Abstract
Wide spread variation in measurement results of total 25-hydroxyvitamin D (25(OH)D) confounds international efforts to develop evidence-based clinical guidelines. Accordingly, NIH Office of Dietary Supplements (ODS) in collaboration with CDC National Center for Environmental Health (NCEH), National Institute of Standards and Technology (NIST) and Ghent University established the Vitamin D Standardization Program (VDSP) in November 2010. VDSP objectives include: (1) standardize 25(OH)D concentration measurements in national health surveys around the world, (2) evaluate survey differences, (3) extend standardization efforts to assay manufacturers, and to clinical, commercial, and research laboratories, (4) promote standardization of emerging metabolites of vitamin D status, and (5) enable the use of standardized data in patient care and public health. An interlaboratory comparison study is being conducted to assess measurement variability among current assays. Participants include national health surveys from Australia, Canada, Germany, Ireland, Mexico, South Korea, UK and USA, 15 assay manufacturers, and two external quality assurance programs. CDC will implement a formal laboratory certification program. Standardization activities will use single-donor, fresh-frozen serum collected using the CLSI C37 protocol. Initial assay performance criteria, based on biological variability data, are ≤10 % imprecision and ≤5 % bias in relation to the reference values. An ancillary study on commutability of NIST SRM 972a, external quality assurance testing materials is included. To increase the comparability of existing data from different national surveys, master equations will be developed to facilitate the conversion of already existing national survey data to the NIST-Ghent University reference measurement procedures.
Introduction
The wide spread method-related differences in results of total 25-hydroxyvitamin D (25(OH)D) concentration measurements confounds international efforts to develop evidenced-based guidelines [Citation1,Citation2].
Accordingly, the Vitamin D Standardization Program (VDSP) was established in November 2010 with the goal of promoting 25-hydroxyvitamin D (25(OH)D) concentration measurements that are accurate (precise and true) and comparable over time, location, and laboratory procedure to improve clinical and public health practice world-wide.
VDSP is an international effort conducted by the US Office of Dietary Supplements, National Institutes of Health (NIH) in collaboration with the US Centers for Disease Control and Prevention (CDC), National Center for Environmental Health (NCEH), US National Institute of Standards and Technology (NIST) and the Belgian Laboratory for Analytical Chemistry, Faculty of Pharmaceutical Sciences, Ghent University. The initial objective of the VDSP is to standardize 25(OH)D concentration measurements in national health surveys around the world. This report will provide an overview of the VDSP, an explanation of our focus on national health surveys, a description of our standardization procedures, introduce the CDC Vitamin D Certification Program and outline the anticipated schedule through the year 2013.
Overview of the VDSP
Total 25(OH)D concentration is the biomarker of vitamin D status and exposure that is used to develop dietary reference intakes [Citation3]. It is the sum of 25-hydroxyvitamin D2 (25(OH)D2), 25-hydroxyvitamin D3 (25(OH)D3) concentrations. In addition, total 25(OH)D concentration is used as the principal biomarker of vitamin D status in developing clinical guidelines for treating vitamin D insufficiency and deficiency. As a result, it is the focus of public health monitoring and surveillance by national health surveys.
The central role of total 25(OH)D concentration in the assessment of vitamin D status leads to the following VDSP objectives to:
Standardize measured 25(OH)D concentrations in national health surveys to the recently developed NIST-Ghent University reference measurement procedures (RMP) [Citation4,Citation5].
Evaluate differences in measured 25(OH)D concentrations among standardized national health surveys.
Expand standardization services from national surveys to include assay manufacturers and clinical and research laboratories.
Promote the standardization of emerging metabolites of vitamin D status, and
Enable the use of standardized data in patient care and public health activities.
