Abstract
Healthcare workers (HCWs) are at high risk of acquiring emerging infections while caring for patients, as has been shown in the recent SARS and swine flu epidemics. Using SARS as an example, we determined the effectiveness of infection control measures (ICMs) by logistic regression and structural equation modelling (SEM), a quantitative methodology that can test a hypothetical model and validates causal relationships among ICMs. Logistic regression showed that installing hand wash stations in the emergency room (p = 0.012, odds ratio = 1.07) was the only ICM significantly associated with the protection of HCWs from acquiring the SARS virus. The structural equation modelling results showed that the most important contributing factor (highest proportion of effectiveness) was installation of a fever screening station outside the emergency department (51%). Other measures included traffic control in the emergency department (19%), availability of an outbreak standard operation protocol (12%), mandatory temperature screening (9%), establishing a hand washing setup at each hospital checkpoint (3%), adding simplified isolation rooms (3%), and a standardized patient transfer protocol (3%). Installation of fever screening stations outside of the hospital and implementing traffic control in the emergency department contributed to 70% of the effectiveness in the prevention of SARS transmission. Our approach can be applied to the evaluation of control measures for other epidemic infectious diseases, including swine flu and avian flu.
Introduction
Severe acute respiratory syndrome (SARS) is a serious viral infection that was recognized as a global threat in March 2003. Healthcare workers (HCWs) were found to be at unusually high risk of acquiring SARS while caring for SARS patients [Citation1–5]. Many infection control measures (ICMs) were implemented to protect HCWs during the panic of the SARS epidemic. Although conventional ICMs, such as personal protective equipment (PPE), including N95 respirators and isolation gowns, and negative pressure isolation rooms (NPIRs), were implemented by various hospitals [Citation1,Citation5,Citation6], nosocomial SARS transmission among HCWs still occurred in 36% (16/48) of hospitals caring for SARS patients during the SARS epidemic in Taiwan [Citation7].
During the epidemic, many ICMs were proposed to halt the spread of the SARS virus. However, no data were available to evaluate the collective effectiveness of these measures in preventing SARS virus transmission. Given the nature of such a virulent infection, HCWs and the general public tend to implement as many protection measures as possible, whether these measures are evidence-based, or as a result of anecdotal experience, or simply a myth spread around the World Wide Web. Unfortunately, over-protection can also be counterproductive [Citation8], and can cannibalize the healthcare resources for other services.
Our preliminary analysis of 99 factors of infection control did not reveal any factor that was statistically significant in protecting HCWs from acquiring SARS (all p > 0.05) (data not shown). In this study, we used 2 different methods: a time-dependent analysis and structural equation modelling (SEM). Time-dependent analysis deals with logistical analysis over time and was used to test our hypothesis that ‘earlier is better’. SEM was used to determine the causal relationship between the ICMs and the outcome (healthcare worker acquiring SARS or not). Based on the hypothesis of ‘earlier is better’ rather than ‘more is better’ in terms of ICM implementation [Citation1,Citation6–8], we analyzed the influence of timing on the effectiveness of ICMs by logistic regression. Furthermore, we used SEM, a quantitative methodology, to test hypothetical infection control models using a causal relationship algorithm among variables. SEM is a combination of path analysis (to test the fit of the correlation matrix against 2 or more causal models) and factor analysis (to determine the latent structure (dimensions) of a set of variables), facilitating the investigation of causal relationships among both measured and latent variables. The particular advantage of SEM involving latent variables is that causal relationship theories can be investigated as they pertain directly to the underlying constructs of interest, rather than to the measured variables whose observed relationships are often attenuated by error of measurement. The use of latent variables allows researchers to explicitly test the unreliability of measurement in the model. Factor analysis, path analysis and regression all represent cases of SEM. SEM has been applied in healthcare research for the determination of causal relationships [Citation9–13]. Calis et al. used SEM to determine what causal factors contributed to severe anaemia among children in Malawi [Citation11]. Hansen et al. demonstrated excellent fit with study data and hypothesized paths that personality disorders have a direct negative effect on HIV symptoms [Citation10].
