Abstract
Objective: The objective of this study is to investigate the occurrence of gastrointestinal (GI) and extraintestinal symptoms in children and adolescents with type 1 diabetes mellitus (DM1) and Down syndrome (DS) and their association with specific antibodies and histopathology of celiac disease (CelD), representing its clinical forms in the iceberg. Material and methods: Cross-sectional study (November 2009–December 2012) conducted at an outpatient care facility in Northeast Brazil including patients [DM1 (n = 111); DS (n = 77)] aged 10 months–18 years old. Measurement of anti-endomysial (EmA) and anti-tissue transglutaminase (anti-tTG) IgA antibodies was performed, as was that of anti-tTG-IgG in the cases with low serum IgA. The patients with antibody positivity were subjected to small intestine biopsy. Results: GI symptoms occurred in 53.7% of the sample, extraintestinal symptoms in 4.3%, and antibody positivity in 28.2% (n = 53). Of those who underwent biopsy (n = 40), histopathological findings of CelD were found in 37.5% [DM1 = 5/111 (4.5%), DS = 10/77 (13.0%)]. GI symptoms were associated with antibody positivity, but not with the histopathology. The GI (32.5%), silent (5.0%), and potential (62.5%) forms of disease were detected. Conclusions: The prevalence of GI symptoms was high in groups DM1 and DS, and the occurrence of such symptoms was associated with antibody positivity. The lack of association between the symptoms and histopatholological findings points to the inconsistency of the former as indicators of CelD. Although the GI form predominated among the cases with active CelD, its contribution to the celiac iceberg was smaller compared with the potential form, which determined the large and submerged base of the iceberg representing the high-risk groups investigated.
Introduction
Celiac disease (CelD) is an immune-mediated systemic disease triggered by gluten in genetically susceptible individuals of any age and ethnic group. It is characterized by the presence of specific antibodies, enteropathy, and a wide clinical spectrum ranging from forms with gastrointestinal (GI) and/or extraintestinal symptoms to the silent, latent, and potential forms, which may be represented as an iceberg [Citation1–3].
The role of gluten in other conditions is increasingly emphasized, as many diseases, the autoimmune ones in particular, are associated with CelD, including type 1 diabetes mellitus (DM1) and chromosomal abnormalities, such as Down syndrome (DS) [Citation2,4–6]. In several countries (including Brazil), the prevalence of CelD in DM1 varies from 3.0% to 16.0% [Citation2,5,7–12] and in DS from 4.0% to 17.0% [Citation2,6,13–17], being considerably higher compared with the overall population (0.5–1.0%) [Citation1,2,18].
From the clinical point of view, most individuals with DM1 and DS are believed to exhibit the silent form of CelD [Citation2,7,14]. However, a diagnosis based on more sensitive and specific serologic screening has allowed the retrospective identification of signs and/or symptoms that were formerly not considered, thus calling attention to the emergence of other clinical profiles [Citation10,13,15,19–23].
The GI and/or extraintestinal symptoms exhibited by patients with DM1 and DS may be considered appropriate to those conditions and thus not taken into account in the CelD diagnosis, which is thus delayed, consequently impairing the quality of life in patients and hindering the prevention of disease complications [Citation10,19,21,24].
Within the current scenario, the clinical presentation of CelD in DM1 and DS is the focus of much interest, necessitating studies that assess concomitantly clinical symptoms, more than one serological marker, histopathology of the small intestine, and their associations to identify CelD in the aforementioned high-risk groups.
In 2009, the Brazilian Health Ministry published a national protocol for investigating CelD. However, the recommendations made are still not satisfactorily met because of the difficulty of their implementation by the Brazilian Public Health System. Thus, CelD screening has not yet been included in the monitoring routine of patients with DM1 and DS in most public health services, and therefore, the presence of signs and symptoms should serve as a warning for the need to investigate CelD [Citation25].
The aim of the present study was to investigate the presence of GI and extraintestinal symptoms suggestive of CelD in children and adolescents with DM1 and DS and their association with specific antibodies and histopathology of disease, thereby visualizing its clinical presentation in the iceberg.