Using the reference measurement procedures recently developed by NIST and Ghent University, we propose to standardize the measurement of the concentrations of total 25(OH)D, 25(OH)D2, 25(OH)D3 and the 3-epi-25-hydroxyvitamin D3 [3-epi-25(OH)D3]. The 3-epi-25(OH)D3 metabolite is of unknown origin, biological value, distribution and clinical significance [3,6–8]. Standardization of 3-epi-25(OH)D3 concentration measurement highlights that, in addition to promoting standardization of total 25(OH)D concentration measurements, a fundamental objective of the VDSP is to conduct research on vitamin D – its metabolism, measures of status and the relationship of its biomarkers of status to clinical and public health endpoints. A further example is that the VDSP will investigate 24–25-dihydroxycholecalciferol [24,25(OH)2D3] as a possible interfering substance in immunoassays and its potential to aid in the assessment of vitamin D status [Citation3,Citation9,Citation10].
To achieve the VDSP goals and objectives, we have designed a program with five components: program coordination, certification program, reference laboratories, national health surveys and international organizations. The Office of Dietary Supplements will provide overall program coordination and support. In addition, it will provide overall scientific direction, coordinate national survey and epidemiology study standardization, and coordinate the VDSP research program. As part of an interagency agreement between the Office of Dietary Supplements and CDC, a Vitamin D Standardization Coordinating Center was established within National Center for Environmental Health.
CDC has more than 50 years of experience in standardization programs dating back to the 1960s [Citation11]. In addition, CDC has extensive experience in developing and maintaining certification programs [12–14]. The CDC Vitamin D Coordinating Center will provide direction on the science of standardization, maintain and manage the certification program, provide logistical support for the VDSP and participate in the VDSP research program.
NIST and Ghent University will provide reference laboratory services for the VDSP. They are both internationally renowned laboratories with extensive experience in developing and maintaining RMPs. RMPs are fundamental to any standardization scheme because these provide a mechanism to evaluate trueness [Citation15,Citation16]. Both laboratories have recently developed RMPs to measure total 25(OH)D, 25(OH)D2, 25(OH)D3 and 3-epi25(OH)D3 concentrations based on LC-MS/MS systems [Citation5,Citation6]. The first RMP was developed by NIST. The RMPs developed by NIST and Ghent University give essentially identical results using slightly different methodology. With these RMPs it is possible to determine concentrations of total 25(OH)D, 25(OH)D2, and 25(OH)D3 in serum or plasma. A prerequisite for determining 25(OH)D3 concentrations is the measurement of the 3-epi-25(OH)D concentration which is not chromatographically resolved from 25(OH)D3 by most routine LC-MS/MS methods. This could result in overestimation of the 25(OH)D3 concentration. Therefore, the reference measurement procedures also produce values for the 3-epi-25(OH)D concentration. The reference laboratories will provide scientific direction on standardization especially the development of new RMPs, participate in the VDSP research program and assist other laboratories in their standardization efforts. For example, NIST conducts a Vitamin D Metabolites Quality Assurance Program [Citation17]. Additionally, NIST, with the support of the Office of Dietary Supplements, has developed standard reference materials (SRM) 972a in human sera and SRM 2972 calibration solutions for 25(OH)D2 and 25(OH)D3 [Citation18].
Eight national health surveys are currently participating in the VDSP (). National surveys from Australia, Canada, Germany, Ireland, Mexico, South Korea, UK and USA are all participating in the program and we hope to recruit more national surveys. The national surveys help to provide scientific direction to the VDSP and participate in the VDSP research program. Also, they help to promote the implementation of measurement procedures within their countries that are traceable to the NIST-Ghent University RMPs.
Table 1. National health and nutrition surveys participating in the Vitamin D Standardization Program (VDSP).
An essential component of this international effort includes collaboration with international organizations. Collaborating with the VDSP are the American Association for Clinical Chemistry (AACC), the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), the College of American Pathologists (CAP) and the Vitamin D External Quality Assessment Scheme (DEQAS). This paper is the outcome of our collaboration with the IFCC and the IFCC's kind invitation to participate in the 13th Bergmeyer Conference: Vitamin D in Health and Disease.