Thus, the objective was to determine the hypothetical infection control model that has the highest correlation to effectiveness in protecting HCWs from SARS using SEM, especially in determining what ICMs contribute most to the control of nosocomial infections in HCWs. In this study, we created a latent variable to represent ‘traffic control in the emergency department’ which has 4 measured variables. By determining what combination of ICMs could cause the termination of SARS nosocomial infection among HCWs, a cost-effective infection control strategy can be better determined based on quantitative data.
Materials and methods
Study hospitals and data acquisition
Forty-eight hospitals that provided hospitalization for SARS patients (total of 664 SARS patients including 119 HCWs who were epidemiologically linked by nosocomial infection) during the 2003 SARS epidemic in Taiwan were surveyed. Sixteen hospitals had episodes of nosocomial infection of SARS in HCWs. After the epidemic ended, infection control practitioners performed a survey in these 48 hospitals. The survey examined what ICMs were implemented in the hospitals and when they were implemented. A total of 99 factors were included in the survey. As the default, if there were data missing in the survey, it was considered that the hospital did not implement the ICM in question. Factors in which missing values exceeded 50% were excluded from further analysis.
We arbitrarily defined 21 April 2003 as day-0 for all analyses in this study; this was the day on which the whole country was alerted to the sudden closure of Ho-Ping City Hospital due to the serious nosocomial infections that had occurred at that hospital [Citation14].
Time-dependent analysis
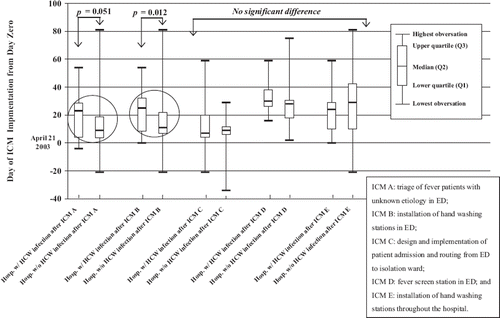
We used a box plot diagram to demonstrate the effect of the time variable when hospitals implemented certain ICMs, because single and multiple variable analyses did not consider time of ICM implementation as a variable. By using an expert panel discussion, we chose 5 major ICMs and determined the correlation of time of implementation and effectiveness of preventing nosocomial SARS transmission in HCWs. These measures were: (A) triage of fever patients with unknown aetiology in the emergency department (ED); (B) installation of hand washing stations in the ED; (C) design and implementation of patient admission and transfer from the ED to the isolation ward; (D) fever screening station in the ED; and (E) installation of hand washing stations throughout the hospital. We calculated the 25% (lower quartile, Q1), 50% (median, Q2), and 75% (upper quartile, Q3) of the 16 hospitals when the hospital implemented the above-mentioned 5 ICMs. Logistic regression was used for analysis using days of ICM implementation as a predictor variable.
Causal relationship modelling
The original survey data determined whether the hospital administration implemented the ICMs or not. We hypothesized that the dependent variable (Y) would be varied over time depending on whether or not all effective ICMs were implemented. We adopted the concept of time series modelling and generated a series of data sets for each hospital. The original surveys were transformed into variables for the purpose of determining what ICMs can effectively protect HCWs. Our data series ended on 5 July 2003 (at T = 55) when the World Health Organization removed Taiwan from its list of areas with recent local transmission of SARS. The period of highest transmission ability from SARS patient to others occurred between the onset of high fever and 7 days following this [Citation15–17]. Thus, we added 7 days to our dependent variable (Y) to better quantify the risk of hospital with the admission of SARS patients.
SPSS AMOS 5.0 software was used for SEM analysis (SPSS Inc., Chicago, IL, USA). For evaluating the performance of the causal relationship models, we defined the correlation coefficient (r) of each ICM as the importance or contribution to the model. There are 3 indicators for the assessment of goodness-of-fit: the comparative fit index (CFI), the standardized root mean-square residual (SRMR), and the R-square (R2). CFI (0–1) compares the existing model fit with a null model that assumes the measured and latent variables in the model are uncorrelated (the ‘independence model’). The CFI value indicates the fit between the null model and the researcher’s model. A CFI of >0.9 is considered a good fit. SRMR is the average difference between the predicted and observed variances and co-variances in the model and is based on standardized residuals. The smaller the SRMR, the better the model fit. SRMR = 0 indicates perfect fit. An SRMR value of <0.05 is widely considered good fit and <0.08 is considered adequate fit. R2 is the squared multiple correlations of observed variables and serves as the reliability indicator of the extent to which each adequately measures its respective underlying model. An R2 value of >0.9 indicates highly correlated, whereas an R2 between 0.6 and 0.9 indicates a medium correlation. The appropriateness of the SEM model was determined, in priority order, by (1) CFI > 0.9; (2) SRMR < 0.05; (3) R2 > 0.9. If all 3 conditions were not fulfilled at the same time, the CFI had to be greater than 0.9 with either SRMR < 0.05 or R2 > 0.9 arbitrary.