Methods
The present cross-sectional study was conducted from November 2009 to December 2012 at the pediatric outpatient clinic of a university hospital (Federal University of Rio Grande do Norte [Universidade Federal do Rio Grande do Norte – UFRN]), which is a regional reference for pediatric specialties, located in Natal, the capital of Rio Grande do Norte (RN) State, Brazil. The state has estimated population of 3.1 million inhabitants, being 77.8% of urban area and 34.2% aged below 19 years. The pediatric outpatient clinic serves around 4000 referrals a month. There is no regional statistics on the percentage of DM1 and DS in this age group. The study population was represented by the universe of children and adolescents enrolled in programs of care for DM1 (group DM1) and DS (group DS), who were consecutively recruited. Individuals from both genders aged 10 months–18 years residing in the capital and the interior of RN, who had regularly ingested gluten within the previous 3 months, independent of the presence or absence of clinical symptoms, were included in the study. The procedures comprised clinical and laboratory assessments and small intestine biopsy (SIB) through upper GI endoscopy (UGIE). The patients with heart disease from group DS were subjected to a cardiological assessment before UGIE, which was not performed in the cases rated as high risk. The patients without laboratory data were excluded from the study.
The study was approved by the research ethics committee of the Onofre Lopes University Hospital, ruling no. 226/08. The participants’ parents or guardians and the patients in group DM1 aged ≥12 years signed an informed consent form.
Clinical assessment (interview, physical examination, and anthropometric assessment)
A questionnaire was applied by four of the authors, who were previously trained to perform this procedure. The data collected included participant’s identification, gender, current age, age at diagnosis of DM1 (if applicable), GI symptoms (abdominal pain, constipation, diarrhea, abdominal distension, vomiting, flatulence, and unsatisfactory weight gain/weight loss), extraintestinal signs and symptoms (reported unsatisfactory growth or any other extraintestinal complaint), age at the onset of symptoms and their duration, length of exclusive breastfeeding, age at the first introduction of gluten into the diet, and occurrence of other diseases.
All the participants underwent a physical examination. The anthropometric assessment in group DM1 was based on the analysis of the height-for-age (HA) and body mass index (BMI)-for-age ratios in percentiles using the programs ANTHRO or ANTHRO PLUS for children under and over 5 years old, respectively; those programs use the World Health Organization (WHO) standard curves (2006/2007) [Citation26] as a reference. For group DS, the percentile curves formulated by Cronk et al. (1988) [Citation27], which are specific for the growth assessment of children with the syndrome, were used to analyze the weight-for-age (WA) and HA ratios.
Laboratory assessment (anti-tTG-IgA/IgG, EmA-IgA and total IgA)
The levels of anti-tissue transglutaminase (anti-tTG) and anti-endomysial (EmA), immunoglobulin (Ig)A antibodies, and serum total IgA were measured. Anti-tTG-IgG was also assessed in the cases with low total IgA to avoid the interference of IgA deficiency. Venous blood samples (5 mL) were collected during the visits in anticoagulant-containing tubes, and sent to the UFRN laboratory for clinical analysis, where they were centrifuged, distributed into aliquots, and stored at −20 °C until analyzed. The anti-tTG (IgA and IgG) antibodies were investigated through an enzyme-linked immunoassay (ELISA) using human recombinant antigen and the cutoff point (≥10 U/mL) recommended by the kit manufacturer. EmA-IgA was measured by the indirect immunofluorescence technique using monkey esophageal tissue as the substrate; uniform fluorescence at a 1:5 dilution was considered positive. Both tests were performed with the commercial kit Orgentec Diagnostika (Mainz, Germany) [Citation28].
The serum IgA levels were analyzed by immunoturbidimetry. The reference values per age were used; values two standard deviations below the mean ( < −2.0 SD) were considered indicative of IgA deficiency, with total deficiency being defined by levels ≤ 7 mg/dL and partial deficiency above this [Citation29].
Histopathological assessment
Only the participants with positive anti-tTG-IgA or IgG and/or EmA were subjected to SIB through UGIE, which was performed in the operating room. Three specimens were taken from the duodenal bulb and four from the second part of the duodenum, fixed with 10% formalin and sent to the UFRN Anatomic Pathology Laboratory, where they were processed in paraffin and stained with hematoxylin–eosin. Two pathologists independently performed the histopathological analysis under an optical microscope, and a third was called in to solve eventual discrepancies. The Marsh [Citation30] classification later modified by Oberhuber et al. [Citation31] was used, whereby the results were graded as follows: Marsh 0, normal mucosa; Marsh 1, infiltrative pattern; Marsh 2, crypt hyperplasia; and Marsh 3, which delineates increasing grades of villous atrophy (3a, 3b, and 3c). Diagnosis of CelD was established as the presence of Marsh 2 or 3 [Citation1].