Why Focus on National Health Surveys?
The reason to focus on national health surveys lies in their purpose [Citation19]. National health surveys are instituted to provide government ministries or/agencies with nationally representative data to inform, evaluate & update government policy & regulatory decisions [Citation20]. In addition, they are used to estimate the burden of disease and therefore are useful in planning public health programs and they provide an excellent data source for epidemiological research. Moreover, they provide reference ranges or intervals. As such, national health surveys can have a profound impact on commercially developed laboratory and health assessment procedures. This effect can help to promote standardization.
For example, in developing dietary reference intakes (DRI) for vitamin D, the Institute of Medicine (IOM) DRI committee examined the distribution of total 25(OH)D concentration in Canada and the USA [Citation3,Citation21]. Surprisingly they found that mean total 25(OH)D concentrations in Canada were higher than in the USA consistently across ages 16 - 79 years (). Are those striking results associated with differences in the ethnic and racial compositions of the Canadian and US populations or to differences in fortification policy and supplement use?
Figure 1. Mean Concentrations of Total plasma 25(OH)D (nmol/L) from the 2007–2009 Canadian Health Measures Survey and serum 25(OH)D (nmol/L) from the 2001–2006 National Health and Nutrition Examination Survey by Age Group. [Citation21] and analysis of NHANES public use data sets, by Sempos CT.
![Figure 1. Mean Concentrations of Total plasma 25(OH)D (nmol/L) from the 2007–2009 Canadian Health Measures Survey and serum 25(OH)D (nmol/L) from the 2001–2006 National Health and Nutrition Examination Survey by Age Group. [Citation21] and analysis of NHANES public use data sets, by Sempos CT.](/cms/asset/e8e4c023-2b80-4b78-9657-c65af5935eca/iclb_a_681935_f0001_b.gif)
In the Canadian Health Measures Survey a DiaSorin Liaison TM assay procedure was used while in the US’ National Health and Nutrition Examination Survey a DiaSorin radio-immunoassay was used. An important question is whether differences in methodology lead to differences in the 25-OHD concentrations obtained in the national surveys. None of these questions can be explored until we have standardized measurements of 25(OH)D concentration to compare.
Importance of Standardization
Before we go too far it is important to define what we mean by “standardization” and how it differs from the term “harmonization” [Citation15]. Harmonization is a familiar concept to analytical and clinical chemists. It means to bring the values of all laboratories into line with each other. In “Standardization” that process is taken one step further by bringing laboratories into alignment with the “true” value as measured by an RMP. That is vitamin D standardization leads to harmonization by bringing into line all laboratories methods, in this case, and assuring that they are traceable to the NIST-Ghent University RMPs.
Standardization allows for the development of and consistent application of evidenced-based medicine guidelines [Citation3,Citation15,Citation16]. It is the first and essential step in comparing and contrasting results from different studies whether they are clinical trials or observational epidemiological studies. With standardization it is possible to achieve long-term stability of measurement procedure results used in both research and patient care.
The best example of this sort of success story is for serum total cholesterol [Citation11,Citation12]. In 1958 CDC established the Cooperative Cholesterol Standardization Program (CCSP). The CCSP was directed by the late Dr. Gerald Cooper. One of Dr. Cooper's first efforts was to select a reference method. After a great deal of research he chose the Abell-Kendall method. Then, in 1962, Dr. James Watt, Director of the NIH National Heart Institute, initiated collaboration with CDC which resulted in the expansion of the CDC standardization efforts to include all epidemiological lipid laboratories supported by the National Heart Institute. There is no better example of this landmark effort than the over 50 years of US trend data for serum total cholesterol collected in the National Health and Nutrition Examination Survey or NHANES [Citation22].
