Abstract
Objective. The authors aimed to investigate the incidence and outcomes of acute upper gastrointestinal bleeding (AUGIB) and to examine the role of drugs potentially associated with AUGIB. Methods. The study was prospective, population-based and consisted of all patients who underwent upper gastrointestinal endoscopy (UGE), during the year of 2010 at the National University Hospital of Iceland. Drug intake of NSAIDs, low-dose aspirin (LDA), warfarin, SSRIs and bisphosphonates prior to GIB was prospectively registered and also checked in a Pharmaceutical Database covering all prescriptions in Iceland. An age- and gender-matched control group consisted of patients who underwent UGE during the study period and were without GIB. Results. A total of 1731 patients underwent 2058 UGEs. Overall, 156 patients had AUGIB. The crude incidence for AUGIB was 87/100,000 inhabitants per year. The most common etiologies were duodenal (21%) and gastric ulcers (15%). Use of LDA (40% vs. 30%), NSAIDs (20% vs. 8%), warfarin (15% vs. 7%), combination of NSAIDs + LDA (8% vs. 1%) and SSRIs + LDA (8% vs. 3%) were significantly more common among bleeders than non-bleeders. Three patients (1.9%) had emergency surgery and two patients died of AUGIB. Independent predictors of clinically significant bleeding were gastric ulcer (OR 6.6, p = 0.012) and NSAIDs (OR 6.6, p = 0.004). Conclusions. LDA, NSAIDs and warfarin play an important role in AUGIB etiology and particularly combinations of drugs. Gastric ulcer and NSAIDs were independent predictors of severe bleeding. Mortality and the need for surgery during hospitalization was low in this population-based setting.
Introduction
Acute upper gastrointestinal bleeding (AUGIB) is a common reason for hospitalization, with reported incidence ranging from 36 to 172/100,000 inhabitants per year [Citation1–4,6–14]. Although the incidence of AUGIB has been rather extensively studied, there are only three prospective population-based studies reporting on the incidence in the last decade [Citation5,8,9]. These studies have shown an incidence of 90–108/100,000 inhabitants per year [Citation5,8,9]. The incidence of AUGIB seems to be declining in the western world [Citation6,8,9,14] which has been considered to be due to decreased incidence of Helicobacter pylori (HP) in western countries and an increase in implementing prevention strategies in users of non-steroidal anti-inflammatory drugs (NSAIDs) [Citation3,7–9]. The role of NSAIDs in GI bleeding is well known [Citation15,16] but data on the severity of bleeding related to NSAIDs are limited. The use of low-dose aspirin (LDA) as an anticoagulant is increasing [Citation17] and an association with increased risk of AUGIB [Citation17–19] has been well documented. Data on the incidence of AUGIB among NSAID and warfarin users in a population-based setting are scarce. SSRIs (selective serotonin reuptake inhibitors) seem to be associated with an increased risk of upper gastrointestinal bleeding (UGIB) in some studies [Citation20–23] but the literature is conflicting on the role of SSRIs [Citation24] and bisphosphonates [Citation25–27] in this context. The mortality of AUGIB ranges from 3% to 14% and commonly an all-cause 30-day mortality of 9–14% has been reported [Citation1–4,9,13,28], with lower mortality directly related to bleeding [Citation8,29].
The aims of the current study were to study the incidence of AUGIB in a population-based setting, with a particular focus on the role of drugs in its etiology. Furthermore, the authors aimed to analyze the overall outcome in these patients.
Material and methods
Population and case finding
The study was prospective and included all patients who underwent upper gastrointestinal endoscopy (UGE) and/or colonoscopy at the National University Hospital of Iceland from 1 January 2009 to 31 December 2010. A control group of non-bleeding patients undergoing UGE during the same time period were matched (2:1) with the bleeders for gender and age (±5 years). Definitions are mentioned below.
Acute upper gastrointestinal bleeding
1) Hematemesis or coffee ground vomiting along with presentation to the emergency room, with or without hospitalization, or occurring in a hospitalized patient.
2) Melena along with hospitalization or occurring in a hospitalized patient.
3) Rectal bleeding with a confirmed cause of bleeding on UGE and a negative colonoscopy along with hospitalization or occurring in a hospitalized patient.
Of all those who underwent UGE many individuals were excluded, they can be divided into five groups:
Individuals undergoing UGE for other reasons than UGIB or suspicion of UGIB.
