Abstract
Objective: Bariatric surgery is the most efficient treatment of severe obesity. We investigated to what extent BMI- or waist-hip ratio (WHR)-related genetic variants are associated with excess BMI loss (EBMIL) two years after Roux-en-Y gastric bypass (RYGB) surgery, and elucidated the affected biological pathways.
Methods: Two-hundred fifty-one obese patients (age: 43 ± 10.7, preoperative BMI: 45.1 ± 6.1 kg/m2, 186 women) underwent RYGB surgery and were followed up after two years with regard to BMI. Patients were genotyped for 32 single-nucleotide polymorphisms (SNPs) that were investigated with regard to their impact on response to RYGB and preoperatively measured Three Factor Eating Questionnaire (TFEQ) scores.
Results: Homozygous T carriers of the SNP rs4846567 in proximity to the Lysophospholipase-like 1 (LYPLAL1) gene showed a 7% higher EBMIL compared to wild-type and heterozygous carriers (p = 0.031). TT-allele carriers showed furthermore lower scores for Hunger (74%, p < 0.001), lower Disinhibition (53%, p < 0.001), and higher Cognitive restraint (21%, p = 0.017) than GG/GT carriers in the TFEQ. Patients within the lowest quartile of Hunger scores had a 32% greater EBMIL compared to patients in the highest quartile (p < 0.001).
Conclusion: The LYPLAL1 genotype is associated with differences in eating behavior and loss of extensive body weight following RYGB surgery. Genotyping and the use of eating behavior-related questionnaires may help to estimate the RYGB-associated therapy success.
Introduction
Obesity is of multifactorial pathogenesis, whereby genetic, epigenetic and non-genetic factors appear to play a significant role. Currently, the most efficient treatment option for severely obese patients is Roux-en-Y gastric bypass (RYGB) surgery. However, large interindividual differences in weight loss are observed after RYGB surgery.[Citation1] The reasons for this observation are not yet fully elucidated. Psychological factors related to eating behavior such as impulse control and hunger affect weight loss.[Citation2,Citation3] Dietary patterns and food preference are linked to obesity and body fat distribution in general and specifically to abdominal obesity.[Citation4,Citation5] The Three Factor Eating Questionnaire (TFEQ) is a tool to assess individual eating habits by evaluating three traits: Hunger, Disinhibition, and Cognitive restraint.[Citation6,Citation7] Several studies indicate that the TFEQ is a useful instrument to explain changes in body weight based on food intake behavior. Using the TFEQ, French et al. detected an association between BMI and Hunger and Disinhibition,[Citation8] while others were able to show that changes in Cognitive restraint are associated with changes in body weight and BMI.[Citation9,Citation10] Whether preoperatively obtained eating behavior scores may be linked to the magnitude of weight loss after bariatric surgery has not yet been proven.[Citation2,Citation3]
Several phenotypes, such as age, surgery type and BMI before surgery, have been identified to influence the success in weight loss after RYGB surgery.[Citation11,Citation12] A genetic influence on the development of obesity and body fat distribution has also been reported in both targeted and genome wide association studies (GWAS).[Citation13–16] Genetic variants detected in these studies are located e.g., in the fat mass and obesity-associated gene (FTO) and leptin, and have been found to be associated with eating behavior.[Citation17,Citation18] The mechanisms by which such variants may influence the risk for obesity are largely unknown.
Hitherto, studies investigated separately the impact of preoperative eating behavior and the influence of genetic risk variants on therapy success in bariatric surgery. Studies investigating the interaction of all these three parameters have not yet been published. In the current study, we investigate to what extent eating behavior, as quantified by the TFEQ, has an impact on excess BMI loss (EBMIL) after RYGB bariatric surgery, and if BMI and waist-hip ratio (WHR)-associated genetic variants may have the ability to influence eating behavior.