Cholesterol standardization was achieved to a certain extent by requiring laboratories to use the same measurement systems. In contrast, the goal of the VDSP is to standardize any and all routine measurement procedures to the RMP. Achieving that goal will require collaboration and cooperation with assay manufacturers. To that end we have enrolled a number of assay manufacturers into the VDSP interlaboratory comparison and commutability studies with 15 different measurement procedures. Collaboration with assay manufacturers, clinical, commercial and research laboratories is an essential focus, therefore, of the VDSP.
By the same token, we are committed to cooperating and collaborating with external quality assessment or performance testing schemes. Members of CAP and DEQAS are scientific consultants to the VDSP and the laboratories of those members are participating in the interlaboratory comparison study. Moreover, performance testing samples from both CAP and DEQAS are part of the ancillary commutability study currently ongoing.
VDSP Standardization Protocols
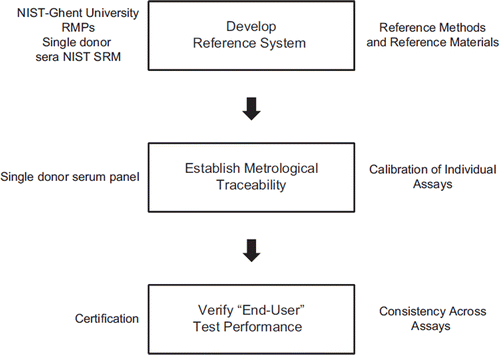
There are two basic protocols: one for current and future measurement procedures and another for standardizing 25(OH)D concentrations from past surveys or studies. The keys to the development of a standardization protocol are the development of a reference system, establishing a metrological chain of traceability from an individual assay to the RMP and then a method for verifying end-user analytical performance (). The reference system includes the RMP, reference materials and the use of single donor sera in the standardization program. It is the set of single donor sera which is the backbone of the system. With this system current and future measurement systems can be standardized to the NIST-Ghent RMP. Using methods developed by the VDSP, those results as well as additional analyses can be used to standardize 25(OH)D concentrations measured in past national health surveys or in past studies of any sort.
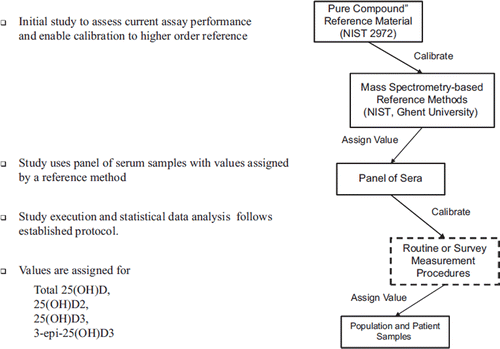
The VDSP reference system includes the NIST-Ghent RMP, the NIST SRMs 972a and 2972 and the single donor serum set (). SRM 2972 is an ethanolic solution of a “pure compound” reference material for the measurement of 25(OH)D2 and 25(OH)D3 concentrations, and could directly be used to calibrate the RMP. Using the RMPs, values for concentrations of total 25(OH)D, 25(OH)D2, 25(OH)D3 and 3-epi-25(OH)D3 were assigned to each of the 50 single donor serum samples for use in the interlaboratory comparison study. Using a protocol to be described below, the single donor serum set was sent by the CDC Vitamin D Standardization Coordinating Center to the reference laboratories and to each participant interlaboratory comparison study for measurement. Results were then sent back to the Coordinating Center for review, comparison to the assigned values, and interpretation. In this way, the VDSP reference system establishes metrological traceability of the current and/or future survey measurement procedures to the hierarchically higher order RMPs and the primary 25(OH)D2 and 25(OH)D3 reference materials. This provides an unbroken chain between the RMPs and the current and/or future survey measurement procedures leading to provisional standardization. Establishing traceability is a higher standard which is possible to achieve in a certification program.