Patients suspected of occult bleeding because of anemia, iron-deficiency anemia and/or positive hemoccult test with no diagnosis on endoscopy.
Patients with melena and no signs of bleeding revealed in UGE and colonoscopy.
Individuals who had overt GIB but were not hospitalized.
Patients with occult bleeding with a confirmed cause of bleeding.
In the calculation of incidence of AUGIB, individuals who did not have a registered home in the greater metropolitan area of Reykjavík were excluded, as the National University Hospital of Iceland is bound to serve that population. Information about the population was provided by the Office for National Statistics in Iceland [Citation30].
Data collection
Before the endoscopic procedure, the gastroenterologist noted the indication for the endoscopy and its findings were recorded in a prospective fashion and later verified with analysis of medical records. The gastroenterologists recorded the indication for the UGE, whether or not GI bleeding was suspected or present and whether or not the bleeding was clinically significant. Bleeding was considered clinically significant if the patients needed blood transfusions (hemoglobin (Hb) < 100 g/l), became hemodynamically unstable (pulse > 100, systolic pressure < 100), were admitted to the intensive care unit, required surgery or died. Other variables noted were: co-morbidities, history of GI bleeding, signs of bleeding during UGE, extent and findings of the endoscopy. Nurses in the endoscopy ward interviewed the subjects thoroughly before the endoscopy regarding their history of drug use. The drugs recorded were the following: NSAIDs, LDA, warfarin, SSRIs, bisphosphonate drugs, platelet inhibitors, low-molecular-weight heparin (LMWH), heparin, corticosteroids and proton pump inhibitors (PPIs).
The use of these drugs needed to be on a regular basis and not only on demand. NSAID users were defined as patients who used NSAIDs daily for a minimum of 5 days.
Laboratory values were collected and were as follows: Hb (g/l), platelet counts, creatinine (µmol/l), prothrombin time (PT, s) and International Normalized Ratio (INR) value. Data on blood transfusions were provided by the Blood Bank of Reykjavík, which provides blood products to the National University Hospital. All information on transfusions is stored in an electronic information system (Prosang, Databyran, Sweden) and full traceability is ensured.
In order to explore the role of drugs in the etiology of UGIB, each patient with UGIB was matched for gender and age (±5 years) with two patients who underwent UGE at the same institution during the same time period without being suspected of or having UGIB. Controls were matched with the bleeders after collection of data from all these patients. The authors were able to match patients two-to-one and there was no selection of non-bleeding patients matched.
The Icelandic Medicines Registry records all prescriptions issued outside of hospitals and nursing homes in Iceland and is run by the Directorate of Health in Iceland. It has been operated since 1 January 2006 but contains data from 2002 and onward, about 2,300,000 registrations are added to the database annually. To further improve the reliability of the drug history, records from the Icelandic Medicines Registry were examined. These records contained information on every prescription issued for the individuals participating in the study from 1 January 2009 until 31 December 2010.
The Icelandic Medicines Agency (IMA) is a governmental agency under the Ministry of Health and Social Securities. IMA holds information on the total amount of every individual drug sold by every wholesaler to every pharmacy, nursing home and hospital in Iceland. Information on the use or sale of prescribed drugs in these institutions was also accessible. These data were accessed and examined by the researchers. The IMA staff was also consulted. For this study, IMA provided information on the amount of LDA sold by wholesalers in the greater metropolitan area of Reykjavík in 2010. A total of 5,204,600 defined daily doses (DDD) of LDA were sold. By dividing 5,204,600 by 365 it was found out that 14,259 DDD were used per day. It was assumed that these 14,259 DDD represented the number of individuals using LDA on a regular basis. The number of individuals using LDA in the AUGIB cohort (they also had to have residency in the greater metropolitan area) was then divided by 14,259, the outcome being the incidence of AUGIB among LDA users. Warfarin is only available by prescription and therefore the IMA was able to provide data on how many individuals were treated with warfarin in hospitals, nursing homes or had a prescription for warfarin. Patients who did not have residency in the greater metropolitan area were excluded in the incidence calculations. The same assumptions and calculations were applied in the analysis of AUGIB incidence among warfarin users.
Every individual medical record was reviewed and all data were verified.
This study was approved by the Data Protection Authority of Iceland and the Bioethics Committee of Iceland.
