Abstract
Objective. The first objective of the present study was to identify opportunities of improvement for clinical practice, assessed by local quality indicators, then to analyze possible reasons why we did not reach defined treatment quality measures. The second objective was to characterize patients, considered unresectable according to present criteria, for future arrangement of interventional studies with improved patient selection. Material and methods. Prospective observational cohort study from October 2008 to December 2010 of patients referred to the authors' institution with suspected pancreatic or periampullary neoplasm. Results. Of 330 patients, 135 underwent surgery, 195 did not, 129 due to unresectable malignancies. The rest had benign lesions. Perioperative morbidity rate was 32.6%, mortality 0.7%. Radical resection (R0) was obtained in 23 (41.8%) of 55 patients operated for pancreatic adenocarcinoma and 6.3% underwent reconstructive vascular surgery. Diagnostic failure/delay resulted in unresectable carcinoma, primarily misconceived as serous cystic adenoma in two patients. One resected lesion turned out to be focal autoimmune pancreatitis. One case with misdiagnosed cancer was revised to be a pseudoaneurysm. Palliative treatment was offered to 144 patients with malignant tumors, 62 due to locally advanced disease and all pancreatic adenocarcinomas. Conclusions. Quality improvement opportunities were identified for patient selection and surgical technique: Too few patients underwent reconstructive vascular surgery. The most important quality indicators are those securing resectional, radical (R0) surgery. Altogether 143 patients (57.9%) of those with malignant tumors were found unresectable, most of these patients are eligible for inclusion in future interventional studies with curative and/or palliative intention.
Introduction
Pancreatic and periampullary malignancies represent specific diagnostic and therapeutic challenges and surgery is still the only treatment with curative potential. Despite this fact, surgical treatment is underutilized in the USA. The National Cancer Data Base (NCDB) recently reported that 38.2% of patients with early stage pancreatic cancer without any identifiable contraindications, failed to undergo surgery [Citation1]. Considerable variability in outcome exists among hospitals performing pancreatectomies [Citation2], but it is difficult to identify the factors responsible for this variation. The lack of standardized examination of resected specimens has been one important confounder of outcome evaluation, until a systematic, detailed technique for handling and evaluation of resected specimens with coloring of the resectional margins was introduced [Citation3]. The International Study Group for Pancreatic Surgery (ISGPS) has developed objective, universally accepted definitions for complications after pancreatic surgery [Citation4–6]. This cohort study was initiated after the implementation of all these standards. The first objective was to identify opportunities of improvement of the clinical practice, assessed by local quality indicators, then to analyze possible reasons why we did not reach defined treatment quality measures. The diagnostic and therapeutic algorithm was critically assessed, focusing on clinical consequences of surgery without preoperative histological diagnosis. Also intraoperative strategy and technique were critically assessed, with rate of R0 resections as end point. The performance of the authors' institution was finally evaluated against expert validated quality indicators [Citation7], which has been developed by the American College of Surgeons' Pancreatic Cancer Quality Development (ACSPCQD).
The second objective was to characterize patients, considered unresectable according to present criteria, for future arrangement of interventional studies with improved patient selection. The concept that neoadjuvant chemoradiation might improve overall survival in patients with marginally respectable [Citation8], or even clearly resectable pancreatic adenocarcinoma [Citation9], is supported by several authors. But the benefit of an R1 resection, compared with no resection, also seems underestimated [Citation10], and different treatment algorithms need prospective investigation.
Material and methods
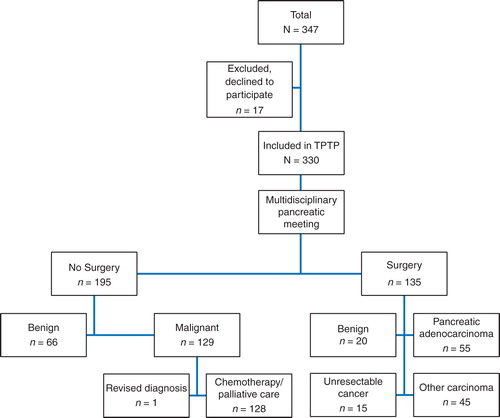
In October 2008, Oslo University Hospital, Ullevaal (a tertiary referral center) established a multidisciplinary research program for patients undergoing investigation for a solid or cystic pancreatic or periampullary neoplasm. The program was approved by the Regional Ethical Committee for registration of clinical data in addition to sampling of blood, tumor tissue, cyst fluid and bone marrow for research purposes. The authors present clinical data for 330 consecutive patients (), referred for evaluation by the multidisciplinary team. Data were prospectively collected from October 2008 to December 2010 after signed informed consent. Seventeen patients did not sign the consent and were excluded.