The CDC Vitamin D Certification Program
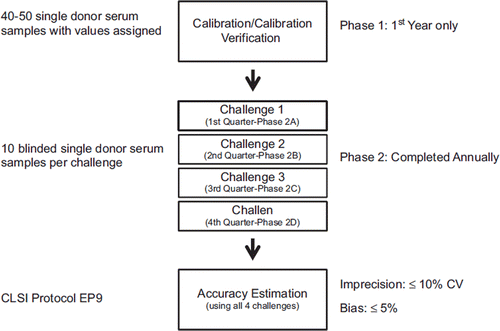
A formal laboratory standardization program will be implemented in 2012 using procedures similar to those used in the CDC Hormone Standardization Program (HoSt Program http://www.cdc.gov/labstandards/hs.html) [Citation13]. The program is used to standardize current and/or future measurement procedures to the RMP. It consists of three phases (). In phase 1 “Calibration/Calibration Verification Phase” the method calibration is verified and recalibrated as necessary using single donor serum samples. For participants of the interlaboratory comparison study, the results from this study can be used for this phase 1 activity. Others can obtain single donor samples from CDC starting fall 2012. Once the assay is calibrated, the participants enters phase 2 which consists of four quarterly challenges using ten blinded single donor serum samples per challenge. At the end of the four challenges, the results from these challenges are combined and analyzed using CLSI 9 protocol [Citation23] to determine the bias and the level of imprecision by the survey laboratory. The results are evaluated to determine if the laboratory in question is meeting the pre-set criteria for imprecision, ≤10 % CV, and accuracy, ≤5 %. These criteria are based on data on biological variability [Citation24] and were reviewed by the VDSP steering committee. Participants meeting these criteria are considered sufficiently accurate and precise and are certified as being standardized to the RMP. Certificates are valid for one year and need to be renewed annually through re-enrollment in phase 2 in the following year. Laboratories that change methods and may need to recalibrate or verify calibration can request samples used in phase 1 at any time during this process.
Figure 4. Centers for Disease Control and Prevention (CDC) Certification Program. Vitamin D Standardization Scheme.
The Office of Dietary Supplements supported the development of the CDC Vitamin D Certification Program for the first year of the VDSP with the interlaboratory comparison study. CDC is expanding its existing standardization programs to include this certification program in 2012. Further details about the program are available from CDC.
VDSP Interlaboratory or Baseline Comparison Study
The VDSP interlaboratory comparison or baseline study can be viewed as the first step along the path towards certification. A set of 50 single donor serum samples was procured from Solomon Park (Seattle, WA, USA) by the CDC Vitamin D Coordinating Center over the summer of 2011. The samples were collected, and prepared following CLSI C37-A guidelines [Citation25]. Based on preliminary measurements performed by the CDC Fat-soluble Vitamins and Nutrients Laboratory the set had a total 25(OH)D concentration interval of approximately 15–145 nmol/L. The concentrations were determined using an LC-MS/MS procedure developed by that laboratory [Citation26]. The single donor serum samples are referred to colloquially as “patient samples”.
In October-November 2011 individual sets were sent out by the Coordinating Center to 36 different participating laboratories. The 36 participating laboratories consisted of the two reference, 8 national survey, 16 assay manufacturers, six commercial, three research/clinical, and two external quality assessment laboratories, one affiliated with CAP and the other with DEQAS. Recruitment of the non-survey laboratories to the interlaboratory comparison study was managed by the CDC Vitamin D Standardization Coordinating Center. The Coordinating Center randomly assigned an ID to each of the patient samples. A run order was also prepared by the Coordinating Center and sent to each of the laboratories.
The value assignment protocol consisted of NIST measuring the 50 patient samples in duplicate on two separate occasions, while Ghent University measured all samples in triplicate on three separate occasions. On each occasion, three independently prepared standard stock solutions were used by both reference laboratories. The set level of imprecision for the RMPs was a CV ≤5 % and a set level of bias ≤1.7 %. The assigned value is the mean of the results from the two reference laboratories.