Materials and methods
Patients
Two-hundred and fifty-one obese patients, as defined by BMI >30kg/m2, were included in the study (mean ± SD; age: 43.0 ± 10.7 years, preoperative BMI: 45.1 ± 6.1 kg/m2, 186 women). All participants underwent RYGB surgery at the Interdisciplinary Obesity Center, St. Gallen, Switzerland. Study participants completed the TFEQ-51 questionnaire at the study center, after an over-night fast, in the morning before the surgical intervention. Two different variants of RYGB surgery were performed: proximal and distal RYGB. In both procedures, the largest part of the stomach was transected, and a small gastric pouch of about 20–30 ml was anatomized to the proximal jejunum with the diameter of the pouch–jejunal anastomosis standardized to about 12 mm. In the proximal RYGB procedure, the biliopancreatic limb was side to side anatomized to the jejunum 150-cm distal from the pouch–jejunal anastomosis (Roux-en Y limb length, 150 cm). In the distal RYGB procedure, the biliopancreatic limb was side to side anatomized to the ileum 60 to 100 cm proximal from Bauhin’s valve (common channel, 60–100 cm). The length to the biliopancreatic limb was approximately 60 cm in the proximal and 60–100 cm in the distal RYGB procedure.[Citation11] Patients who obtained the operation for a second time or underwent an alternative intervention (e.g., gastric banding) were not included. Height and weight were measured at baseline and at the two-year follow-up visit with patients wearing light clothing and no shoes. Characteristics of the study population are summarized in .
Table 1. Clinical characteristics of the study cohort.
The TFEQ
The original version of the Three Factor Eating Questionnaire was used to assess eating behavior prior to RYGB surgery. The TFEQ comprises 51 questions, which measure three dimensions of eating behavior: Hunger (14 questions), Disinhibition (16 questions), and Cognitive restraint (21 questions). Each question is dichotomously evaluated (score 0 or 1). Scores are subsequently summed up to a final score for each of the three categories leading to maximum reachable points of 14, 16, and 21, respectively.
Genotyping
Two-hundred thirt-seven patients of the RYGB cohort were genotyped for 32 single-nucleotide polymorphisms (SNPs) that have been earlier associated with larger BMI or WHR in two comprehensive GWA studies. Only SNPs with a reported minor allele frequency of at least 15% were taken into consideration.[Citation13,Citation14] Genotyping was performed using the Illumina iSelect genotyping array (Illumina Inc.). Thirteen SNPs were not determinable because of technical reasons and were, thus, excluded from further analysis. The list of investigated genetic variants is provided in Supplementary Table S1. All determined SNPs were in the Hardy–Weinberg equilibrium.
Statistics
To determine which of the BMI- or WHR-associated SNPs have a significant impact on the TFEQ factor outcome, the genetic variants were included as covariates in a multiple linear regression model, besides age, sex, and preoperative BMI. Initially, a genetic additive effect model was assumed and included in the analyses, coding the genotypes as 0 (two major alleles), 1 (heterozygote), and 2 (two minor alleles), respectively. In case that the plotted result clearly indicated a recessive or dominant relationship (e.g., the score from two genotypes were visually similar and different from the third), the SNP was recoded accordingly (i.e., 0 for homozygous and heterozygous major allele carriers, 1 for homozygous minor allele carriers) and considered in the analyses. p Values were adjusted for multiple testing using Bonferroni correction.
Genetic variants that were shown to have a significant impact on TFEQ outcome were further investigated and included as a covariate in a multiple linear regression model to study their impact on relative weight loss two years after surgery (EBMIL). EBMIL was calculated as 100 − [(final BMI −25/initial BMI −25) × 100]. The model was adjusted for age, sex, initial BMI, and surgery type.[Citation19] Percent BMI loss was calculated according to [Citation19] and was used as a confirmatory dependent variable in genetic association analyses.
The impact of the preoperative TFEQ factor scores on EBMIL was analyzed using a multiple linear regression model, adjusting for age, sex, presurgery BMI, and surgery type. Post hoc t-tests were performed to compare if the quartiles of TFEQ scores were associated with EBMIL. Adjusted p values <0.05 were considered significant and calculated using the p adjust-function in R.[Citation20] Analyses were otherwise performed using SPSS Statistics (version 22.0 for Windows, IBM, Chicago, IL).
Results
The average EBMIL was over 80% two years after bariatric surgery (mean ± SD: 83.8 ± 17.9). Age was inversely associated with EBMIL (p = 0.03). The TFEQ scores were normally distributed, reaching average scores of 7.1 ± 3.3 for Hunger, 8.5 ± 3.2 for Disinhibition, and 9.0 ± 4.0 for Cognitive restraint. Increasing age was associated with Cognitive restraint (p = 0.04) but not with Hunger or Disinhibition. No sex differences were observed for the TFEQ or weight loss outcomes ().