Figure 1. Diagnostic and therapeutic flowchart for patients with pancreatic and periampullary tumors (n = 330) evaluated at the multidisciplinary conference.
Diagnostic and therapeutic algorithm
Standard evaluation included physical examination, routine laboratory tests, tumor markers (CEA, CA 19-9, chromogranin A) and contrast-enhanced helical computed tomography (CT) of the abdomen and thorax in three vascular phases. Tumors with infiltration of the celiac trunk or superior mesenteric artery and metastatic disease were considered unresectable [Citation11]. Tumors with infiltration of the superior mesenteric vein (SMV) or portal vein (PV) were considered resectable if there was patent vein above and below the occlusion to allow interposition grafting [Citation12–14]. Tumors were never considered borderline resectable, as international definitions of this concept were published during the inclusion period. Patients underwent laparotomy when presumed operable, the others referred for palliative chemotherapy, without later reassessment of resectability. Preoperative percutaneous biopsy/cytology was not performed in resectable patients because of the risk of tumor seeding [Citation15]. Patients with cystic lesions underwent endoscopic ultrasound (EUS), and if possible, aspiration of cystic fluid for cytology and analyses of mucin, CEA and amylase.
All patients were presented at the weekly multidisciplinary pancreatic conference, attended by radiologist, hepato-pancreato-biliary (HPB) surgeon, gastroenterologist and oncologist. Neoplasms in the body or tail were preferably resected laparoscopically, lesions in the head with open pylorus preserving pancreaticoduodenectomy (PPPD). The relationship between the resection plane and the tumor was guided by intraoperative ultrasound in addition to direct vision during all laparoscopic procedures [Citation16]. Intraoperative evaluation of indication/surgical approach for vascular resection/reconstruction was done in co-operation with the liver transplantation team or vascular surgeons. The pancreatic resectional margin was investigated intraoperatively by frozen sections. Total pancreatectomy was chosen only when mandatory to achieve negative resectional margins. Histological confirmation of the lesion was secured from the resected specimen in all operated patients. For those not operated, EUS or percutaneous biopsy/cytology was recommended in all cases, but some did not accept it. After documentation of malignant cytology or histology, unresectable patients were referred to the Department of Oncology for palliative chemotherapy and/or radiotherapy. Patients with pancreatic adenocarcinoma who underwent curative surgery, received adjuvant chemotherapy (leucovorin every second week for 6 months, according to the Nordic FLV regimen). Follow-up was performed with CT scan of the chest and abdomen every sixth month or if the patient had symptoms suspected of a recurrence.
TNM classification and assessment of surgical radicality
All tumors were classified according to the TNM criteria of the AJCC (American Joint Committee on Cancer) 2010 cancer system [Citation17]. The margin resection status (R-status) was defined according to the revised pathological specimen evaluation with inking of resection margins and R1 defined as a distance of the tumor from the resection margin of < 1 mm. The pathologist participated in the surgical procedure, marking every plane of resection, thereafter sectioning the specimen for final assessment of R-status.
Complications
Complications during the preoperative investigation in the authors' hospital or after surgery were recorded. ISGPS definitions of postoperative fistula (grade A–C) [Citation4], delayed gastric emptying [Citation5] and postoperative hemorrhage [Citation6] were used. Grade C fistulas from the pancreaticojejunal anastomosis were treated by resection of the pancreatic remnant.
Local guidelines/quality measures
The diagnostic part of the algorithm included all guidelines described above: the whole patient cohort of patients with pancreatic and periampullary tumors underwent preoperative investigation, aiming at surgical resection as fast as possible, without percutaneous biopsy/cytology [Citation18], and without neoadjuvant chemoradiation. The positive prediction of resectability, based on preoperative radiology, was required in at least 85%, based on previous experience and literature [Citation19,20]. The maximum accepted interval from baseline to start of surgical treatment was set to 3 weeks, and maximum 4 weeks were accepted for investigation in advance of baseline.
Formally developed quality indicators
During the inclusion period, a set of 43 quality indicators was published, developed and validated by the ACSPCQD Expert Panel [Citation7]. These have been compared with the authors' local quality indicators and utilized to assess the performance of their institution. Finally, rating of clinical relevance and importance of all quality indicators has been performed, based on the present outcome data.