The protocol for the participating laboratories consisted of analyzing the 50 patient samples in duplicate on three days. It was recommended that a different calibrator lot be used on each of the three days. The initial assay performance criteria for routine measurement procedures are ≤10 % imprecision and ≤5 % bias in relation to the reference values. A comparison of the routine or “survey” and reference laboratory results will be used to develop a set of master equations that can be used to convert routinely measured survey results to the NIST-Ghent University RMPs.
Ancillary Commutability Study
Do reference materials perform like patient samples? That is the fundamental issue surrounding the assessment of commutability [Citation27,Citation28]. For the purpose of this study, commutability is defined as the ‘equivalence of the mathematical relationships between the results of different measurement procedures for a reference material and for representative samples from healthy and diseased individuals’. The commutability of a reference material is measurement procedure specific and its assessment requires special experimental designs.
Reference materials are commonly pooled or otherwise modified. This includes NIST prepared SRMs and materials used in the CAP and DEQAS external quality assessment schemes. Sometimes measurement procedures provide accurate results when used to analyze patient samples but inaccurate results when measuring pooled samples. Pooled samples are generally considered to be non-commutable unless shown otherwise.
Commutability is assessed by analyzing pooled reference materials and routine patient samples using the same study protocol as is used in an interlaboratory comparison study. It is recommended to assess the commutability of pooled reference materials prior to their use as calibrators or trueness controls. However, because of the costs and complexities of assessing commutability formal testing of reference materials is done infrequently.
The VDSP interlaboratory study provided a unique opportunity to test the commutability of NIST's new SRM 972a, 5 CAP and 8 DEQAS reference materials. Eleven laboratories participated in the commutability study – eight laboratories using immunoassay and three laboratories using LC-MS/MS assays. The experiment was designed using CLSI C53-A guidelines [Citation29] which will be used in the analysis of the results.
Standardizing 25(OH)D Values from Past Surveys
Serum total 25(OH)D concentrations have been measured previously in past cycles on national health surveys or in epidemiological studies including clinical trials. The question arises: Can those measurements be standardized to the NIST-Ghent RMPs? The answer is “Yes” given that banked sera from the past surveys are available for re-analysis.
We have developed two options for standardizing values from past surveys.
Option 1 pertains to the surveys that are currently participating in the interlaboratory comparison study. In that case a three step process can be used to standardize past values to the NIST-Ghent RMPs. The three steps are:
Use results from the interlaboratory comparison study to develop a master equation to convert values based on the current measurement procedure to the NIST-Ghent RMPs;
Re-measure a statistically defined subsample of the stored sera from the past survey and develop an equation to convert past values to the current measurement procedure; and
Merge the two sets of equations to form a single equation that is used to convert past survey values to the NIST-Ghent RMPs. A fourth option step would be to send a duplicate set of samples to a certified traceable laboratory for confirmation.
Option 2 also consists of three steps, namely:
Select a higher order laboratory that has been certified traceable to the NIST-Ghent University RMPs, possibly a reference laboratory;
Use a statistically defined sampling procedure to select a small subset of the banked sera for re-measurement by the certified laboratory; and
Develop a mathematical equation to convert the past survey results to the results obtain by the higher order laboratory which is traceable to the NIST-Ghent RMPs.
In addition, we have also developed a statistical algorithm for estimating the number of stored samples that need to be reanalyzed based on the inter-assay coefficient of variation (CV) of the routine measurement procedure. Moreover, we have developed a uniform sampling procedure for selecting the appropriate samples. We are preparing a paper which will report in more detail about the procedures they have developed.
Option 1 is the more cost effective protocol for survey laboratories by eliminating the need for shipping and the costs of contracting with a higher order laboratory. Moreover, some national laboratories are prohibited from shipping participant samples out of the country. As a result, Option 2 is not available to some national health surveys.
Based on , it is clear that there is a great deal of data from past national health surveys which might profit from standardization. Work is underway to standardize results from many of the past surveys conducted in Canada, Germany, Ireland, and the US. Other surveys and epidemiological studies, as resources become available, may choose to standardize data from some of the past surveys. In any event, standardized past survey data will become a great resource for research providing an opportunity to compare 25(OH)D concentrations across time and countries.
