Abstract
1. Humanized-liver mice, in which the liver has been repopulated with human hepatocytes, have been used to study aspects of human liver physiology such as drug metabolism, toxicology and hepatitis infection. However, the procurement of human hepatocytes is a major problem in producing humanized-liver mice because of the finite nature of the patient-derived resource.
2. In order to overcome this limitation, the human hepatic cell line HepaRG® were evaluated as promising donor cells for liver reconstitution in the TK-NOG mouse model.
3. We demonstrate that, in vivo, transplanted confluent culture or differentiated HepaRG® cells proliferated and differentiated toward both hepatocyte-like and biliary-like cells within the recipient liver. In contrast, proliferative HepaRG® cells could engraft TK-NOG mouse liver but could differentiate only toward biliary-like cells. The differentiation to hepatocyte-like cells was characterized by the detection of human albumin in the recipient mouse serum and was confirmed by immunohistochemical staining for human leukocyte antigen, human albumin, cytochrome P450 3A4, and multidrug resistance-associated protein 2. Biliary-like cells were characterized by positive staining for cytokeratin-19.
4. These results indicated that the differentiated HepaRG® cells are a possible cell source for generating humanized-liver mice, which are a useful model for in vivo studies of liver physiology.
Introduction
The liver performs many complex functions, including carbohydrate, urea and lipid metabolism, storage of essential nutrients, production of plasma proteins and secretion of bile acids, metabolism of drugs and other compounds and excretion of the metabolites into bile canaliculi. Although primary cultured hepatocytes are the standard model for xenobiotic metabolism and toxicity studies, an in vitro culture method that maintains the in vivo functions of the hepatocytes has not been established (Nibourg et al., Citation2012). To overcome this issue, we and other groups have developed humanized-liver mice in which the liver is reconstituted with human liver cells for studying in vivo drug metabolism and liver regeneration (Azuma et al., Citation2007; Dandri et al., Citation2001; Hasegawa et al., Citation2011; Mercer et al., Citation2001). The reconstituted livers also express enzymes found in human hepatocytes, and they can generate human-specific metabolites of test substrates, including steroids. One of the problems in generating humanized-liver mice is the cell source for liver reconstitution. Commercially available cryopreserved human hepatocytes are the easiest to use for generating humanized-liver mice at present; however, it is well known that individual differences not only affect the success rate of generating chimeric mice but also influence the drug-metabolizing properties of the humanized livers. As primary human hepatocytes never successfully proliferate in vitro, it is difficult to stably generate humanized-liver mice with finite hepatocytes.
Novel cell sources that can proliferate in vitro and reconstitute the liver in vivo are needed to achieve steady generation of humanized-liver mice. In this study, we focused on HepaRG® cells as a cell source for generating humanized-liver mice. HepaRG® is an immortalized cell line that was isolated from a hepatic-differentiated grade 1 Edmonson hepatocholangiocarcinoma (Gripon et al., Citation2002). Previous studies have demonstrated that bipotent progenitor HepaRG® cells that have the ability to differentiate into both hepatocyte-like and biliary-like epithelial phenotypes in vitro (Cerec et al., Citation2007). Because fully differentiated HepaRG® cells express physiologic functions similar to primary cultured human hepatocytes, they are regarded as an in vitro model of drug metabolism (Guillouzo et al., Citation2007; Kanebratt & Andersson, Citation2008). A few studies have reported the successful engraftment of HepaRG® cells into the mouse liver (Cerec et al., Citation2007; Jiang et al., Citation2010) and have described the in vivo expression of human serum albumin from the transplanted HepaRG® cells. However, the engraftment of HepaRG® cells was confirmed by immunohistochemical staining with the mature hepatocyte marker albumin, and the expression of drug-metabolizing enzymes in vivo has not been investigated. Thus, it remains unclear whether HepaRG® cells engrafted into the mouse liver preserve their capacity to undergo complete hepatocyte maturation in vivo.
Recently, we developed a novel humanized model with inducible liver injury platform consisting of the targeted expression of the herpes simplex virus type 1 thymidine kinase (HSV-TK) in the liver of severely immunodeficient NOG mice (TK-NOG) (Hasegawa et al., Citation2011). A brief exposure to a non-toxic dose of ganciclovir (GCV) causes mouse liver cells expressing the transgene to be ablated. Then, the transplanted human hepatocytes are stably maintained within the liver of TK-NOG mice in the absence any exogenous drug or immunosuppressive treatments. However, the procurement of human hepatocytes is a major problem in producing humanized-liver mice because of the finite nature of the patient-derived resource. Therefore, we evaluate HepaRG® cells as promising donor cells for liver reconstitution in the TK-NOG mouse model to over come this problem.
Materials and methods
HepaRG® cells
In accordance with the manufacturer’s protocol, HepaRG® cells were maintained in HepaRG® maintenance medium (Biopredic International, Rennes, France). For differentiation, HepaRG® cells were seeded onto six-well plates at a density of 2 × 104 cells/cm2. Cells were cultured in maintenance medium until the seventh day after plating, when the medium was replaced with HepaRG® differentiation medium containing 1.7% dimethyl sulfoxide (DMSO) (Biopredic International, Rennes, France). Both the maintenance medium and the differentiation medium were changed every 2 days.
Real-time quantitative reverse transcription polymerase chain reaction for expression of drug metabolism-related genes
Total RNA was obtained from HepaRG® cells for each day of differentiation using the RNeasy Mini Kit (Qiagen K.K., Tokyo, Japan). Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed using the High Capacity cDNA Reverse Transcription kit (Life Technologies Corporation, Grand Island, NY). The TaqMan Gene Expression Master Mix and TaqMan Gene Expression Assays (Life Technologies Corporation, Grand Island, NY) were used for RT-PCR, and amplification was then carried out using an ABI Prism 7000 Sequence Detection System (Life Technologies Corporation, Grand Island, NY). The amount of cDNA was normalized to the expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The TaqMan Assay number is listed in Supplementary Table 1.