CelD classification
CelD was classified following the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [Citation1] as GI, extraintestinal, silent, and potential forms. The occurrence of GI symptoms and exclusively extraintestinal symptoms characterized the GI and extraintestinal forms, respectively, and the absence of symptoms corresponded to the silent form; all this with screening and enteropathy consistent with CelD. The potential form was defined as positive serology, with or without symptoms, and the absence of enteropathy in the duodenal specimens. The clinical forms were represented in the celiac iceberg.
Statistical analysis
The quantitative variables were described as the mean ± SD and the categorical variables as the frequency and percentage. The mean values of the variables with normal distribution (Kolmogorov–Smirnov test) were compared by Student’s t test, and the values without a normal distribution by the Mann–Whitney test. Pearson’s Chi-square or Fisher’s exact test analyzed the association between independent categorical variables and outcome (dependent variables), and the prevalence ratio was calculated. The significance level was set as alpha value = 0.05 and a 95% confidence interval; p-values < 0.05 were considered significant.
Results
In total, 188 children and adolescents were included for analysis. The mean age was 8.95 ± 4.74 years (range: 10 months–18 years old), 102 (54.3%) were female, and 95 (50.5%) resided in the interior of RN. The distribution of the sample was as follows:
Group DM1: 111 patients; mean age: 10.84 ± 4.48 years old; an average length of DM1: 3.83 ± 3.31 years. Thirty-two patients exhibited positive markers [EmA IgA = 17/111 (15.3%), anti-tTG-IgA = 12/111 (10.8%), anti-tTG and EmA (3/111(2.7%)]; 25 (78.1%) underwent SIB, the children’s guardians did not give consent in the other seven cases; and histopathological findings of CelD were found in five (4.5%) patients.
Group DS: 77 patients; mean age: 5.97 ± 3.66 years old. A total of 21 patients exhibited positive markers [EmA IgA = 10/77(13.0%), anti-tTG-IgA = 5/77(6.5%), anti-tTG and EmA (6/77(7.8%)]; 15 (71.4%) underwent SIB, the children’s guardians did not give consent in three cases and the cardiological assessment was unfavorable in an additional three cases. Histopathological findings of CelD were found in 10 (13.0%) patients.
The means of the following biodemographic variables exhibited significant difference: current age (in years) (p < 0.01); age (in years) at the onset of symptoms [DM1 = 8.34 ± 3.90; DS = 3.70 ± 3.16 (p < 0.01)]; age (in months) at the first introduction of gluten into the diet [DM1 = 9.35 ± 4.47; DS = 7.78 ± 4.82 (p = 0.02)]; and length (in months) of exclusive breastfeeding [DM1 = 4.67 ± 3.11; DS = 3.75 ± 3.04 (p = 0.03)]. Statistical significance was not found relative to the mean duration of symptoms (in years) [DM1 = 2.40 ± 2.21; DS = 2.46 ± 3.07 (p = 0.60)].
GI symptoms were present in 101 (53.7%) patients [DM1 = 52 (46.8%); DS = 49 (63.6%); p = 0.02]. The frequency of these symptoms per group is described in .
Table I. Frequency of gastrointestinal symptoms in children and adolescents with type1 diabetes mellitus (DM1 group) and Down syndrome (DS group) at the Pediatric Hospital/UFRN. Natal/Brazil.
Extraintestinal symptoms were present in 30/188 (16.0%) patients, in eight cases (4.3%) alone and in 22 (11.7%) associated with GI symptoms. The distribution of the extraintestinal symptoms was as follows: acquired hearing loss (1), epileptic seizures (1), chronic fatigue (1), and unsatisfactory growth (17) in group DM1; arthralgia (1), arthritis (1), epileptic seizures (1), and unsatisfactory growth (7) in group DS.
In group DM1, short stature was found in 16 (13.4%) patients, underweight in two (1.8%), and overweight in 21 (18.9%). Of the five children in group DM1 with CelD, one exhibited short stature, two normal weight, and three overweight. In group DS, short stature was found in three (3.9%) patients, underweight in two (2.6%), and overweight in five (6.5%). All 10 children in group DS with CelD exhibited adequate body weight and height.