Results
Time-dependent analysis
Sixteen hospitals in this study had episodes in which SARS was transmitted to HCWs. All 16 hospitals had implemented the 5 ICMs, as described in the Materials and methods section, and as required by the Taiwan Centers for Disease Control (CDC). However, even after all 5 ICMs were implemented, 25% (4/16) of hospitals still experienced nosocomial transmission of the SARS virus to HCWs. When we determined the effectiveness of the 5 major ICMs using the day of implementation as the predictor, we found that only ICM B (installation of hand washing stations in the ED) was significantly associated (p = 0.012, odds ratio 1.07; 95% CI 1.02–1.14) with a hospital that had nosocomial transmission of SARS in HCWs, while ICM A (triage of fever patients with unknown aetiology in the ED) was closely associated (p = 0.051, odds ratio 1.04; 95% CI 1.0–1.08) (). ICMs C, D and E did not have a significant impact on the hospital (data not shown).
SEM model with optimal CFI
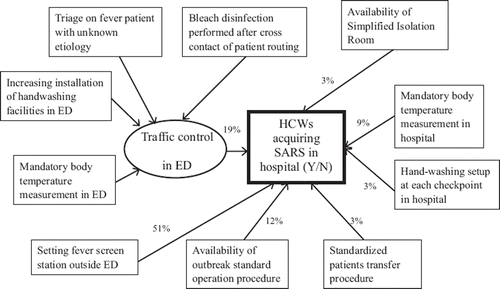
The CFI, SRMR, and R2 of our SEM model were 0.976, 0.0253, and 0.67 (), respectively. Our results fulfilled the first 2 criteria (CFI > 0.9; SRMR < 0.05), but the R2 of 0.67 only indicated medium correlation. The SEM model included 1 latent variable, traffic control in the ED, and 6 independent factors. Since ‘traffic control in the ED’ had a significant impact on the prevention of SARS among HCWs [Citation7], we selected several factors to form a latent variable ‘traffic control in the ED’ to represent this concept. The most important factor (highest proportion of effectiveness) was installation of a fever screening station outside the ED (51%). Other factors included a latent variable of traffic control in the ED (19%), availability of outbreak standard operation protocol (12%), mandatory patient body temperature surveillance in hospital (9%), hand washing setup at each checkpoint in the hospital (3%), availability of a simplified isolation room (3%), and a standardized patient transfer protocol (3%) ().
More than 100 different combinations of ICMs associated with traffic control in the ED were tested for determination of the latent variable. The following 4 measures were obtained based on the highest correlation to the latent variable of traffic control in the ED: triage for patients with fever of unknown aetiology (correlation r = 0.87), increasing installation of hand washing facilities in the ED (r = 0.75), bleach disinfection performed after crossing clean zones and during (cross-contact) patient transfer (r = 0.86), and mandatory body temperature surveillance in the ED (r = 0.82).
Discussion
Healthcare workers are at risk of acquiring a SARS infection while caring for SARS patients. Many rational ICMs were implemented to protect HCWs during the panic of the SARS epidemic. For example, NPIR, PPE, and hand washing were all implemented by each hospital as required by the health department. However, nosocomial transmission still occurred despite the above-mentioned measures [Citation1,Citation5,Citation6,Citation14]. Yen et al. formulated the concept of traffic control, an integrated infection control strategy involving triaging patients (using barriers and zones of risk) and checkpoint spots for hand washing [Citation7]. Traffic control was shown to significantly reduce the rate of SARs in HCWs (0.03 cases/bed vs HCWs in other hospitals 0.13 cases/bed) during the 3-week study period [Citation7]. However, there were limitations in the study; the authors were unable to demonstrate that traffic control was the key factor in reducing the number of HCWs acquiring the SARS infection. Understanding the causal relationship is important to validate the effectiveness of each ICM among so many integrated measures.