Statistics
All data were processed in Microsoft Office Excel 2010® and IBM SPSS statistics®. The χ² goodness-of-fit test, the Fisher's exact test and the Freeman–Halton test were used to test differences between groups regarding dichotomous variables. Unpaired Mann–Whitney U test and the Kruskal–Wallis test were used to compare continuous variables. Variables with a significant p-value in the univariate analysis were entered into a multiple logistic regression analyses in an attempt to identify independent predictors of having an AUGIB and clinically significant bleeding. All tests were two-tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile range (IQR) or means and standard deviation (SD).
Results
A total of 1731 individuals underwent UGE during the study period and 482/1731 (22.8%) had a suspicion of, or confirmed GI bleeding (). In all, 249 (14.4%) of those 1731 had confirmed UGIB and 156 patients met the inclusion criteria for AUGIB (). Of the 156 bleeders, 71% were hospitalized due to bleeding, 24% were already hospitalized and 5% presented to the emergency room but were not hospitalized. The crude incidence of AUGIB was 87/100,000 inhabitants per year, with dramatic increase with increasing age (). The AUGIB cohort consisted predominantly of males, 58% vs. 42% females (p = 0.0547), with a median age of 71 (IQR 55.8–80.3). The controls had a median age of 71 (IQR 55.8–80.3), males were 58%. In all, 15% had a history of GI bleeding and 90% anemia at baseline. Further information on laboratory parameters can be found in Supplementary Table I. The most common primary indication for AUGIB was hematemesis or coffee ground vomiting (61%) followed by melena (37%) and rectal bleeding (1.9%). Peptic ulcer was the most common diagnosis (40%) with duodenal ulcer being more common than gastric ulcer (), whereas 4.5% had both gastric and duodenal ulcer (). Other common diagnoses were Mallory–Weiss syndrome (12.2%) and esophagitis (9.6%), vascular ectasia and esophageal varices were relatively uncommon or 5.1% and 3.8% of all diagnoses, respectively (). The cause of AUGIB was not identified during UGE in 6.4% of the patients. Overall, 62 patients had peptic ulcer disease (). In 44 of those patients, a CLO test (Campylobacter-like organism test) was performed. Overall, 12 (32%) patients had a positive CLO test. Patients diagnosed with gastric and duodenal ulcer were positive in 20% and 41% of the cases, respectively.
Figure 1. A flow chart showing patients who were included in the study and the patient groups that were excluded. UGE = upper gastrointestinal gastroscopy; AUGIB = acute upper gastrointestinal bleeding.
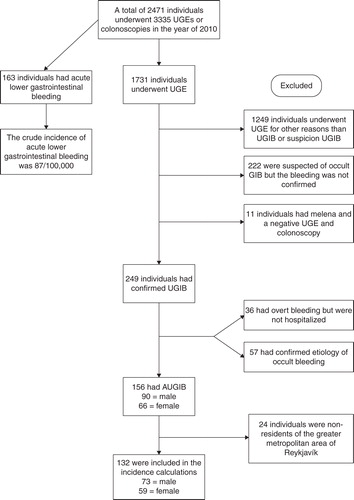
Table I. The crude annual incidence of AUGIB is illustrated as well as age standardized incidence rates for certain age groups.
Table II. The diagnoses found in the total study cohort.
Bleeders were significantly more likely than controls to use NSAIDs, LDA and warfarin, as well as the combination of LDA with NSAID or SSRIs (). Among those patients who had peptic ulcer, 14/62 (23%) were on NSAIDS versus 24/312 (8%) in controls (p = 0.0018) and similarly 33/62 (53%) versus 93/312 (30%), among controls (p = 0.0006) were on LDA, respectively. In a logistic regression analysis, use of the following drugs was independently related to bleeders: NSAIDs (odds ratio (OR) 2.086, 95% confidence interval (CI) 1.1–4.1, p = 0.034), warfarin (OR 2.441, 95% CI 1.3–4.6, p = 0.006) and combination of LDA and SSRI (OR 2.840, 95% CI 1.1–7.1, p = 0.026).
Table III. The use of drugs considered a priori to be potentially related to increased risk of GI bleeding.