The screening found positive serology results in 53/188 (28.2%) patients; upon considering each marker, a significant difference was not found between the groups, DM1 = 32 (28.8%) and DS = 21 (27.3%) [p = 0.81]. Among the 53 patients, 38 (71.7%) exhibited GI symptoms. There was an association of GI symptoms with positive serology results (), which did not occur in the case of the extraintestinal symptoms (p = 0.47). Low serum IgA levels were found in 23 (12.2%) patients, a finding indicative of partial IgA deficiency. Anti-tTG-IgG was assessed in 18/23 patients, and the titers were negative in all of them. The other five had previously shown positive anti-tTG-IgA titers.
Table II. Gastrointestinal symptoms and their association with serological markers and histopathological findings of celiac disease in children and adolescents with type1 diabetes mellitus (DM1 = 111) and Down syndrome (DS = 77) at the Pediatric Hospital/UFRN. Natal/Brazil.
In total, 40/53 (75.5%) patients with positive serological markers underwent biopsy. CelD was confirmed in 15/40 (37.5%) of these patients, whose duodenal mucosa exhibited Marsh-Oberhuber stages 3a and 3b. In the other 25 patients, the duodenal mucosa exhibited Marsh–Oberhuber stages 0 or 1. An association was not observed between histopathological findings of CelD and GI () or extraintestinal (p = 0.90) symptoms.
The proportion of patients with histopathological findings of CelD was larger in group DS (13.0%) compared with DM1 (4.5%) [p = 0.03]. Among the 10 patients with CelD in group DS, one exhibited autoimmune thyroiditis and alopecia areata and another already had clinical remission from acute lymphocytic leukemia. None of the five patients with CelD from group DM1 exhibited any other associated autoimmune disorder.
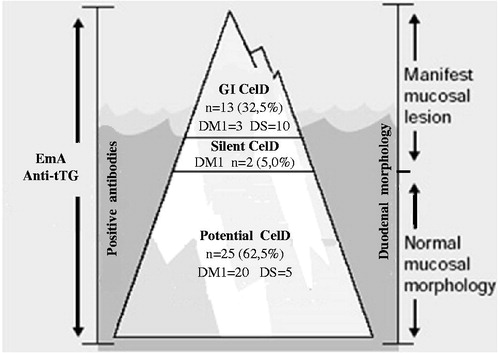
The GI, silent, and potential forms of CelD were found in the investigated population and represented in the iceberg (). No case of extraintestinal form was identified.
Figure 1. Diagram of celiac disease (CelD) iceberg originating from the clinical forms of children and adolescents with type 1 diabetes mellitus (DM1) and Down syndrome (DS) which were subjected to biopsy (n = 40). The visible portion is represented for the gastrointestinal (GI) form and the submerged portion for the silent and potential forms of CelD.
Discussion
The present investigation reports on the clinical presentation of CelD in patients with DM1 and DS from specialized care. Up to the time of study inclusion, they had never undergone screening for CelD.
Analysis of the biodemographic characteristics showed that the time elapsed since the onset of symptoms was similar between groups DM1 and DS, leading to the assumption that the children’s first visit to the specialized service was already late. Weaning from breastfeeding was too early, and gluten was first introduced into the children’s diet after the age of 6 months, both of which are considered risk factors for CelD [Citation32–34]. Because DM1 and DS are high-risk groups for CelD, their presence presumably increases the risk of developing the disease. However, the hypothesis that breastfeeding and the age at first introduction of gluten behave as preventive factors against CelD is not supported by recent scientific evidence [Citation35,36].
The frequency of symptoms was high in the population assessed. GI symptoms are known to occur in both DM1 and DS. In the case of DM1, symptoms may be the result of GI motility disorders, changes in visceral sensitivity and secretion of neurotransmitters, in addition to diseases affecting the GI system, such as CelD [Citation37]. These facts accounted for the high prevalence of abdominal pain in group DM1, as was previously reported in some studies [Citation10,21,23]. Clinical symptoms of CelD are estimated to occur in 50% of individuals with DM1, with abdominal pain in 16% of the cases [Citation19]. Reduced intestinal muscle tone, which occurs in individuals with DS, and the possible occurrence of previously undiagnosed CelD may contribute to the higher frequency of constipation and flatulence in this group of patients [Citation13,15].