Our analysis showed that the timing of the ICM implementation was critically important. Hospitals in which HCW SARS transmission did not occur implemented ICM B earlier than those experiencing SARS transmission to HCWs (). The hypothetical causal relationship model between the ICMs and the HCWs acquiring SARS was supported by our SEM analysis in which the following ICMs were the causes of preventing SARS transmission: traffic control in the ED (including triage on patients with fever of unknown aetiology, increasing installation of hand washing facilities in the ED, bleach disinfection performed after cross-contact in patient transfer, and mandatory body temperature surveillance), installation of a fever screening station outside the ED, availability of an outbreak standard operation protocol, mandatory body temperature surveillance in hospital, hand washing setup at each checkpoint in the hospital, standardized patient transfer procedure, availability of a simplified isolation room. It appears that successful control of SARS infection is not based on an individual measure, but on the integration of several measures.
Installation of a fever screening station outside the ED was the most important factor, and contributed 51% of the effectiveness towards the prevention of SARs in HCWs. During the SARS epidemic, as patients overwhelmingly flooded into hospitals and caused chaos within the ED [Citation6,Citation18] it was rational for each hospital to screen the patients outside the hospital. The outside fever screening station was first developed by the Singaporeans [Citation19]; this was done in accordance with the ancient concept of quarantine against the black plague during the 14th century. Our finding quantitatively validated this approach. The fever screening station acted as the security guard in the front line of protection.
The second most important factor was the traffic control measures in the ED (19%). HCWs in the ED are at the front line of contact with SARS patients and the most likely to be infected [Citation6,Citation18,Citation20]. The environmental survey also found a high sample positive rate for SARS coronavirus RNA in the ED [Citation6]. Our finding validated the need to protect HCWs in the ED using traffic control, as this is an important factor for protecting all HCWs from hospital-acquired SARS. The retrospective observation also confirmed our finding that the nosocomial transmission of SARS in Taiwan declined significantly after the implementation of traffic control in the ED of hospitals as mandated by the Department of Health. By implementing traffic control in the ED, direct contact of SARS patients and contamination of the environment can be minimized by screening patients with fever, transferring and isolating patients, and disinfecting the environment.
Hospital management is important since it affects the other factors associated with protecting HCWs from SARS, including availability of outbreak standard operation protocols (12%), mandatory body temperature surveillance in hospital (9%), hand washing setups at each checkpoint in hospital (3%), availability of a simplified isolation room (3%), and standardized patient transfer protocols (3%). During the SARS epidemic, body temperature measurement and hand washing were encouraged spontaneously, and the compliance rate was higher during the non-epidemic period. Standardized outbreak control and patient transfer procedures were part of the hospital standard operation protocols. Hospitals with better management are less likely to have HCWs acquiring SARS or any other nosocomial infections. The Six Sigma process may be implemented into the hospital infection control procedures to prevent further unknown infections [Citation21–23].
The major weakness of this study is that the survey form did not go through rigorous reliability and validity testing before the study, given the panic of the epidemic. In addition, no molecular subtyping was performed in this study so we were unable to prove that the HCWs who acquired SARS were epidemiologically linked to the SARS patients to whom they were exposed.
In summary, traditional statistics for association analysis cannot clarify causal relationships. We used structural equation modelling to validate the measures that halted the SARS transmission among HCWs. In addition, early implementation of ICMs proved important for preventing transmission. This approach can be applied to the evaluation of control measures for other epidemic infectious diseases, including swine flu and avian flu. Outdoor epidemic screening/quarantine stations and detention wards can be developed as a first line of protection. The concept of traffic control can be applied in the emergency department, and even throughout the hospital, to prevent direct or casual contact of infected patients during transportation and treatment. When HCWs are protected from nosocomial outbreaks, healthcare services can remain available to the patients.
Acknowledgement
The authors thank Dr Victor L. Yu at University of Pittsburgh for his review and criticisms.
Declaration of interest: All authors on this manuscript report no conflict of interest.