A comparison of clinically and non-clinically significant bleeding revealed that gastric ulcers were more common in the former group (). Interestingly, the use of NSAIDs and combined use of NSAIDs and LDA were associated with more severe bleeding (). Regarding H. pylori, 75% (9/12) of patients positive for H. pylori had a clinically significant bleeding versus 69% (22/32) of patients negative for H. pylori (p = NS). In a stepwise multiple logistic regression with clinically significant bleeding as the dependent variable, the following variables were found to be independent predictors of clinically significant bleeding: gastric ulcer (OR 6.566, 95% CI 1.508–28.588, p = 0.012) and NSAIDs (OR 6.610, 95% CI 1.821–23.991, p = 0.004).
Table IV. Comparison between patients with clinically significant bleeding and patients with non-clinically significant bleeding.
The Rockall score [Citation31] was calculated for the bleeders, 22 (14%) individuals had a low-risk score (0–2), 121 (78%) had a score of 3–7 and 13 (8%) a high-risk score (8 or more). An analysis of the Rockall score revealed a statistical difference in hemoglobin (120 vs. 92 vs. 78 g/l, p < 0.0001), requirement of blood transfusion (9% vs. 65% vs. 92%, p < 0.0001) and clinically significant bleeding (32% vs. 72% vs. 85%, p = 0.000426) between the low-, intermediate- and high-risk groups, respectively.
In Iceland, LDA was the most sold drug in 2010 with 8 million DDDs sold from wholesalers [Citation32]. The incidence of AUGIB among LDA and warfarin users was 371/100,000 and 1429/100,000 inhabitants per year, respectively. Of the 14,259 individuals in the greater metropolitan area treated with LDA, 53 had a bleeding episode and therefore 1 in every 269 patients treated experienced AUGIB. In all, 21 of the 1466 treated with warfarin had a bleeding episode, meaning 1 in every 70 individuals treated experienced AUGIB. Of patients using NSAIDs and LDA, 68% and 63% of the cases purchased the drugs over the counter, respectively. Approximately 60% of the AUGIB cohort required blood transfusion and most patients required 2–4 units of blood with a median value of 4 (IQR 2–5). Of the 93 patients requiring blood transfusion, 45 (48.4%) had peptic ulcer disease, only 5.4% had Mallory–Weiss syndrome and 7.5% had esophagitis. Almost all patients were diagnosed with the first UGE (94.4%) and signs of active or recent bleeding were present in 36%. A total of 37 (24%) patients required endoscopic hemostasis, the most common reason being peptic ulcer disease (57%), followed by esophageal varices (16%) and vascular ectasia (14%). Further information on endoscopic hemostasis is available in Supplementary Table II.
Only three patients (1.9%) underwent urgent surgery. Two patients had an AUGIB-related death (1.3%). One (M77) patient with end-stage obstructive pulmonary disease had three bleeding gastric ulcers and the bleeding caused a cardiac arrest (). The second patient (M57) had an incarcerated hernia which resulted in abdominal compartment syndrome and ischemia of the stomach and small bowel (). Out of the two patients who were successfully surgically treated, one patient had a perforated gastric ulcer (M31) whereas the other had a bleeding duodenal ulcer (M58) ().
Table V. General data on patients undergoing acute surgery or had an AUGIB-related death.
Discussion
In this prospective population-based study, the incidence for AUGIB was 87/100,000 inhabitants per year, which is similar to recent prospective studies. AUGIB was more prevalent among men and older people. The most common etiologies for AUGIB were peptic ulcer, Mallory–Weiss syndrome and esophagitis. LDA, NSAIDs and warfarin play an important role in AUGIB, and NSAIDs might increase the risk of severe GI bleeding. One in every 70 warfarin users, and 1 in every 269 users of LDA experienced AUGIB, respectively. Only three (1.9%) patients had to undergo surgery and mortality was very low.
The previously reported incidence of AUGIB has varied greatly, from 36 to 172/100,000 [Citation1–4,6–14]. Partly, this might be explained by the retrospective nature of some of these studies. It has been demonstrated that case finding of GI bleeders with a prospective method was clearly superior to case finding based on ICD codes [Citation1]. Recently, a study from the Netherlands has confirmed this [Citation33]. Socioeconomic background has been found to be important in some studies as the most socially deprived were two times more likely than the least socially deprived to have an UGIB [Citation2,4], which might play a part in the explanation for the varying incidence. The incidence of AUGIB is decreasing in the western world according to most time-trend analyses [Citation3,5,7–9] but not all [Citation4]. This is most likely related to a decrease in the prevalence of peptic ulcer disease, due to decreased prevalence of H. pylori and increased use of PPIs along with NSAIDs [Citation3,7–9]. All of these time-trend analyzing studies had their former time period in the 80s or 90s and the latter time period after 2000 [Citation3,7–9]. In the last 10 years, only three well-designed prospective population-based studies have analyzed the incidence of AUGIB. The incidence was found to have a narrow range of 90–108/100,000 inhabitants per year [Citation5,8,9]. The results of 87/100,000 are remarkably similar to other population-based studies. Given that the incidence has been similar in these four prospective studies performed in the last decade, it is a relevant question whether AUGIB incidence has reached its plateau after the decrease in incidence in recent decades.
