediate risk (10-year risk of 10–20%) would represent the most promising candidates for additional testing to increase or decrease their actual risk. Such markers however, may not only be used for improved risk stratification but also for the early detection of subclinical disease, for the diagnosis of acute or chronic ischemic syndromes (ACS), for monitoring disease progression or response to therapy, and finally for the selection of an appropriate therapy. However, these different areas of clinical biomarker application certainly require different criteria for biomarker qualification and utilization.
Blood biomarkers
Considering the large number of emerging blood biomarkers available in the area of primary prevention of CVD, recently the NACB LMPG committee (National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines) [Citation3] has selected a subset of markers that seem to be most promising for further evaluation: lipoprotein subclasses and particle concentration, Lp¬-PLA2, apoA1 and apoB, high-sensitive C-reactive protein (CRP), fibrinogen, white blood cell count, (WBCC), homocysteine, brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP), creatinine, microalbuminuria, and cystatin C. This represents only a small list out of a large panel of potential candidates and even for most of these markers the clinical utility has not been fully established in all areas of CVD at present.
We have been overwhelmed with a large number of prospective studies indicating the potential usefulness of a new biomarker, but realize that the sole presentation of a moderately increased relative risk (RR) associated with a marker, usually in the order of 1.5 to 3, is not sufficient. For further evaluation of the potential clinical utility of an emerging biomarker other criteria like discrimination, calibration, and reclassification are even more important than simply an increased RR [Citation4].
Many of the new biomarkers have not shown a strong association with cardiovascular endpoints even after adjusting for potential confounders. Therefore, considering the complexity of the pathophysiology of CVD, it is conceivable to believe that the integration of different biomarkers reflecting various cardiovascular pathways might be superior to the measurement of a single biomarker in risk stratification and prediction of CVD events. However, new biomarkers, used individually or in combination, clearly have to demonstrate that they provide incremental information over and above traditional cardiovascular risk factors contained in the various scores.
Combination of blood biomarkers in apparently healthy subjects
One of the first attempts to investigate a multimarker approach in primary prevention comes from the Framingham Heart Study (FHS) [Citation5] in which 10 biomarkers related to CVD were studied in more than 3,200 subjects followed for a median of 7.4 years. Biomarkers included CRP, natriuretic peptides such as BNP, NT-proBNP, fibrinogen, D-dimer, plasminogen activator inhibitor-1 (PAI-1), homocysteine, and the urinary albumin to creatinine ratio. The biomarkers that most strongly predicted major cardiovascular events were BNP and the urinary albumin to creatinine ratio. A high value of the multimarker score was clearly predictive for death or cardiovascular events. However, the addition of the multimarker score to conventional risk factors resulted in only minimal increases in the ability to classify risk as measured by C-statistics. This study has been discussed controversially for the modest number of endpoints, which in addition contained a combination of hard endpoints like fatal and non-fatal MI as well as softer endpoints, i.e. angina pectoris and congestive heart failure (CHF).
Recently, Zethelius et al. [Citation6] have evaluated the contribution of multiple biomarkers for the prediction of hard outcomes such as death from all causes and from CVD over a 10-year follow-up period. Four biomarkers, related to cardiac and renal disease as well as inflammation, such as troponin I, NT-proBNP, cystatin C, and CRP were measured within the Uppsala Longitudinal Study of Adult Men (ULSAM), a community-based cohort of 1,135 elderly men, aged 71 years at baseline, of whom 661 subjects were without prevalent CVD at baseline. During a follow-up of 10 years, 315 participants died, 136 deaths were from CVD. In this analysis, a one standard deviation (SD) increase in the concentration of each biomarker, if taken separately, was significantly associated with death from CV. All causes after multivariable adjustment and the magnitude of the associations was almost the same within the whole cohort and after exclusion of subjects with prevalent CVD at baseline. Using state-of-the-art statistical tools, the authors could further show an improvement in risk stratification for future cardiac and total mortality by adding biomarkers to a model that included established risk factors, both in the whole sample and in participants without CVD at baseline. Furthermore, in the subgroup of subjects without prevalent disease at baseline, the addition of all four biomarkers to the established risk factors resulted in 133 appropriate and in 69 inappropriate risk reclassifications. The clinical implications of such reclassifications, however, remain unclear at present. Finally, model calibration and global fit also confirmed the incremental value of the combination of biomarkers beyond conventionally available cardiovascular risk factors. Thus, the strong independent associations seen in this study and the clear incremental value of several blood biomarkers in addition to the FRS suggest that these markers may be more relevant for fatal outcomes than for non-fatal endpoints.