Statistics
Values are given as median and range. Survival curves were illustrated as Kaplan–Meier plots. Survival differences between groups were analyzed with the Log rank test. The level of significance was set at p < 0.05.
Results
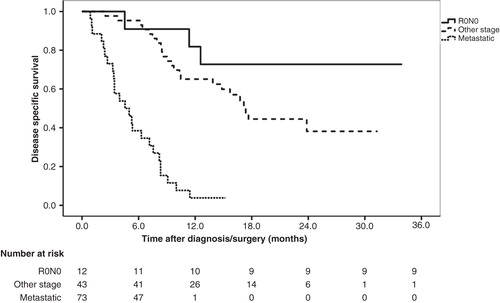
The study population consisted of 330 consecutive patients seen in the multidisciplinary clinic, 165 (50%) men, 165 (50%) women, median age 66 years (range 21–86 years). In 135 patients (41%), a surgical procedure was performed. Resection of a malignant tumor was done in 100 patients, 20 (14.8%) underwent pancreatic resection of a benign lesion and 15 (11.1%) patients were found unresectable at laparotomy due to unrecognized metastases (n = 9) or a more locally advanced tumor (n = 6) than preoperatively expected (). In 195 patients (59.1%), surgical treatment was not found indicated, 66 due to final benign diagnosis, 129 due to locally advanced (n = 56) or metastatic (n = 73) disease (). Patient demographics, surgical procedures, R-status and TNM classification in the 100 patients who underwent resection of a malignant tumor are listed in , 55 (52.9%) were resected for pancreatic adenocarcinoma. Whipple procedures or total pancreatectomies were performed in 95 patients, 6 of these (6.3%) combined with reconstructive vascular surgery. Operation time was median 295 min, range 181–480 min, median blood loss 855 ml, range 230–4000 ml and ASA (American Society of Anesthesiologists) score was median 2.0, range 1–3. R0 resection was achieved in only 41.8% of the pancreatic adenocarcinomas (). The analysis of potential improvements of surgical technique is focused on the 32 R1 resections, particularly 10 cases with the combination: tumor diameter less than 30 mm, N0 and R1 status. Disease-specific survival was median 21.3 months for pancreatic adenocarcinoma with R0 resection and N0 status (n = 12) as compared with median 17.4 months for patients with R1 or N1 status (n = 43) (). The difference is not statistically significant (p = 0.17) with these limited patient numbers. Disease-specific survival for carcinomas at the papilla of Vater (n = 21) was analyzed separately. Estimated 3-year survival was 78%.
Table I. Patient demographics, surgical procedures, R-status and TNM classification in 100 patients resected for malignant tumors.
Figure 2. Kaplan–Meier survival curves for patients with pancreatic adenocarcinoma. Those with resection status R0/N0 (n = 11) are compared with those with R0/N1 or R1/N1 status (n = 43) (p = 0.17) and those with metastatic disease at baseline (n = 56) (p < 0.001).
Postoperative complications were recorded in altogether 44 patients, one of these died on the fourth postoperative day from multiorgan failure, that is, mortality was 0.7% and total morbidity was 32.6% (). Most complications were associated with the Whipple procedure, except from one pancreatic fistula grade A, observed after open distal resection. Six pancreatic fistulas after pancreaticoduodenectomy were grade A and B, and two were grade C. In one patient with grade C pancreatic fistula, removal of the pancreatic remnant was performed the first postoperative day. Final diagnosis in this patient was autoimmune pancreatitis (AIP). The other grade C fistula also resulted in reoperation and removal of the pancreatic remnant. In this case, preoperative EUS-guided FNA of an endocrine carcinoma resulted in duodenal perforation and retroperitoneal abscess. The patient who died 4 days after surgery was primarily operated for a locally advanced endocrine carcinoma. The superior mesenteric artery and the celiac trunk were injured during a debulking procedure, resulting in death, despite successful vascular reconstruction. Delayed gastric emptying was severity grade A in 10, grade B in 4 patients and none were grade C.
Table II. Postoperative complications in 135 patients undergoing surgery.
Unresectable malignant tumor was found in 144 patients, 129 (89.6%) recognized at the multidisciplinary meeting, additional 15 patients (10.4%) during laparotomy. A misdiagnosed cancer was revised, and 143 patients were discharged with the diagnosis unresectable pancreatic carcinoma. In 18 patients (12.6%), histological documentation was lacking. The authors accepted this in patients who denied biopsy, but informed all patients about the uncertainty of a final diagnosis, based only on radiological and clinical criteria.