In the current study, peptic ulcer disease was the most common cause of bleeding (in approximately 40% of cases) which is in agreement with most studies where the proportion of peptic ulcer disease as a cause of bleeding has been reported to be 22–67% [Citation1,3–7,9–13,28,29,34]. In all studies except one [Citation34], duodenal ulcers were more common than gastric ulcers. The proportion of esophagitis as a cause of bleeding had a wide range of occurrence (1.3–17.5%) in previous studies [Citation1,2,4,13,28,29], and the approximately 10% of patients with bleeding esophagitis in the current study is in line with those results. In only 6.4% of patients, a cause of bleeding was not identified, which is lower than in most [Citation1–3,6,28,34] but not all previous studies [Citation9,13].
The association between the use of LDA and AUGIB is well known [Citation17–19] and the current study confirms previous findings. However, the incidence of AUGIB in LDA users is, to the authors' knowledge, unknown. AUGIB in LDA was found to occur in 1 of every 269 users. The incidence was 371/100,000 which is 4 times the incidence in the general population and 1.8 times higher than in the age group 60–79 years (), but the mean age for LDA users was 72 years (±13.1). Thus, it seems that LDA also increases the risk of AUGIB in a population-based setting. A larger portion of the bleeders were using warfarin than non-bleeders. Most case–control studies evaluating the risk of AUGIB during warfarin therapy have a cohort of individuals who have atrial fibrillation, have undergone coronary stenting or have had a heart valve replacement. Case–control population-based studies that cover the general population are largely lacking. One population-based retrospective case–control study performed in the UK demonstrated an increased risk of AUGIB for individuals using warfarin [Citation35]. The incidence of AUGIB in warfarin users was 1429/100,000 in the current study, which is 16 times higher than in the general population and 6.7 times higher than in the age group 60–79 years (), but the mean age for warfarin users was 76 years (±7.1). Therefore, the authors concluded that warfarin might increase the risk of AUGIB in the population-based setting.
The authors' methodology by which the incidence of AUGIB in drug users was obtained is not without flaws. There are at least three factors that might have confounded the results: 1) the authors were uncertain that all of the DDD in pharmacies, nursing homes or hospitals were sold or administered. However, LDA is the most sold drug in Iceland and although there might have been remnants in stock at the end of 2010, there should also have been remnants in stock from 2009 to the beginning of 2010. This point does not apply to warfarin since the amount of DDD was obtained by serviced prescriptions and not by wholesale; 2) pharmacies in the greater metropolitan area tend to be cheaper than the ones outside it, which means that some amount of DDD might have been sold to individuals with residency outside the greater metropolitan area. This point does not apply to warfarin since the authors were able to exclude patients with residency outside of the greater metropolitan area in the warfarin calculations. They found that approximately 10% of individuals buying warfarin in the metropolitan area did not have residency in the greater metropolitan area; 3) some individuals will use the drug on a contemporary basis. Factors 1) and 2) make the incidence seem higher than it should be, factor 3) makes the incidence seem lower than it should be. Therefore, it cannot be ruled out that the incidence of AUGIB is in reality lower in LDA users than the authors reported and higher for warfarin users.
NSAIDs are the most well-documented drugs associated with AUGIB [Citation16,36,37] and the results also confirm that well-known association. Notably, the use of NSAIDs was associated with a clinically severe bleeding. To the authors' knowledge, previous studies have not analyzed the association with markers of severity of bleeding although studies have compared NSAID users with non-NSAID users. Wilcox and Clark showed that NSAID users were less likely than non-NSAID users to be subjects to re-bleeding or death and also had a shorter hospital stay [Citation38]. This study results are not in accordance with this, but more studies are needed to show whether NSAID users are at risk of more severe GI bleeding.