The largest attempt to study an integrated approach of various novel biomarkers reflecting different pathophysiological mechanisms comes from the MORGAM Biomarker study [Citation7]. In the MORGAM Consortium, 30 biomarkers reflecting distinct pathophysiological pathways – inflammation oxidative stress, lipid metabolism, renal function, cardiovascular function and remodelling, metabolic processes, coagulation, vitamins and myocardial cell damage – were prospectively evaluated in 7,867 individuals coming from the FINRISK 97 study who had no history of MI or stroke at baseline. During a follow-up of 10 years, 336 incident cardiovascular events occurred in men and 136 in women. The CVD endpoint was defined as a composite outcome variable including fatal or non-fatal MI, unstable angina, the need for coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) and finally ischemic stroke. Using fully adjusted Cox proportional hazards models, the strongest association with this combined endpoint was seen in men for NT-proBNP, CRP, D-dimer, midregional pro-atrial natriuretic peptide (MRproANP), troponin I and in women additionally for interleukin (IL)-1 receptor antagonist (RA) and cystatin C (all p< 0.01). When examined as single markers, only D-dimer and troponin T significantly improved C-statistics among men and NT-proBNP among women. Integrated discrimination improvement (IDI) suggested better discrimination of the model after adding NT-proBNP, CRP, MRproANP to a model already including the classical risk factors for men. Among women, adding NT-proBNP improved the model discrimination. Combination of the five best markers significantly improved C-statistics in men (from 0.8154 to 0.8238, p< 0.002).
A further example comes again from a Nordic population [Citation8]. In the Malmö area in Sweden, a cohort study of 5,067 participants with a mean age of 58 years at baseline (1991 – 1994) without CVD had been set up. Participants underwent measurement of CRP, cystatin C, midregional pro-adrenomedullin (MR-proADM) and NT-proBNP, as well as lipoprotein-associated phospholipase A2 (Lp-PLA2). During a median follow-up of 12.8 years 418 incident cardiovascular and 230 coronary events occurred. Data were analyzed by multivariate Cox proportional hazards regression and measures of model discrimination, calibration and reclassification (net reclassification index, NRI) and integrated discrimination improvement (IDI) were included. Biomarkers retained in backward elimination models were CRP and NT-proBNP for cardiovascular events and MR-proADM and NT-proBNP for coronary events which increased the C-statistic by 0.007 (p=0.04) and 0.009 (p=0.08). Overall, the proportion of participants reclassified was modest (8% for CV risk and 5% for coronary risk). However, greater improvements were seen in those at intermediate risk (7.4% for CV events, p=0.03 and 14.6% for coronary events, p=0.003). Still, correct reclassification was almost entirely confined to down-classification of individuals without events rather than up-classification of those with events. Thus, the authors concluded that although selected biomarkers may be used to predict future cardiovascular events the gains over conventional risk factors are minimal.
Finally, in a relatively small cohort of 302 elderly subjects from a population- based study from the UK, in addition to traditional risk factors, homocysteine, folic acid, CRP and IL-6 were measured [Citation9]. The main endpoint was cardiovascular mortality. The first major result showed that the FRS in this elderly population was not able to discriminate between those being at low, intermediate, and high risk. Looking at the four emerging risk markers, homocysteine, folic acid, CRP, and IL-6, cumulative cardiovascular mortality was clearly increased in those with high homocysteine levels and was elevated borderline in those with high folic acid levels. However, no significant association was seen with either elevated CRP or IL-6 levels. Looking into discrimination by calculating the area under the curve (AUC) from ROC analysis of three prediction models for 5-year cardiovascular mortality based on the FRS, the addition of plasma concentrations of homocysteine rather than classic risk factors showed an increase in the AUC and thus may potentially be used to select older people for primary preventive intervention. However, these findings need to be validated in an independent cohort.