A benign final diagnosis was found in 87 patients, evaluated at the multidisciplinary meeting, 20 underwent pancreatic resection. In the remaining 66 patients, the benign diagnosis was based upon radiology, histology/cytology, cyst fluid analysis and clinical course of the condition. The collective frequency of malignant lesions in this heterogenic patient cohort was 73.6%.
Quality-of-care analyses of patient selection revealed failure in five patients (1.5%) and the preventable negative outcome is listed in . Diagnostic delay of malignant disease, recorded from baseline, was found in two patients, misdiagnosis of cancer occurred in one and one patient had serious complications after surgery for a benign lesion, another after laparotomy for locally advanced, unresectable disease. R1 status was the outcome of nine pancreaticoduodenectomies and one laparoscopic distal resection of tumors with diameter less than 30 mm, which represent a quality improvement opportunity.
Table III. Preventable negative clinical outcome.
The performance of the authors' hospital was evaluated against 43 quality indicators, identified by the ACSPCQD Expert Panel [Citation7]. The local guidelines were similar, but generally the local time limits for investigation and treatment were shorter. Judged by these quality indicators, the authors' hospital showed good performance in 42 but did not adhere to the certification requirement: indicator 4 requires that the surgeons should be certified by an international organization. In Norway, this is performed by a national organization.
Discussion
The first purpose of diagnostic and therapeutic interventions in patients with pancreatic and periampullary tumors is permanent cure, second to offer the best possible palliation. The roles of surgery and chemotherapy are crucial. As shown in , the net outcome of the pre- and peroperative practice was 135 patients undergoing surgery, whereas 125 patients with malignancies were considered unresectable, 73 because of metastases, 56 due to locally advanced tumors. An opportunity of improvement for patients with locally advanced tumors might be, either neoadjuvant chemoradiation for downstaging, followed by surgery, or upfront surgery including more advanced dissection techniques for distal resections [Citation21] or more comprehensive reconstructive vascular surgery on mesenteric vessels. Increased response rate and overall survival have also been documented in patients with metastatic disease and good performance status treated with the FOLFIRINOX regimen [Citation22].
The present observational study is searching for opportunities of improvement in diagnostic and therapeutic practice for the collective cohort of patients with solid and cystic pancreatic and/or periampullary tumors. Altogether 243 patients (73.6%) had malignant lesions, illustrating that diagnostic accuracy is critically important: ability to clarify whether or not a lesion is malignant and if it is resectable. Identification and implementation of improved diagnostic and therapeutic algorithms are prerequisites for future interventional studies. Quality indicators enabling faster and more accurate diagnosis/staging, then radical surgery, are crucial.
The authors' first critical analysis focused on the intraoperative technique described above, resulting in the outcome shown in and . The heterogeneity of periampullary tumors influence outcome, measured as rate of R0 resections. All carcinomas of the papilla of Vater, duodenal carcinomas and cholangiocarcinomas were resected with free margin with one exception, whereas 32 patients, 58% of those with pancreatic adenocarcinoma, were R1. illustrates that survival is profoundly influenced by treatment modality, as all patients with metastatic pancreatic adenocarcinoma died during the first year after diagnosis. R0N0 resection was found only in 12 (21%) of 55 patients, resected for pancreatic adenocarcinoma. The aggressive tumor biology of pancreatic adenocarcinoma is well known [Citation23]. In the group of 12 patients with R0/N0 status (), 3 patients died within the first year after radical surgery and adjuvant chemotherapy, 1 additional after 13 months. These observations make the question of neoadjuvant chemotherapy highly relevant. The residual nine patients are recurrence free after median 23.5 (15–36) months follow-up. A recent report from a more comprehensive database reported 54.5% 5-year survival without neoadjuvance in a similar subgroup of patients, resected for pancreatic adenocarcinoma, who also had low lymph node ratio and G1-differentiation [Citation24]. With focus on the quality of care analysis, illustrates how the surgical technique has failed: small tumors, that is, with diameter less than 30 mm, have been resected without a free margin in 10 patients. An improvement opportunity would be to resect the SMV/PV whenever a close anatomical relationship between tumor and vessel makes radicality questionable. A prerequisite for this strategy is that the vascular reconstructions are performed with minimal rate of added complications. The present frequency (6.3%) of reconstructive vascular surgery during right-sided pancreatic resection has probably been too low during this investigation, also compared with other pancreatic centers [Citation25,26], even though different patient selection makes direct comparison of frequencies difficult. PV infiltration may be predicted radiographically [Citation27] and survival benefits of radical surgery, also in this situation, have again been documented. Improvements of local clearance have been reported by other centers after vascular resection without neoadjuvant chemotherapy [Citation12], and resected patients experience prolonged survival together with improved patient reported quality of life during the first 2 years after diagnosis [Citation28]. A recent report support the hypothesis that vascular invasion above all is an indicator of unfavorable topography, rather than a robust parameter for adverse tumor biology [Citation29].