The literature concerning SSRIs and the risk of AUGIB shows conflicting results. There are data to support increased risk of AUGIB associated with the use of SSRIs in some [Citation20–23] but not all studies [Citation24]. It is possible that SSRIs introduce a modest risk for AUGIB [Citation21,39] but the study results do not support that notion. In the current study, the authors found an increased risk of AUGIB in combination with LDA, as previous studies have confirmed [Citation20,40,41].
Endoscopic hemostasis was undertaken in 24% of the patients which is lower than in previous studies [Citation8,29]. A potential explanation might be that in these two studies peptic ulcer disease was the cause of bleeding in 53% of cases, whereas it accounted only for 40% in the current study. The population-based nature of the current study may also be a partial explanation. Urgent surgery for AUGIB used to be common, but the need for acute surgery has steadily been decreasing in the last decades [Citation13]. The need for acute surgery in this study was only 1.9%, which is the lowest proportion reported to date, but similar to recent figures reported by Hearnshaw et al. from the UK [Citation28]. The administration of blood transfusion was very similar to several other studies [Citation5,8,29] but higher than in some [Citation11,28]. A relatively high mortality rate for AUGIB is usually reported ranging from 9% to 14% [Citation1–4,8,28], which may be explained by the fact that mortality in these studies was defined as all-cause 30-day mortality. If deaths, only associated with bleeding are included in the calculations, then the mortality rate amounts to 2.3–2.6% [Citation8,29], which is similar to the 1.3% proportion reported here.
The main limitation of the current study is lack of power, especially regarding drug intake. However, this limitation is ameliorated by a very thorough drug history. First, patients were interviewed regarding drug intake, second, their medical records were reviewed and third, information was obtained from a Pharmaceutical Database holding information on every prescription made in Iceland from 2002 onward. Another limitation of this study is that individuals with AUGIB might have died without undergoing endoscopy, although this is rare. The authors were uncertain that the mortality reported by them might in fact be higher. The main strengths of this study are its prospective and population-based design.
In conclusion, peptic ulcers remain the most common cause of AUGIB with duodenal ulcers being more common than gastric ulcers. The incidence of 87/100,000 inhabitants per year is similar to recent prospective studies which might indicate that AUGIB incidence has reached its plateau. AUGIB remains a common reason for hospitalization but the risk of surgery or death is low. NSAIDs and LDA are important factors for AUGIB. Warfarin, as well as SSRIs combination with LDA or warfarin, seem to have a role in AUGIB while SSRIs alone and bisphosphonates do not seem to increase risk of AUGIB.
Supplementary Material
Download MS Word (60.5 KB)Acknowledgements
The authors would like to thank Magdalena Sigurðardottir RN, Elisabet Lilja Haraldsdottir secretary and the doctors and nurses at the Endoscopic Department at the National University Hospital of Iceland. The authors would also like to thank Runar Gudlaugsson Msc.pharm. at the Icelandic Medicines Agency. This study was supported with a grant from the National University Hospital of Iceland Research Fund.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995;311:222–6.
- Blatchford O, Davidson LA, Murray WR, Blatchford M, Pell J. Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ 1997;315:510–14.
- van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol 2003;98:1494–9.
- Button LA, Roberts SE, Evans PA, Goldacre MJ, Akbari A, Dsilva R, Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment Pharmacol Ther 2011;33:64–76.
- Paspatis GA, Konstantinidis K, Chalkiadakis I, Tribonias G, Chlouverakis G, Roussomoustakaki M. Changing trends in acute upper gastrointestinal bleeding in Crete, Greece: a population-based study. Eur J Gastroenterol Hepatol 2012;24:102–3.
- Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1995;90:206–10.
- Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol 2009;104:1633–41.
- Loperfido S, Baldo V, Piovesana E, Bellina L, Rossi K, Groppo M, Changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc 2009;70:212–24.
- Theocharis GJ, Thomopoulos KC, Sakellaropoulos G, Katsakoulis E, Nikolopoulou V. Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J Clin Gastroenterol 2008;42:128–33.
- Paspatis GA, Matrella E, Kapsoritakis A, Leontithis C, Papanikolaou N, Chlouverakis GJ, An epidemiological study of acute upper gastrointestinal bleeding in Crete, Greece. Eur J Gastroenterol Hepatol 2000;12:1215–20.
- Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 1995;90:568–73.