Combination of blood biomarkers in patients with manifest atherosclerosis
Moving from apparently healthy subjects investigated in population-based cohorts to secondary prevention, one of the largest examples of a multimarker approach comes from the Heart Outcomes Prevention Evaluation (HOPE) study in patients with either manifest atherosclerosis or a history of diabetes mellitus [Citation10]. The concentration of ten biomarkers was measured in 3,199 patients with stable CHD, followed over 4.5 years for recurrent CV events. In Cox regression analysis, including traditional risk factors and several biomarkers, only four of them (NT-proBNP, soluble intercellular adhesion molecule (sICAM)-1, sIL-1 RA, and fibrinogen) remained significantly associated with the primary outcome. However, adding these four biomarkers to a basic model consisting of simple traditional risk factors did not further improve model accuracy, which was already achieved by including NT-proBNP (increase of AUC from 0.65 to 0.71 (p<0.001)).
Thus, based on these controversial results from several large studies, there is no clear evidence that the addition of even a large number of emerging biomarkers to existing risk prediction models, mainly based on the FRS, clearly improves risk prediction.
Markers of subclinical atherosclerosis
Various imaging methods have been applied to improve cardiovascular risk prediction (Table 1). Among them, the most important are measurement of the intima-media thickness (IMT) by high-resolution carotid ultrasonography (US) and the determination of coronary artery calcium (CAC) by electronic beam computed tomography (EBCT) which more recently has been substituted by multislice computed tomography (MSCT).
Carotid ultrasound
Although a number of studies do exist that have prospectively evaluated the association between increased IMT and further cardiovascular outcome [Citation11], the vast majority of studies has not accounted for all potential confounders and thus may be subject to residual bias.
Plichart et al. [Citation12] carried out the largest prospective study investigating 9,249 subjects within the Three-City Study for carotid atherosclerosis and prediction of clinical CHD. They not only assessed IMT but also determined the presence or absence of carotid plaque. Since earlier carotid US studies had produced somewhat conflicting results with regard to the optimal localization of measurements (common carotid artery, CCA, bifurcation, internal carotid artery, ICA) or using the mean or maximum IMT and carrying out measurements for IMT at a site free of plaque, the present study used bilateral carotid US examinations measuring CCA-IMT at a site free of any plaque and assessed the presence or absence of plaque. Such data were collected in 6,631 subjects aged 65 to 85 and, after excluding a past history of CHD, 5,895 subjects remained for final analysis. Mean IMT was 0.71 mm and 25% presented more than one plaque, 19% one plaque, and 56% showed no plaque at all. During a median follow-up of 5.4 years 223 incident CHD events occurred. In age, gender and study centre adjusted Cox proportional hazard models as well as in a fully adjusted model considering other risk factors, those presenting with more than one plaque had a 2.6-fold and a 2.2-fold increased risk for future CHD, respectively. No association was seen between carotid IMT measurements and future CHD. In ROC analysis, a model with plaque showed a significantly increased AUC compared to a model without (0.729 to 0.744, p<0.05). NRI based on the method by Pencina et al. [Citation13] for events was not significant with 1.8% but was significant for non-events with 7.4% (p<0.01). Thus, the authors concluded that in this large population-based cohort, carotid plaque but not CCA- IMT was an independent predictor of CHD.