VDSP Plans and Schedule through 2013
We are currently in the process of completing the interlaboratory comparison study and the ancillary commutability study. Our plan is to complete those analyses in time to hold a public symposium in March-August of 2013 to report the results. The symposium is scheduled to take place in Bethesda, MD on the NIH campus. Between now and then we hope to recruit more national health surveys into the VDSP. Once results from the interlaboratory comparison study for the eight national surveys becomes available we plan to evaluate the impact of standardization on 25(OH)D distributions and to begin evaluating the survey characteristics that appear to explain differences between and among surveys. Announcements about the VDSP will be made through the Office of Dietary Supplements webpage http://ods.od.nih.gov/Research/VitaminD.aspx, the Federal Register, and professional organizations, e.g. AACC, IFCC, the American Institute of Nutrition, and the Endocrine Society.
The CDC Vitamin D Certification Program is open to assay manufacturers, clinical and commercial, national survey and research laboratories. Enrollment is currently ongoing. Announcements about the program will be made through CDC/VDSP website and professional organizations, e.g. AACC, IFCC and Endocrine Society.
Please do not hesitate to contact us if you have any questions about the VDSP including enrolling in the CDC Vitamin D Certification Program.
Questions and Answers
J Wielders, Netherlands
We all agree there is a need for standardisation, but why choose a mixture of two species as the measurand?
C Sempos
Because both have biological value. If you wish to know a person's vitamin D status, you have to be able to measure their total vitamin D. The immunoassays all measure total as they have antibodies to both 25(OH)D2 and 25(OH)D3 forms. Information from the DQAS scheme indicates the antibodies do not bind to the epimer, so, if you wish to know vitamin D status, you must measure the total vitamin. The ultimate objective is to learn about the concentrations of 25(OH)D2, 25(OH)D3 and the epimer.
R Vieth, Canada
I believe too much emphasis is being placed on the epimer. It does not naturally occur in most adults. It is rare to see in proficiency samples unless they have been spiked. It is of interest to those using MS methods. The compound of interest which large-scale automated analysers are measuring is 24,25,(OH)2D, which has not been mentioned. It is a big interferent.
C Sempos
In general, the epimer concentration is 3–5 % of the total. In some individuals it can be 50–60 % or more. Thienpont has found it in people of all ages. It is important that we learn its source and if it is a contaminant and if it interferes with 25(OH)D metabolism. Secondly, when using MS methods, if we do not correct for the epimer concentration, 25(OH)D concentration will be overestimated. Laboratories around the world are using a wide variety of methods, both immunological and MS methods, so to develop reference guidelines which are independent of time, place and assay methodology, a standardisation programme is absolutely required.
http://informahealthcare.com/doi/abs/10.3109/00365513.2012.681935
Download PDF (35.9 KB)Dedication: To the memory of Dr. Mary Frances Picciano, 1946-2010.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC, the NIH, the US Department of Health and Human Services, NIST, or the US Department of Commerce.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Carter GD. Accuracy of 25-hydroxyvitamin D assays: Confronting the issues. Current Drug Targets 2011;12: 19–28.
- Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: Current procedures, performance characteristics and limitations. Steroids 2010;75:477–488.
- IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC. The National Academies Press2011.
- Tai SS-C, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010;82:1942–1948.
- Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 2011;57:441–448.
- Stepman HCM, Vanderroost A, Stöckl D, Thienpont LM. Full-scan mass spectral evidence fo 3-epi-25-hydroxyvitamin D3 in serum and infants. Clin Chem Lab Med 2011;49: 253–256.
- Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 epimer of 25-hydroxyvitamin D in present in adult serum. J Clin Endocrinol Metab 2012;97:163–168.
- Strathman FG, Sadilkova K, Laha TJ, LeSourd SE, Bornhorst JA, Hoohnagle AN, Jack R. 3-epi-25hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta 2012;413:203–206.