Postoperative morbidity and mortality are important quality indicators, found valid by the ACSPCQD Expert Panel (quality indicator 28 and 29). The present figures are listed in , illustrating that the second highest postoperative complication rate, 40%, was recorded after double bypass in unresectable carcinoma. Combined with , illustrating that survival for metastatic pancreatic adenocarcinoma was 5.2 (3.1–8.2) months, this information puts focus on an obvious staging problem: In 9 (60%) patients, of those found unresectable during laparotomy, preoperative radiology did not recognize distant metastasis. Adequate palliation should have been offered these patients by endoscopic stenting [Citation30]. Postoperative recovery is protracted in this group [Citation31]: preoperative level of quality of life was regained 12 weeks after surgery. Failed preoperative staging have thus forced unresectable patients to spend more than half of their residual lifetime on unnecessary postoperative recovery. The most dramatic quality failure in this series is the intraoperative injury of mesenteric vessels, resulting in death 4 days later. This case is included in , listing avoidable negative outcome. During the failure analysis, the authors concluded that closer cooperation with the liver transplantation team should focus on early dissection of the superior mesenteric artery/celiac trunk, if this is mandatory during a debulking procedure. This can almost always be performed without long-lasting ischemia of abdominal organs, which was the main reason for intractable postoperative problems in the present case.
The best outcome for patients with any of the malignant tumors in the present cohort is early, radical surgical removal, that is, before spread of tumor cells, as illustrated by the survival curve for R0N0 resected adenocarcinoma (). Several quality indicators are focused on the diagnostic process, one of them, applicable to the whole preoperative work-up, is time, as published by the ACSPCQD Expert Panel (quality indicator 26) [Citation7]: if a patient is to receive treatment, then the time from diagnosis to surgery or first treatment should be less than 2 months. The local limit for this time interval is 3 weeks. The present data support a defined time limit as a valid quality indicator, and surgical delay should be as close to zero as possible. But accuracy requirements must also be taken care of during a fast preoperative investigation.
Histological documentation of cancer was lacking in 12.6% of those discharged with the diagnosis of unresectable pancreatic cancer. These patients may receive incorrect prognostic information, and even wrong treatment. In one of these patients, referred to the authors' clinic for a second opinion, the primary diagnosis of an unresectable locally advanced pancreatic tumor was revised to a pseudoaneurysm, secondary to pancreatitis (). The present data also underline the risk of misdiagnosis when applying radiological follow-up for cystic lesions. In the two cases of misconceived serous cystic neoplasms/pseudocysts/branch duct IPMN (intraductal papillary mucinous neoplasm), diagnostic delay resulted in unresectable pancreatic carcinoma. But also the opposite problem is illustrated by the patient with AIP, undergoing total pancreatectomy. The accuracy of the preoperative workup has to be improved. Preoperative analysis of IgG4 level and EUS-guided biopsy should always be performed [Citation32,33] in advance of surgical resection of a pancreatic focus, possible caused by AIP. But sensitivity and specificity are limited, and resection of some benign lesions is still accepted in most pancreatic centers [Citation18,34,35].
Removal of the malignant tumor is the greatest available benefit of treatment, recently documented also in a comprehensive report 5736 cases who underwent an oncologic resection compared with 31,399 who did not [Citation36]. The main question, generated from this observational study, is how outcome can be improved for the 143 patients with unresectable malignant tumors. There is evidence that patients with locally advanced disease might benefit from neoadjuvant chemotherapy or upfront surgery, when the risk of R1 resection seems higher. But in the neoadjuvant protocols more than 50% of included patients lost the possible benefit of an R0/R1 resection, that is, never became resectable. This underlines the need of prospective investigation of both alternatives, as the postoperative complications rates after pancreatic surgery [Citation37] are improving. The benefit of FOLFIRINOX should also be further investigated, particularly the downstaging capacity in metastatic disease.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173–80.