A genetic variant in proximity to the gene Lysophospholipase-like 1 (LYPLAL1) is associated with the strength of Hunger feelings and disinhibition before bariatric surgery
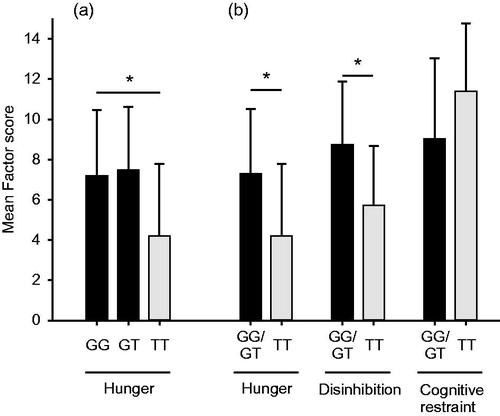
Forty BMI- and WHR-associated SNPs were used as covariates in a multiple linear regression model with Hunger, Disinhibition, and Cognitive restraint as the independent variable, respectively. The SNP rs4846567, located on chromosome 1 downstream of the gene Lysophospholipase-like 1 (LYPLAL1),[Citation14] was significantly associated to Hunger (p = 0.036, Bonferroni correction, adjusted for 32 tests). Initially an additive effect model was assumed for this variant in the univariate linear regression model with Hunger as dependent variable. The result plot indicated a recessive effect of the T-allele on the Hunger factor (). The additive effect model was therefore recoded to a recessive model (GG + GT =0, TT =1) and used in subsequent analyses. As shown in , TT-allele carriers of the variant rs4846567 showed a 74% lower Hunger-associated score compared to GG + GT carriers (TT: 4.2 ± 3.6, GG + GT: 7.3 ± 3.2, p < 0.001 [p = 0.018, adjusted for 32 tests]). TT-allele carriers of SNP rs4846567 showed furthermore a 53% decrease in Disinhibition (T = 5.7 ± 2.9, G = 8.7 ± 3.2, p < 0.001 [p = 0.038, adjusted for 32 tests]). No significant impact on Cognitive restraint was observed after adjustment for multiple testing.
Figure 1. LYPLAL1 and hunger sensations. Two of the presurgical-assessed factor scores (Hunger, Disinhibition) evaluated by the Three Factor Eating Questionnaire are associated with the LYPLAL1 genotype rs4846567. Homozygous SNP carriers showed a 74% lower TFEQ Hunger score (p = 0.018) and 53% lower Disinhibition score (p = 0.038) than GG- and GT-allele carriers. Cognitive restraint increased with 21% but did not withstand multiple testing adjustment. (a) Hunger was first analyzed using an additive genetic effect model. (b) The three factors were further analyzed using a recessive effect model subgrouping patients into G-allele carriers and noncarriers of the G-allele. The figure shows mean scores and standard deviations. All p values were adjusted using Bonferroni correction. *p < 0.05.
The genetic variant rs4846567 is associated with larger weight loss two years after surgery
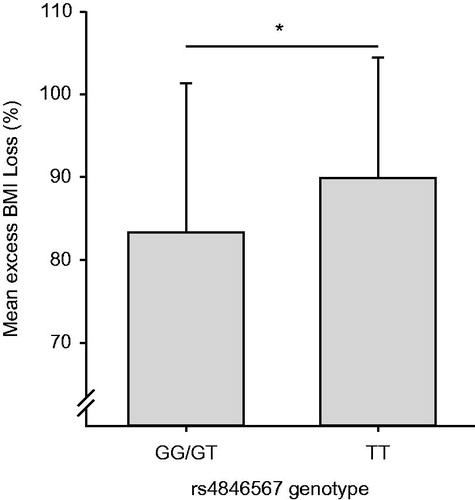
To investigate if rs4846567 had an impact on EBMIL, we inserted this variant in an univariate linear regression model with EBMIL as the dependent variable. Homozygous carriers of the SNP rs4846567 showed a 7% higher EBMIL two years after RYGB surgery (90 ± 15%) compared to GG- and GT-allele carriers (83 ± 18%; p = 0.031, ). When using percent BMI loss as independent variable, TT-allele carriers lost 41 ± 7% compared to GG- and GT-allele carriers 36 ± 8%, p = 0.050).