Coronary artery calcium scoring (CAC)
Prospective studies have shown that the presence and extent of CAC is strongly associated with non-fatal and fatal CHD as well as total mortality, in most studies independently of traditional risk factors. In some of these studies, in particular the South Bay Heart Watch Study [Citation14] the addition of CAC scoring to global risk assessment by the Framingham algorithm clearly increased the AUC from 0.71 to 0.81. Of note, in women considered to be at low risk based on the FRS [Citation15], data from the Multi-Ethnic Study of Atherosclerosis (MESA) showed that in particular the presence of advanced CAC identified a subgroup at high risk of future CHD. A recent publication from MESA [Citation16] in which 6,722 initially-healthy subjects were followed for incident coronary events during a median follow-up of 3.8 years, demonstrated a strong and independent association between increasing CAC and any coronary event with hazard ratios (HR) of 3.6, 7.7 and 9.7 in those with Agatston scores of 1-100, 101-300 and >300, respectively. However, despite such strong associations, the AUC in particular in Caucasians showed no incremental value over and above the FRS. Thus, the latest AHA Scientific Statement concluded that EBCT or MSCT may be reasonable in measuring atherosclerosis burden in clinically selected intermediate risk patients for refined clinical risk prediction and to select patients for more aggressive target values for lipid lowering therapies (class 2b, level of evidence b) [Citation17].
Erbel et al. [Citation18] presented 5-year outcome data from the Heinz-Nixdorf RECALL Study during last year's ACC Meetings. In this population-based cohort study, 4,814 men and women aged 45-75 years were included. CAC scoring was done by EBCT and risk factors were assessed according to ATP III guidelines. Primary endpoint was fatal (n=29) and non-fatal MI (n= 64). CAC score categories (Agatston score) were strongly related to outcome even in multivariable adjusted models. ROC analyses for men and women combined showed an incremental value of CAC scoring with a significant increase in the AUC from 0.667 (ATP III alone) to 0.754 (ATP III + CAC score). In gender specific analyses, results were less clear for women which may have to do with the power of the study (n= 30 primary endpoints in women).
Combination of markers of subclinical disease with blood biomarkers
Since results regarding the clinical utility of carotid IMT measurements for future cardiovascular risk prediction are still controversial, it is not surprising that there is only one study that looked into the combination of a blood biomarker, in this case CRP and measurement of carotid atherosclerosis (IMT and plaque) by Cao et al. [Citation19]. Within the Cardiovascular Health Study (CHS) simultaneous measurement of carotid IMT, plaque characteristics and CRP was done and all three variables were related to 12-year-incidence of CVD and all-cause mortality in 5,888 elderly subjects. Main results showed that all parameters were correlated with one another and each parameter independently predicted risk of CVD and mortality in multivariable models which included all three measures and traditional risk factors. Being in the top tertile of the carotid IMT distribution was more predictive for various events than having a CRP > 3 mg/L or being in the high risk group on the basis of carotid plaque characteristics. However, elevated CRP was a particularly useful predictor in the presence of subclinical atherosclerosis with a 72% increase in risk for CVD and a 52% increase in total mortality. Cumulative event rates suggested a possible additive interaction for incident CVD and all-cause mortality with an excess rate attributable to the interaction of CRP and subclinical disease of 54% for CVD death and 79% for all-cause mortality. By contrast, CRP did not add predictive power in the absence of carotid atherosclerosis. Finally, both CRP and subclinical atherosclerosis added only modest incremental information to risk prediction when adjusted for the effect of conventional risk factors with either C- statistics or AUC derived from ROC analysis.
Since the clinical utility of CAC measurements for improved cardiovascular risk prediction has not been finally demonstrated, there are only two studies that investigated the combination of a blood biomarker with CAC score. Park et al. [Citation20] studied 1,461 participants without CHD at baseline for the presence of CAC and measured the CRP concentration. During follow-up of 6.4 years, in subjects with baseline CRP ≤ 10 mg/L, the presence of CAC was a predictor of cardiovascular outcome as was CRP. Further analysis showed that there was an increasing risk with increasing calcium and CRP. Relative risks for the medium-calcium/low CRP risk group to the high CAC/high CRP group ranged from 1.8 to 6.1 for MI/ coronary death and 2.8 to 7.1 for any cardiovascular event. The authors concluded that CRP and CAC scoring both contributed independently towards the incidence of cardiovascular events. Most recently, Wong et al. [Citation21] presented data on the combined effect of CAC scoring and measurement of myeloperoxidase (MPO), a strong marker of oxidative stress. Altogether 1,302 asymptomatic adults, mean age 59 years, 47% women without known CVD were followed for 3.8 years. Main results showed that mean MPO levels increased according to CAC categories and incident CVD events were more likely in those at or above vs. below the median MPO level even after adjustment for age, sex, CAC score, and risk factors. Combining CAC and MPO categories, CVD events ranged from 0.6% in those with a CAC score of 0-9 to 7.1% (adjusted hazard ratio, HR, 9.2, p<0.001) in those with CAC scores of ≥ 100 and MPO below the median and 14% (adjusted HR 19.5, p<0.0001) in those with CAC scores of ≥ 100 and MPO at or above the median. Thus, the authors concluded that imaging of subclinical atherosclerosis combined with assessment of a biomarker of plaque vulnerability might help improve CVD risk stratification.