- Bentrem DJ, Brennan MF. Outcomes in oncologic surgery: does volume make a difference? World J Surg 2005;29:1210–16.
- Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–7.
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13.
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–8.
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–5.
- Bilimoria KY, Bentrem DJ, Lillemoe KD, Talamonti MS, Ko CY. Assessment of pancreatic cancer care in the United States based on formally developed quality indicators. J Natl Cancer Inst 2009;101:848–59.
- Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–46.
- Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist 2012;17:192–200.
- Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Fernandez-del CC, Deshpande V, Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “True” R0 resection? Ann Surg 2013;257:731–6.
- Boggi U, Del CM, Croce C, Vistoli F, Signori S, Moretto C, Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery 2009;146:869–81.
- Muller SA, Hartel M, Mehrabi A, Welsch T, Martin DJ, Hinz U, Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2009;13:784–92.
- Weitz J, Kienle P, Schmidt J, Friess H, Buchler MW. Portal vein resection for advanced pancreatic head cancer. J Am Coll Surg 2007;204:712–16.
- Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Buchler MW, Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882–93.
- Torreggiani WC, Lyburn I, Harris AA, Zwirewich CV. Seeding of pancreatic cancer along the path of a surgical drain: case report and literature review. Can Assoc Radiol J 2000;51:241–3.
- Marangos IP, Buanes T, Kazaryant A, Rosseland A, Gladhaug I, Mathisen O, Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in high rate of R0 resection and long postoperative survival [in press]. Surgery 2012.
- Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4.
- Barone JE. Pancreaticoduodenectomy for presumed pancreatic cancer. Surg Oncol 2008;17:139–44.
- Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg 2004;91:1410–27.
- Hsu CC, Wolfgang CL, Laheru DA, Pawlik TM, Swartz MJ, Winter JM, Early mortality risk score: identification of poor outcomes following upfront surgery for resectable pancreatic cancer. J Gastrointest Surg 2012;16:753–61.
- Yamamoto Y, Sakamoto Y, Ban D, Shimada K, Esaki M, Nara S, Is celiac axis resection justified for T4 pancreatic body cancer? Surgery 2012;151:61–9.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25.
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801–6.
- Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311–19.
- Hartwig W, Hackert T, Hinz U, Hassenpflug M, Strobel O, Buchler MW, Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg 2009;250:81–7.
- Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: prognostic value of the length of venous resection. Surgery 2009;145:417–25.
- Nakao A, Kanzaki A, Fujii T, Kodera Y, Yamada S, Sugimoto H, Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg 2012;255:103–8.
- Pezzilli R. Total pancreatectomy and quality of life. JOP 2011;12:616.
- Rehders A, Stoecklein NH, Guray A, Riediger R, Alexander A, Knoefel WT. Vascular invasion in pancreatic cancer: tumor biology or tumor topography? Surgery 2012;152:S143–51.
- Larssen L, Medhus AW, Hjermstad MJ, Korner H, Glomsaker T, Soberg T, Patient-reported outcomes in palliative gastrointestinal stenting: a Norwegian multicenter study. Surg Endosc 2011;25:3162–9.
- Nieveen van Dijkum EJ, Kuhlmann KF, Terwee CB, Obertop H, de Haes JC, Gouma DJ. Quality of life after curative or palliative surgical treatment of pancreatic and periampullary carcinoma. Br J Surg 2005;92:471–7.
- Pezzilli R, Cariani G, Santini D, Calculli L, Casadei R, Morselli-Labate AM, Therapeutic management and clinical outcome of autoimmune pancreatitis. Scand J Gastroenterol 2011;46:1029–38.
- Detlefsen S, Zamboni G, Frulloni L, Feyerabend B, Braun F, Gerke O, Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: a study of 114 surgically treated European patients. Pancreatology 2012;12:276–83.
- Pancreatic Section, British Society of Gastroenterology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005;54:v1–16.
- Barens SA, Lillemoe KD, Kaufman HS, Sauter PK, Yeo CJ, Talamini MA, Pancreaticoduodenectomy for benign disease 9. Am J Surg 1996;171:131–4.
- Katz MH, Hu CY, Fleming JB, Pisters PW, Lee JE, Chang GJ. Clinical calculator of conditional survival estimates for resected and unresected survivors of pancreatic cancer. Arch Surg 2012;147:513–9.
- Hoem D, Viste A. Improving survival following surgery for pancreatic ductal adenocarcinoma – a ten-year experience. Eur J Surg Oncol 2012;38:245–51.