The positive serology results found in the present study was higher than that in other studies conducted in Brazil, which varied from 2.5% in Rio de Janeiro [Citation9] to 21.0% in Recife [Citation12] for patients with DM1 and was 7.0% among children and youths with DS in Southern Brazil [Citation17]. The histopathological assessment confirmed the high prevalence rate of CelD in groups DM1 and DS, being comparable with the rates found in some European countries [Citation5,6]. In Brazilian studies with patients with DM1, such as the investigations conducted by Baptista et al. [Citation11] and Whitacker et al. [Citation10], the prevalence rate was 4.8% and 4.0%, respectively, similar to that found in the present study (4.5%). For DS, the prevalence of CelD was the same in the studies by Lobe et al. [Citation16] and Nisihara et al. [Citation17] (5.6%) and lower than that detected in the present study (13.0%).
The high frequency of positive serological markers yielded a spectrum, one end of which comprised 15 patients with positive antibodies and CelD proven biopsy and the other end of which comprised 25 patients with positive antibodies and normal biopsy results, most of whom were from group DM1, indicating the potential form of CelD. It is believed that screening through high-sensitivity and high-specificity markers contributed to the extension of these findings, which would not have been possible had single and isolated techniques been used. Thus, it seems reasonable to suggest that in the case of high-risk groups, screening for CelD is more reliable and efficacious when association of antibodies are investigated, in addition to the analysis of serum IgA in the patients with negative serological markers who use this fraction.
The cross-sectional design of the study might have influenced the frequency of the potential form of CelD. Such patients are at high risk for subsequently developing villous atrophy, and thus, monitoring their clinical condition and serological markers (associated or not with additional biopsies) is crucial for defining the diagnosis. Barera et al. [Citation38] monitored a large population of diabetic children with positive serology for CelD over 6 years and found that 60% were seropositive by the time DM1 was diagnosed, whereas the remaining 40% were diagnosed with CelD in the following 4 years.
Other studies also found potential CelD in patients with DM1, such as Franzese et al. [Citation39], who detected it in 12.2% of the participants in their multicenter study in Italy. In Brazil, potential CelD was found in 3.8% of patients with DM1 [Citation11].
It should be observed that although the behavior of specific antibodies in the overall population of celiac patients is satisfactorily known, there is little evidence relative to such behavior in specific high-risk groups, in which case, dysregulation of the immune system may contribute to the production of autoantibodies and the consequent increase in their positivity. Thus, the possibility of false-positive results for CelD antibodies found in this study should be kept in mind, i.e., their detection is not necessarily associated with present or future occurrence of CelD. Sardy et al. [Citation40] observed that false-positive reactions for anti-tTG can occur without any relationship to CelD in patients with autoimmune diseases.
The presence of GI symptoms might have contributed to the selection of patients with positive markers, inasmuch as such symptoms allegedly reflect the presence of CelD. The association found between them was significant for abdominal pain, abdominal distension, diarrhea, and unsatisfactory weight gain (), whose signs and symptoms are mainly related to malabsorption phenomena. However, this association should be viewed with caution because, although the symptoms may sometimes be profuse, the subjectivity of certain complaints should be considered, as it may lead to mistaken interpretations and limitations in establishing the relevance of such a relationship. Therefore, there is no basis to infer that a given symptom may indicate the positivity of a certain marker.
The lack of an association between the presence of symptoms and histopathological findings of CelD reinforces the inconsistency of symptoms as disease indicators, as they can also represent manifestations of DM1 and DS. However, the undervaluation of symptoms may delay the CelD diagnosis and increase the duration of exposure to gluten. Thus, GI and extraintestinal symptoms appear to behave more as confounding than as elucidative variables in the aforementioned high-risk groups.
The clinical presentation of CelD was variable and heterogeneous in groups DM1 and DS. The GI form prevailed among the cases with active disease, which was detected in all the group DS patients and in most of the group DM1 patients, thus contributing to the visible portion of the iceberg. When all forms of the disease were analyzed in combination, the higher prevalence of potential CelD was largely determinant of the large and submerged base of the iceberg (), which indicates that the silent form may not be the most frequent, as indicated by previous studies [Citation2,7,14,19,21]. The disease profile might have changed after the advent of more sensitive and specific serological markers, such as EmA and anti-tTG, which allow identifying patients with positive antibodies, but still without compatible histopathological findings.