- Czernichow P, Hochain P, Nousbaum JB, Raymond JM, Rudelli A, Dupas JL, Epidemiology and course of acute upper gastro-intestinal haemorrhage in four French geographical areas. Eur J Gastroenterol Hepatol 2000;12:175–81.
- Thomopoulos KC, Vagenas KA, Vagianos CE, Margaritis VG, Blikas AP, Katsakoulis EC, Changes in aetiology and clinical outcome of acute upper gastrointestinal bleeding during the last 15 years. Eur J Gastroenterol Hepatol 2004;16:177–82.
- Ahsberg K, Hoglund P, Kim WH, von Holstein CS. Impact of aspirin, NSAIDs, warfarin, corticosteroids and SSRIs on the site and outcome of non-variceal upper and lower gastrointestinal bleeding. Scand J Gastroenterol 2010;45:1404–15.
- Hernandez-Diaz S, Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000;160:2093–9.
- Wilcox CM, Alexander LN, Cotsonis GA, Clark WS. Nonsteroidal antiinflammatory drugs are associated with both upper and lower gastrointestinal bleeding. Dig Dis Sci 1997;42:990–7.
- Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population-based case-control study. BMJ 2006;333:726.
- Garcia Rodriguez LA, Lin KJ, Hernandez-Diaz S, Johansson S. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation 2011;123:1108–15.
- Sorensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK, Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol 2000;95:2218–24.
- de Abajo FJ, Garcia-Rodriguez LA. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry 2008;65:795–803.
- Targownik LE, Bolton JM, Metge CJ, Leung S, Sareen J. Selective serotonin reuptake inhibitors are associated with a modest increase in the risk of upper gastrointestinal bleeding. Am J Gastroenterol 2009;104:1475–82.
- Tata LJ, Fortun PJ, Hubbard RB, Smeeth L, Hawkey CJ, Smith CJ, Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding? Aliment Pharmacol Ther 2005;22:175–81.
- Wessinger S, Kaplan M, Choi L, Williams M, Lau C, Sharp L, Increased use of selective serotonin reuptake inhibitors in patients admitted with gastrointestinal haemorrhage: a multicentre retrospective analysis. Aliment Pharmacol Ther 2006;23:937–44.
- Barbui C, Andretta M, De Vitis G, Rossi E, D'Arienzo F, Mezzalira L, Antidepressant drug prescription and risk of abnormal bleeding: a case-control study. J Clin Psychopharmacol 2009;29:33–8.
- Etminan M, Levesque L, Fitzgerald JM, Brophy JM. Risk of upper gastrointestinal bleeding with oral bisphosphonates and non steroidal anti-inflammatory drugs: a case-control study. Aliment Pharmacol Ther 2009;29:1188–92.
- Donahue JG, Chan KA, Andrade SE, Beck A, Boles M, Buist DS, Gastric and duodenal safety of daily alendronate. Arch Intern Med 2002;162:936–42.
- Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 2000;160:517–25.
- Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011;60:1327–35.
- Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol 1997;92:924–8.
- Hagstofa Íslands (Statistics Iceland). Available at http://hagstofa.is/Hagtolur/Mannfjoldi.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996;38:316–21.
- Icelandic Medicines Agency (Lyfjastofnun). Available at http://www.imca.is/imca/statistics/2012.
- Valkhoff VE, Coloma P, Mosseveld M, Kuipers EJ, Sturkenboom MC, Trifiro G. Su1803 validation of the diagnosis for upper gastrointestinal bleeding in a Dutch medical record database. Gastroenterology 2012;142:S-507.
- Enestvedt BK, Gralnek IM, Mattek N, Lieberman DA, Eisen G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc 2008;67:422–9.
- Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug–drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ 2007;177:347–51.
- Carson JL, Strom BL, Soper KA, West SL, Morse ML. The association of nonsteroidal anti-inflammatory drugs with upper gastrointestinal tract bleeding. Arch Intern Med 1987;147:85–8.
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol 2010;24:121–32.
- Wilcox CM, Clark WS. Association of nonsteroidal antiinflammatory drugs with outcome in upper and lower gastrointestinal bleeding. Dig Dis Sci 1997;42:985–9.
- Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry 2010;71:1565–75.
- van Walraven C, Mamdani MM, Wells PS, Williams JI. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ 2001;323:655–8.
- Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med 2003;163:59–64.