Figure 2. LYPLAL1 and excess BMI loss. Rs4846567, close to the Lysophospholipase-like 1 (LYPLAL1) gene, is significantly associated with mean excess BMI loss after RYGB surgery. TT-allele carriers lost 90.0 ± 15% (mean ± SD) of their excess weight two years after RYGB surgery compared to 83.3 ± 18% observed in G-allele carriers (p = 0.031). *p < 0.05.
TFEQ outcome scores are associated with EBMIL
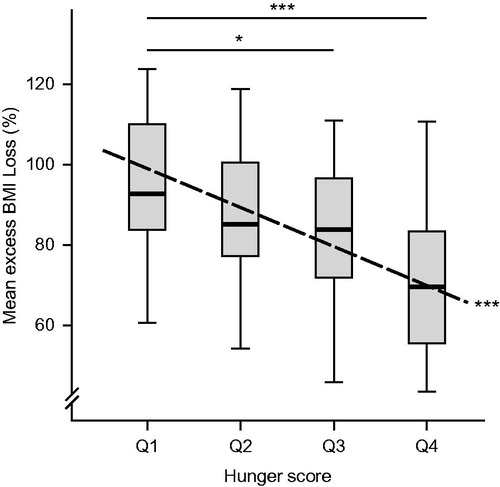
An inverse association was observed between Hunger scores and EBMIL after surgery, using univariate linear regression analysis (β = −2.52, p < 0.001, , dashed line). Patients with low Hunger scores lost most weight after surgery. When divided into quartiles, patients in the lowest Hunger score quartile (score <5, Q1) lost 32% more excess weight than individuals with scores >9 (p < 0.001, Q4; ). Between the first and third quartile, the difference was 16%, p = 0.038. Similar results were obtained when regressing hunger scores and quartiles of the Hunger score against percent BMI loss (p < 0.001, respectively; p = 0.050). No significant associations were observed between Disinhibition, Cognitive Restraint, and EBMIL.
Figure 3. The TFEQ Hunger score is negatively associated with excess BMI loss (dashed line, β = −2.52; p < 0.001). Patients with the lowest hunger scores (quartile 1, Q1) lost 32% more excess weight compared to individuals with the highest score quartile (Q4, p < 0.001). Patients in Q1 lost 16% more compared to patients in Q3 (p = 0.038). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In the current study, we demonstrate that TT-allele carriers of the LYPLAL1-associated variant rs4846567 show lower Hunger and Disinhibition scores in the TFEQ. We also show the impact of preoperative appetite sensations on EBMIL after RYGB surgery. Rs4846567 was significantly associated with both EBMIL and preoperative eating behavior, which corresponds well with Heid et al., who reported the T-allele to be associated with lower WHR.[Citation13] All results were confirmed when using percent BMI loss as dependent variable. This is the first study that associates both eating behavior and genetics to therapy success in bariatric surgery patients. Interestingly, rs4846567 has been recently reported to interact with a healthy diet-score and to be associated with BMI-adjusted WHR in a meta-study including 68000 adults of European decent.[Citation21] Furthermore, another genetic variant in linkage disequilibrium with rs4846567, rs2605100, has been associated with weight loss after lifestyle interventions.[Citation22] These results strongly support our findings of a significant impact of this genetic variant on eating behavior and weight loss. Associations between eating behavior and BMI have been described earlier.[Citation10] It can be speculated that appetite feelings are a result of a complex interplay between metabolic processes and/or psychological traits, which may partly explain why some individuals have a stronger predisposition to accumulate weight than others. This would also explain some of the large interindividual differences in weight loss observed after RYGB surgery.
Patients undergoing bariatric surgery show altered meal patterns and eating behavior after the intervention. Several studies could demonstrate that RYGB surgery patients significantly improve their TFEQ scores one year after surgery, using the TFEQ-R18 and R21 version of the questionnaire, which are measuring the same traits.[Citation23–25] Similar results were published for gastric banding patients.[Citation26,Citation27] Hitherto, only one study investigated the relationship between presurgical TFEQ scores and weight loss after surgery.[Citation2] In contrast to our study, Burgmer et al. reported no association between presurgery TFEQ-scores and weight loss one year after the intervention. The different study outcome may be a result of the shorter follow-up interval. Sjöström et al. reported that the nadir of weight is reached around one year after the operation and is generally followed by a regain of weight.[Citation28] Based on these results, the two year postoperative observation period chosen by us may lead to more reliable results in TFEQ weight loss association analyses.