Limitations of the application of biomarkers at presence
The rapidly increasing literature on biomarkers in CVD has provided us with valuable new information regarding the pathophysiology of this complex disorder. Such information comes both from blood biomarkers and from imaging biomarkers. However, before such information can be translated in the routine clinical setting, a number of criteria have to be fulfilled. It is not sufficient to simply demonstrate a more or less strong association between a biomarker and cardiovascular outcome. Hlatky et al. [Citation4], in a Scientific Statement from the AHA, recently published criteria for the evaluation of novel markers of cardiovascular risk. They defined various areas of evaluation of a novel biomarker;
First a proof of the concept which relates to a difference in levels of a marker between subjects with and without outcome.
Second, the prospective validation: does the novel biomarker predict development of future outcomes in a prospective cohort or nested case cohort/case control study?
Third, the demonstration of an incremental value: does the novel biomarker add predictive information to established standard risk markers?
Fourth, the demonstration of clinical utility: does the novel risk marker change predicted risk sufficiently to change recommended therapy? Such data so far are only available for troponins in ACS, natriuretic peptides in CHF, and CRP in primary prevention based on results from the JUPITER study.
Fifth, clinical outcomes: does the use of the novel risk marker improve clinical outcomes especially when tested in a randomized trial?
Finally, cost effective issues: does the use of the marker improve clinical outcome sufficiently to justify the additional cost of testing and treatment?
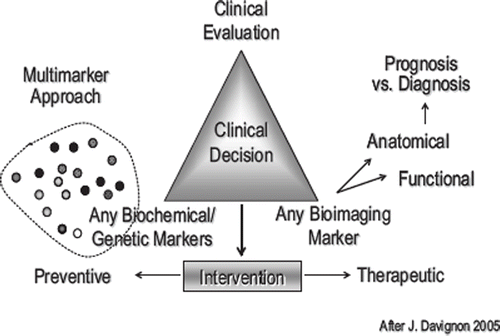
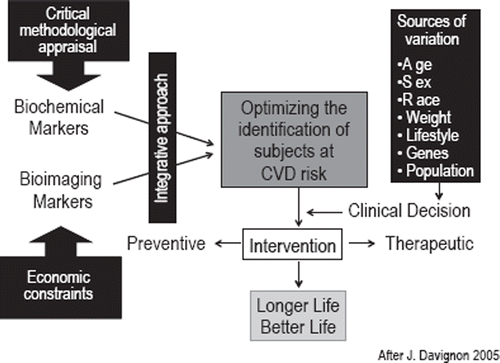
Since such data are not available for the vast majority of emerging biomarkers if tested against the background of any global risk assessment algorithm in primary prevention or a basic clinical score in those with manifest disease, it is not surprising that consistent data on the potential of integrating various biomarkers either among blood biomarkers or the combination between blood and imaging biomarkers is lacking. and present the conceptual framework for such an integrated approach. Further large and comprehensive studies are needed that combine measurements of subclinical atherosclerosis using cardiac CT or ultrasound, possibly magnetic resonance imaging (MRI) of the abdominal aorta or the carotid artery, together with extensive evaluation of plasma and serum for new blood biomarkers and genetic markers. This is already being done in the BioImage Study as part of the HRP initiative [Citation22]. Such comprehensive collection of data, analyzed by innovative methods developed in bioinformatics, might lead to new predictive models of outcome.