In conclusion, the frequency of the GI form was high among the cases with active CelD, particularly in group DS, while the potential form of disease was the most prevalent, especially in group DM1, thereby demonstrating the emergence of other clinical profiles. The contribution of the GI form to the celiac iceberg was smaller compared with the potential form. The lack of an association between symptoms and histopathological findings of CelD stresses the inconsistency of the former as disease indicators, even though they were associated with antibody positivity.
The high frequency of the potential form of CelD reinforces the critical need to perform systematic serological screening in high-risk groups such as patients with DM1 and DS. The follow-up of such patients with respect to the future development of active CelD is recommended. The high prevalence of CelD in DM1 and DS reinforces the importance of serological screening in the diagnostic approach in specialized services and should call attention to the need to introduce such screening within Brazil’s public healthcare system.
Acknowledgements
The authors would extend their thanks to Teresa Neuma de Souza Brito and Luanda Ferreira Canário for performing laboratory analysis, to Dr. Paulo Matos de Castro and Dr. Regina Fotim Barros for conducting the GI endoscopy, and to Dr. Angela Fernandes Ferreira for her contribution to the statistical analysis execution.
Declaration of interest
The authors report that they have no conflicts of interest. The study was financially supported by the Research Support Foundation of Rio Grande do Norte State (Fundação de Apoio à Pesquisa do Estado do Rio Grande do Norte-FAPERN).
References
- Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60.
- Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med 2012;367:2419–26.
- Nenna R, Tiberti C, Petrarca L, Lucantoni F, Mennini M, Luparia RP, et al. The celiac iceberg: characterization of the disease in primary schoolchildren. J Pediatr Gastroenterol Nutr 2013;56:416–21.
- Helms S. Celiac disease and gluten-associated diseases. Altern Med Rev 2005;10:172–92.
- Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther 2014;40:1123–32.
- Mårild K, Stephansson O, Grahnquist L, Cnattingius S, Söderman G, Ludvigsson JF. Down syndrome is associated with elevated risk of celiac disease: a nationwide case–control study. J Pediatr 2013;163:237–42.
- Aktay AN, Lee PC, Kumar V, Parton E, Wyatt DT, Werlin SL. The prevalence and clinical characteristics of celiac disease in juvenile diabetes in Wisconsin. J Pediatr Gastroenterol Nutr 2001;33:462–5.
- Gonçalves CBCD, Silva IN, Tanure MG, Bahia M. Study of prevalence of celiac disease in children with type1 diabetes mellitus: result of 10 years of follow-up. Arq Bras Endocrinol Metab 2013;57:375–80.
- Mont-Serrat C, Hoineff C, Meirelles RMR, Kupfer R. Diabetes and autoimmune diseases: prevalence of celiac disease in children and adolescents with type1 diabetes mellitus. Arq Bras Endocrinol Metab 2008;52:1461–5.
- Whitacker FC, Hessel G, Lemos-Marini SHV, Paulino MFVM, Minicucci WJ, Guerra-Júnior G. Prevalence and clinical aspects when it comes to the association between type1 diabetes mellitus and celiac disease. Arq Bras Endocrinol Metab 2008;52:635–41.
- Baptista ML, Koda YK, Mitsunori R, Nisihara RM, Ioshii SO. Prevalence of celiac disease in Brazilian children and adolescents with type1 diabetes mellitus. J Pediatr Gastroenterol Nutr 2005;41:621–4.
- Brandt KG, Silva GA, Antunes M. Celiac disease in a group of children and adolescents with type1 diabetes mellitus. Arq Bras Endocrinol Metab 2004;48:823–7.
- Bhat AS, Chaturvedi MK, Saini S, Bhatnagar S, Gupta N, Sapra S, et al. Prevalence of celiac disease in Indian children with Down syndrome and its clinical and laboratory predictors. Indian J Pediatr 2013;80:114–17.
- Book L, Hart A, Black J, Feolo M, Zone JJ, Neuhausen SL. Prevalence and clinical characteristics of celiac disease in Down syndrome in a US study. Am J Med Genet 2001;98:70–4.
- Bonamico M, Mariani P, Danesi HM, Crisogianni M, Failla P, Gemme G, et al. Prevalence and clinical picture of celiac disease in Italian Down syndrome patients: a multicenter study. J Pediatr Gastroenterol Nutr 2001;33:139–43.