Other studies have shown a genetic impact on eating behavior. The heritability of cognitive restraint, emotional eating, and uncontrolled eating in a twin study is estimated to be around 59%, 60%, and 45%, respectively.[Citation29] Gast et al. reported a gene variant, located within the gene GRM8, to be associated with BMI and Cognitive restraint in a cohort of 548 healthy subjects.[Citation30] These findings support our concept that genetic variants may have the potential to impact weight via a change of eating behavior.
It can only be speculated how rs4846567 mediates the impact on eating behavior and weight loss. LYPLAL1 is expressed in adipose tissue and is overexpressed in obese patients.[Citation31] Furthermore, LYPLAL1 has a regulatory effect on ion channels through its depalmitoylation ability.[Citation32] Rs4846567 is located within a short, 401 base pair long insulator segment,[Citation33] which has the ability to disturb the interaction between promoter and enhancer regions and to block the propagation of heterochromatic structures and DNA-methylation in adjacent chromatin.[Citation34] It is possible that rs4846567 leads to a less available promoter region and consecutively to an altered expression of LYPLAL1. LYPLAL1 is strongly expressed in the hypothalamus, brain stem, and substantia nigra [Citation35] – regions that are known to regulate energy metabolism.[Citation36–38] Altered expression of LYPLAL1 may therefore affect the reward systems and the strength of hunger feelings, resulting in an altered TFEQ score and an increased weight loss after bariatric surgery.
We investigated patients undergoing bariatric surgery in a one center setup. This is the first study that associates both eating behavior and genetics to therapy success in bariatric surgery. The Hunger factor of the TFEQ gave a clear indication of the clinical relevance of the questionnaire. Patients scoring <5 (quartile 1) reached normal weight, while the higher scorers had an up to 32% less EBMIL, which would translate into a difference of 4.0 kg/m2. The difference in EBMIL for the SNP rs4846567 was 7%. These findings suggest a relevant effect of the genetic variant on RYGB outcome, translating into about 1.2 kg/m2 weight loss. The previously reported association of rs4846567 to BMI-adjusted WHR when interacting with diet [Citation21] and WHR,[Citation13] further strengthen our findings. Still, our study is of correlative nature, and further investigations are needed to confirm our findings and explain the molecular biological mechanisms underlying the effect.
In summary, we demonstrate that the gene variant rs4846567 is associated with the strength of eating behavior before surgery and the magnitude of EBMIL after RYGB surgery. Both a preoperative genotyping and a TFEQ-based evaluation of eating behavior may help to assess the chances of therapy success after bariatric surgery.
Ethics, consent, and permissions
The study was performed according to the 1964 Declaration of Helsinki and its later amendments. All study participants provided written informed consent to the use of their clinical data and blood samples for genetic analyses. The study protocol was approved by the Cantonal Ethic committee St. Gallen.
Supplementary Table
Download MS Excel (13.8 KB)Acknowledgements
We thank all of our patients who provided the blood samples and clinical information. Genotyping was performed by the SNP&SEQ Technology Platform in Uppsala. The platform is part of Science for Life Laboratory at Uppsala University and is supported as a national infrastructure by the Swedish Research Council. The study was supported by the Swedish Research Foundation, The Åhlens Foundation, and the Swedish Brain Research Foundation.
Disclosure statement
The authors declare no competing interest.
References
- Benoit SC, Hunter TD, Francis DM, et al. Use of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgery. Obes Surg. 2014;24:936–943.
- Burgmer R, Grigutsch K, Zipfel S, et al. The influence of eating behavior and eating pathology on weight loss after gastric restriction operations. Obes Surg. 2005;15:684–691.
- Elfhag K, Rössner S, Barkeling B, et al. Sibutramine treatment in obesity: initial eating behaviour in relation to weight loss results and changes in mood. Pharmacol Res. 2005;51:159–163.
- Bamia C, Orfanos P, Ferrari P, et al. Dietary patterns among older Europeans: the EPIC-Elderly study. Br J Nutr. 2005;94:100–113.
- Mostad IL, Langaas M, Grill V. Central obesity is associated with lower intake of whole-grain bread and less frequent breakfast and lunch: results from the HUNT study, an adult all-population survey. Appl Physiol Nutr Metab. 2014;39:819–828.