Summary and conclusions
We are still trying to prove the clinical utility of various biomarkers in addition to global risk assessment for CVD, beyond gaining further mechanistic insight.
Single markers in general are less likely to show an incremental effect on risk prediction, based on the complex pathophysiology of atherothrombosis. Thus, multimarker panels are needed but we have not yet identified the most appropriate candidates. This may be easier for type 2 diabetes mellitus than for cardiovascular disease.
Candidates may vary from population to population, based on differences of classical risk factors and other morbidities but also with regard to outcome (fatal/non-fatal disease, coronary/cardiovascular events and total mortality).
There is no consistent evidence yet for an added value with an integrated approach using presently available analytical techniques.
We need to improve statistical methods to assess the clinical utility of emerging biomarkers (discrimination, C-statistics, ROC, calibration, predictiveness model, classification and regression tree (CART), analysis, and reclassification).
“Omics” technology may provide more promising candidates (for risk prediction and as therapeutic targets) as presently available but does not solve the existing bioinformatics problem.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- ACCF/AHA/ACP 2009 Competence and training statement: A curriculum on prevention of cardiovascular disease: A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on competence and training (Writing Committee to Develop a Competence and Training Statement on Prevention of Cardiovascular Disease). Circulation. 2009;120:e100–e126.
- De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, . European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601–10.
- , NACB LMPG Committee MembersMyers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, Grundy SM, . National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378–84.
- Hlatky M, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, . Criteria for evaluation of novel markers of cardiovascular risk. A scientific statement from the American Heart Association. Circulation. 2009; 119: 2408–16.
- Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques P, . Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9.
- Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, . Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16.
- Salomaa V, Saarela O, Zeller T, Kuulasmaa K, Kee F, Blankenberg S; for the MORGAM Project. Prospective assessment of 30 novel biomarkers in the prediction of relative and absolute risk of cardiovascular events: the MORGAM biomarker study. Circulation 2009;119;e271–e366.
- Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engström G, Persson M, Smith J, Magnusson M, Christensson A, Struck J, Morgenthaler N, Bergmann A, Pencina M, Wang T. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA 2009;302:49–57.
- de Ruijter W, Westendorp R, Assendelft W, den Elzen W, de Craen A, le Cessie S, Gussekloo. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083.
- Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, Rupprecht HJ, . for the HOPE Study Investigators. Comparative impact of multiple biomarkers and N-Terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2006; 114:201–8.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67.
- Plichart M, Zureik M, Gariepy J, Dartigues JF, Jouven X, Ritchie K, Tzourio C, . Respective contribution of carotid intima-media thickness and plaques to incident coronary heart disease in community-dwelling elderly. The Three-City Study. Eur Heart J 2009, Abstract Supplement.
- Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;30:157–72.
- Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–5.
- Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, . Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: The multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167:2437–42.
- Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, . Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45.
- Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, . Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the Amer. Heart Ass. Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91.
- Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Dragano N, Stang A, ., for the Heinz Nixdorf Recall Study Investigative Group. Signs of subclinical coronary atherosclerosis measured as coronary artery calcification improve risk prediction of hard events beyond traditional risk factors in an unselected general population: The Heinz Nixdorf Recall Study – 5-year outcome data. American College of Cardiology, Scientific Sessions; 2009.
- Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, . Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116: 32–8.
- Park R, Detrano R, Xiang M, Fu P, Ibrahim Y, LaBree L, Azen S; Combined use of computed tomography coronary calcium scores and C-reactive protein levels in predicting cardiovascular events in non-diabetic individuals. Circulation. 2002;106:2073–7.
- Wong ND, Gransar H, Narula J, Shaw L, Moon JH, Miranda-Peats R, Rozanski A, . Myeloperoxidase, subclinical atherosclerosis, and cardiovascular disease events. J Am Coll Cardiol. Cardiovasc Imaging. 2009;2:1093–9.
- Plump A, Lum P. Genomics and cardiovascular drug development. J Am Coll Cardiol. 2009;59:1089–100.