References
- Chen YC, Chen PJ, Chang SC, Kao CL, Wang SH, Wang LH, . Infection control and SARS transmission among healthcare workers, Taiwan. Emerg Infect Dis 2004; 10:895–8.
- Chia SE, Koh D, Fones C, Qian F, Ng V, Tan BH. Appropriate use of personal protective equipment among healthcare workers in public sector hospitals and primary healthcare polyclinics during the SARS outbreak in Singapore. Occup Environ Med 2005;62:473–7.
- Kissoon N. The threat of severe acute respiratory syndrome (SARS). West Indian Med J 2003;52:91–4.
- Lim WS, Anderson SR, Read RC. Hospital management of adults with severe acute respiratory syndrome (SARS) if SARS re-emerges—updated 10 February 2004. J Infect 2004;49:1–7.
- Twu SJ, Chen TJ, Chen CJ, Olsen SJ, Lee LT, Fisk T, . Control measures for severe acute respiratory syndrome (SARS) in Taiwan. Emerg Infect Dis 2003;9:718–20.
- Chen YC, Huang LM, Chan CC, Su CP, Chang SC, Chang YY, . SARS in hospital emergency room. Emerg Infect Dis 2004;10:782–8.
- Yen MY, Lin YE, Su IJ, Huang FY, Ho MS, Chang SC, . Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp Infect 2006;62:195–9.
- Chow CB. Post-SARS infection control in the hospital and clinic. Paediatr Respir Rev 2004;5:289–95.
- Hayashi N, Yamashina M, Taguchi H, Ishige N, Igarashi Y. Schizophrenic outpatient perceptions of psychiatric treatment and psychotic symptomatology: an investigation using structural equation modeling. Psychiatry Clin Neurosci 2001;55:587–93.
- Hansen NB, Vaughan EL, Cavanaugh CE, Connell CM, Sikkema KJ. Health-related quality of life in bereaved HIV-positive adults: relationships between HIV symptoms, grief, social support, and Axis II indication. Health Psychol 2009;28:249–57.
- Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, . Severe anemia in Malawian children. N Engl J Med 2008;358:888–99.
- Nyamathi A, Stein JA, Schumann A, Tyler D. Latent variable assessment of outcomes in a nurse-managed intervention to increase latent tuberculosis treatment completion in homeless adults. Health Psychol 2007;26:68–76.
- Rhodes SD, Arceo R. Developing and testing measures predictive of hepatitis A vaccination in a sample of men who have sex with men. Health Educ Res 2004;19: 272–83.
- Anonymous. Severe acute respiratory syndrome—Taiwan, 2003. MMWR Morb Mortal Wkly Rep 2003;52:461–6.
- Chen MI, Tan IB, Ng YY. Modelling the utility of body temperature readings from primary care consults for SARS surveillance in an army medical centre. Ann Acad Med Singapore 2006;35:236–41.
- Liu CL, Lu YT, Peng MJ, Chen PJ, Lin RL, Wu CL, . Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest 2004;126:509–17.
- Pang X, Zhu Z, Xu F, Guo J, Gong X, Liu D, . Evaluation of control measures implemented in the severe acute respiratory syndrome outbreak in Beijing, 2003. JAMA 2003;290:3215–21.
- Chang WT, Kao CL, Chung MY, Chen SC, Lin SJ, Chiang WC, . SARS exposure and emergency department workers. Emerg Infect Dis 2004;10:1117–9.
- Chong WC, Tham KY, Goh HK, Seow E. Presentation of severe acute respiratory syndrome (SARS) patients in a screening centre. Singapore Med J 2005;46:161–4.
- Wang FD, Chen YY, Lee YM, Chan YJ, Chen TL, Lue JF, . Positive rate of serum SARS-CoV immunoglobulin G antibody among healthcare workers. Scand J Infect Dis 2007;39:152–6.
- Eldridge NE, Woods SS, Bonello RS, Clutter K, Ellingson L, Harris MA, . Using the six sigma process to implement the Centers for Disease Control and Prevention Guideline for Hand Hygiene in 4 intensive care units. J Gen Intern Med 2006;21(Suppl 2):S35–42.
- Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg 2005;201:349–58.
- Simmons-Trau D, Cenek P, Counterman J, Hockenbury D, Litwiller L. Reducing VAP with 6 Sigma. Nurs Manage 2004;35:41–5.