- Lobe MCS, Perini LD, de Noronha MGO, Krueger MB, Castellen NR. Prevalence of autoimmune diseases in patients with Down syndrome. AMRIGS 2013;57:5–8.
- Nisihara RM, Kotze LM, Utiyama SR, Oliveira NP, Fiedler PT, Messias-Reason IT. Celiac disease in children and adolescents with Down syndrome. J Pediatr 2005;81:373–6.
- Catassi C, Gatti S, Fasano A. The new epidemiology of celiac disease. J Pediatr Gastroenterol Nutr 2014;59:S7–9.
- Szaflarska-Poplawaska A. Coexistence of coeliac disease and type 1 diabetes. Prz Gastroenterol 2014;9:11–17.
- Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type1 diabetes mellitus patients. Diabetol Metab Synd: Clin Res Rev 2012;6:70–6.
- Volta U, Tovoli F, Caio G. Clinical and immunological features of celiac disease in patients with Type 1 diabetes mellitus. Expert Rev Gastroenterol Hepatol 2011;5:466–79.
- Deja G, Myrda A, Jarosz-Chobot P, Siekiera U. The assessment of autoimmunological status and prevalence of different forms of celiac disease among children with Type 1 diabetes mellitus and celiac disease. Mediat Inflamm 2008;1:1–6.
- Narula P, Porter L, Langton J, Rao V, Davies P, Cummins C, et al. Gastrointestinal symptoms in children with type1 diabetes screened for celiac disease. Pediatrics 2009;124:e489–95.
- Bakker SF, Tushuizen ME, Stokvis-Brantsma WH, Aanstoot HJ, Winterdijk P, Van Setten PA, et al. Frequent delay of coeliac disease diagnosis in symptomatic patients with type 1 diabetes mellitus: clinical and genetic characteristics. Eur J Intern Med 2013;24:456–60.
- Pimentel H, Silveira LRM, Gadelha MIP, Pereira MLB, Vargas PR. Protocolo Clínico e Diretrizes Terapêuticas da Doença Celíaca. Ministério da Saúde. http://portal.saude.gov.br/portal/arquivos/pdf/pcdt_doença_celíaca. (Cited 22 June 2010).
- WHO AnthroChild Growth Standards. http://www.who.int/childgrowth/software (Cited 22 June 2010).
- Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, et al. Growth charts for children with down syndrome: 1 month to 18 years of age. Pediatrics 1988;81:102–10.
- Pavlovic M, Radlovic N, Lekovic Z, Stojsic Z, Puleva K, Berenji K. When to screen children with Down syndrome for celiac disease? J Trop Pediatr 2010;56:443–6.
- Naspitz CK, Solé D, Sampaio C, Sales MM, Gonzalez GH. Níveis séricos de IgG, IgM, IgA em crianças brasileiras normais. J Pediatr (Rio J) 1982;52:121–6.
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992;102:330–54.
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185–94.
- Ludvigsson JF, Fasano A. Timing of introduction of gluten and celiac disease risk. Ann Nutr Metab 2012;60:22–9.
- Akobeng AK, Ramanan AV, Buchan I, Heller RF. Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch Dis Child 2006;91:39–43.
- Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005;293:2343–50.
- Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, Amarri S, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014;371:1295–303.
- Vriezinga SL, Auricchio R, Bravi E, Castillejo G, Chmielewska A, Crespo Escobar P, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014;371:1304–15.
- Rodrigues MLC, Motta MEFA. Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus. J Pediatr (RioJ) 2012;88:17–24.
- Barera G, Bonfanti R, Viscardi M, Bazzigaluppi E, Calori G, Meschi F, et al. Occurrence of celiac disease after onset of type 1 diabetes: a 6-year prospective longitudinal study. Pediatrics 2002;109:833–8.
- Franzese A, Iafusco D, Spadaro R, Cavaliere O, Prisco F, Auricchio R, et al. Potential celiac disease in type1 diabetes: a multicenter study. Diabetes Res Clin Pr 2011;92:53–6.
- Sárdy M, Csikós M, Geisen C, Preisz K, Kornseé Z, Tomsits E, et al. Tissue transglutaminase ELISA positivity in autoimmune disease independent of gluten-sensitive disease. Clin Chim Acta 2007;376:126–35.