- Wagner D, Hanwell HE, Schnabl K, Yazzanpanah M, Kimball S, Fu L, Sidhorn G, Rouseau D, Cole DEC, Vieth R. The ratio of serum 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J Steroid Biochemistry and Molecular Biology. 2011; 126:72–77.
- Edouard T, Husseini A, Glorieux FH, Rauch F. Serum 24,25-dihydroxyvitamin D concentrations in osteogenesis imperfect: relations to bone parameters. J Clin Endocrin Metab. First published ahead of print as doi:10.1210/jc.2011–3015
- Cooper GR. CDC Cholesterol Standardization Program celebrates 50 years of service. Clinical Laboratory News 2008;34:14.
- Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: A model for standardization and improvement of clinical laboratory measurements. Clin Chem 2000; 46:1762–1772.
- Vesper HW, Botelho JC, Shacklady C, Smith, Myers GL. CDC project on standardizing steroid hormone measurements. Steroids 2008;73:1286–1292.
- Vesper HW, Botelho JC. Standardization of testosterone measurements in humans. J Steriod Biochem Mol Biol 2010;121:513–519.
- W. Greg Miller, Myers GL, Gantzer ML, Kahn SE, Schönbrunner ER, Thienpont LM, Bunk DM, Christenson RH, Eckfeldt JH, Lo SF, Nűbling CM, Sturgeon CM. Roadmap for harmonization of clinical laboratory methods. Clin Chem 2011;57:1108–1117.
- Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem 2009;55:1067–1075.
- NIST Vitamin D Metabolites Quality Assurance Program: http://www.nist.gov/mml/analytical/vitdqap.cfm (accessed on March 21, 2012).
- Phinney, KW, Bedner M, Tai SS, Vamatheven VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Pfeiffer CM, Betz JM, Coates PM, Picciano MF. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem 2012;84:956–962.
- Murphy RS, Michael GA. Methodological considerations of the National Health and Nutrition Examination Survey. Am J Clin Nutr 1982;35:1255–1258.
- Sempos CT, Briefel RR, Flegal KM, Johnson CL, Murphy RS, Woteki CE. Factors involved in selecting a dietary survey methodology for national nutrition surveys. Australian J Nutr Dietet 1992;49:96–101.
- Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Reports 2010;21:47–55.
- Carrol MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, Grundy SM, Johnson CL. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA 2005;294: 1773–1781.
- NCCLS. Method comparison and bias estimation using patient samples; Approved guideline – Second Edition. NCCLS document EP9-A2 (ISBN 1-56238-392–2). NCCLS, Clinical and Laboratory Standards Institute. 950 West Valley Road, Suite 2500 Wayne, PA 19087 USA, 2002.
- Stöckl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chem Acta 2009;408:8–13.
- NCCLS. Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures; approved guidelines. NCCLS document C37-A (ISBN 1-56238-392-2). NCCLS, Clinical and Laboratory Standards Institute. 950 West Valley Road, Suite 2500 Wayne, PA 19087 USA, 1999.
- Chen H, McCoy LF, Schleicher RL, Pfeiffer CM. Measurement of 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2) in human serum using liquid chromatography-tandem mass spectrometry and its comparison to a radioimmunoassay method. Clin Chim Acta 2008;391:6–12.
- Vesper HW, Miller WG, Myers GL. Reference materials and commutability. Clin Biochem rev 2007;28:139–147.
- Miller WG, Jones GRD, Horowitz GL, Weykamp C. Proficiency testing/external quality assessment: Current challenges and future directions. Clin Chem 2011;57:1670–1680.
- Clinical and Laboratory Standards Institute (CLSI). Characterization and qualification of commutable reference materials for laboratory medicine; Approved guideline. CLSI document C53-A (ISBN 1-56238-726-X). Clinical and Laboratory Standards Institute. 950 West Valley Road, Suite 2500 Wayne, PA 19087 USA 2010.