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83.
- Lindroos AK, Lissner L, Mathiassen ME, et al. Dietary intake in relation to restrained eating, disinhibition, and hunger in obese and nonobese Swedish women. Obes Res. 1997;5:175–182.
- French SA, Mitchell NR, Wolfson J, et al. Questionnaire and laboratory measures of eating behavior. Associations with energy intake and BMI in a community sample of working adults. Appetite. 2014;72:50–58.
- Drapeau V, Provencher V, Lemieux S, et al. Do 6-y changes in eating behaviors predict changes in body weight? Results from the Québec Family Study. Int J Obes Relat Metab Disord. 2003;27:808–814.
- Gallant AR, Tremblay A, Perusse L, et al. The Three-Factor Eating Questionnaire and BMI in adolescents: results from the Québec family study. Br J Nutr. 2010;104:1074–1079.
- Thurnheer M, Bisang P, Ernst B, et al. A novel distal very long Roux-en Y gastric bypass (DVLRYGB) as a primary bariatric procedure–complication rates, weight loss, and nutritional/metabolic changes in the first 355 patients. Obes Surg. 2012;22:1427–1436.
- Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg. 2014;24:1729–1736.
- Heid IM, Jackson AU, Randall JC, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42:949–960.
- Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948.
- Jacobsson JA, Schioth HB, Fredriksson R. The impact of intronic single nucleotide polymorphisms and ethnic diversity for studies on the obesity gene FTO. Obes Rev. 2012;13:1096–1109.
- Rask-Andersen M, Jacobsson JA, Moschonis G, et al. The MAP2K5-linked SNP rs2241423 is associated with BMI and obesity in two cohorts of Swedish and Greek children. BMC Med Genet. 2012;13:36. doi: 10.1186/1471-2350-13-36.
- Dougkas A, Yaqoob P, Givens DI, et al. The impact of obesity-related SNP on appetite and energy intake. Br J Nutr. 2013;110:1151–1156.
- Llewellyn CH, Trzaskowski M, van Jaarsveld CH, et al. Satiety mechanisms in genetic risk of obesity. JAMA Pediatrics. 2014;168:338–344.
- Deitel M, Greenstein RJ. Recommendations for reporting weight loss. Obes Surg. 2003;13:159–160.
- R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014.
- Nettleton JA, Follis JL, Ngwa JS, et al. Gene × dietary pattern interactions in obesity: analysis of up to 68 317 adults of European ancestry. Hum Mol Genet. 2015;24:4728–4738.
- Delahanty LM, Pan Q, Jablonski KA, et al. Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diabetes Care. 2012;35:363–366.
- Petereit R, Jonaitis L, Kupcinskas L, et al. Gastrointestinal symptoms and eating behavior among morbidly obese patients undergoing Roux-en-Y gastric bypass. Medicina (Kaunas). 2014;50:118–123.
- Laurenius A, Larsson I, Bueter M, et al. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond). 2012;36:348–355.
- de Lauzon B, Romon M, Deschamps V, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134:2372–2380.
- Nickel C, Widermann C, Harms D, et al. Patients with extreme obesity: change in mental symptoms three years after gastric banding. Int J Psychiatr Med. 2005;35:109–122.
- Schindler K, Prager G, Ballaban T, et al. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur J Clin Investig. 2004;34:549–554.
- Sjöström L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752.
- Tholin S, Rasmussen F, Tynelius P, et al. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. Am J Clin Nutr. 2005;81:564–569.
- Gast MT, Tonjes A, Keller M, et al. The role of rs2237781 within GRM8 in eating behavior. Brain Behav. 2013;3:495–502.
- Steinberg GR, Kemp BE, Watt MJ. Adipocyte triglyceride lipase expression in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E958–E964.
- Tian L, McClafferty H, Knaus HG, et al. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels. J Biol Chem. 2012;287:14718–14725.
- Rosenbloom KR, Armstrong J, Barber GP, et al. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 2015;43:D670–D681.
- Ghirlando R, Giles K, Gowher H, et al. Chromatin domains, insulators, and the regulation of gene expression. Biochim Biophys Acta. 2012;1819:644–651.
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399.
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543.
- Ono T, Nakamura K, Nishijo H, et al. Hypothalamic neuron involvement in integration of reward, aversion, and cue signals. J Neurophysiol. 1986;56:63–79.
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