Induction of liver damage
All mouse studies were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the Central Institute for Experimental Animals, and the experimental protocols were approved by the Animal Care Committee of CIEA (Permit Number: 11029A). All surgeries were performed under isoflurane anesthesia, and all efforts were made to minimize suffering. The TK-NOG strain (Hasegawa et al., Citation2011) was maintained by breeding female Tg TK-NOG mice with male non-Tg TK-NOG littermates, and the transgenic offspring were selected by genotyping with the following TaqMan probe set: forward primer (TaqMan-TKF), 5′-CCATGCACGTCTTTATCCTGG-3′; reverse primer (TaqMan-TKR), 5′-TAAGTTGCAGCAGGGCGTC-3′; and TaqMan probe (TK-FAM), 5′-FAM-AATCGCCCGCCGGCTGC-MGB-3′. Adult 8–10-week-old male TK-NOG mice were injected intraperitoneally with GCV sodium (6 mg/kg, Denosine-IV; Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) every other day to ablate mouse liver cells expressing HSV-TK transgene. One week after GCV treatment, the degree of liver damage was examined by determining serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values using an automated clinical chemistry analyzer FUJI DRI-CHEM 7000 (Fuji Photo Film Co. Ltd, Tokyo, Japan).
Transplantation
On Days 1, 7, 21 or 35 after plating, differentiated HepaRG® cells were dissociated by 0.25% trypsin–EDTA and transplanted into TK-NOG mouse.
Cryopreserved human hepatocytes (HEP187170; 26 years, female; Biopredic International, Rennes, France) were used as a positive control for transplantation.
A total of 1 × 106 cells in 40 μl of Hank’s Balanced Salt Solution (HBSS, Life Technologies Corporation, Grand Island, NY) were intrasplenically injected using a Hamilton syringe with a 26 gauge needle, as previously described (Suemizu et al., Citation2008). The successful engraftment of the HepaRG® cells and the HEP187170 hepatocytes was evaluated by measuring increases in the mouse blood level of human albumin (hAlb) with a Human Albumin ELISA Quantitation Kit (Bethyl Laboratories, Montgomery, TX), according to the manufacturer’s protocol. The extent of reconstitution with human hepatocytes was estimated as a function of the hAlb concentration, which was shown to correlate with the extent of human liver replacement (Hasegawa et al., Citation2011). Twelve weeks after transplantation, recipient livers were recovered and fixed with 10 nM Mildform formaldehyde solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan). To evaluate the growth potential of the HepaRG® cell lines in vivo, TK-NOG mice received a subcutaneous (s.c.) injection of 1 × 106 HepaRG® cells suspended in 0.1 ml of HBSS and 0.1 ml of Matrigel (BD Biosciences, Bedford, MA). The human hepatocellular carcinoma cell line HepG2 (obtained from the American Type Culture Collection; Manassas, VA) that has the ability to form subcutaneous xenografts was used as positive control. A total of 1 × 104 HepG2 cells were suspended in 0.1 ml of HBSS:Matrigel in a 1:1 solution and injected s.c. Apparent tumors were measured biweekly. The tumor volume (TV) was calculated using the formula TV = ½ × A × B2 [A: length (mm); B: width (mm)].
Histology and immunohistochemistry
Formalin-fixed tissues were embedded in paraffin and then sliced and analyzed by either hematoxylin–eosin staining (H&E) or immunohistochemistry. Some sections were autoclaved for 10 min in a target retrieval solution (0.1 M citrate buffer, pH 6.0; 1 mM EDTA, pH 9.0) and then placed at room temperature for 20 min. The following antibodies were used for immunohistochemical analysis: monoclonal mouse anti-human leukocyte antigen (HLA) Class I (A, B and C) (clone EMR8-5; Hokudo, Sapporo, Japan), polyclonal goat anti-human albumin (Bethyl Laboratories, Montgomery, TX), anti-cytokeratin-19 (CK-19, Novocastra Laboratories Ltd., Newcastle, UK), polyclonal rabbit anti-cytochrome P450 3A4 (CYP3A4) (Abcam Inc., Cambridge, MA) and anti-human multidrug resistance-associated protein 2 (MRP2) (clone M2 III-6; Merck Millipore, Billerica, MA). The antibodies for mouse, goat and rabbit immunoglobulins were visualized using amino acid polymer/peroxidase complex-labeled antibodies [Histofine Simple Stain Mouse MAX PO (M, G and R); Nichirei Bioscience, Tokyo, Japan] and a diaminobenzidine (DAB; Dojindo Laboratories, Kumamoto, Japan) substrate (0.2 mg/mL 3,3′-diaminobenzidine tetrahydrochloride, 0.05 M Tris–HCl, pH 7.6, and 0.005% H2O2). Sections were counterstained with hematoxylin. A periodic acid-Schiff (PAS) staining kit (Muto Pure Chemicals, Tokyo, Japan) was used for visualizing glycogen. The images were captured under an upright microscope Axio Imager (Carl Zeiss, Thornwood, NY) equipped with AxioCam HRm and AxioCam MRc5 CCD cameras (Carl Zeiss). The HepaRG® cell colonies containing >20 HLA-positive cells on the cross-sections of three to five lobes of the TK-NOG mouse liver were counted. The areas (in centimeters) of the immunohistochemical sections were measured by using the Image Processing and Analysis in Java software (ImageJ version 1.46, http://imagej.nih.gov/ij/). Colony formation was evaluated as the number of colonies per area of observation (colonies/cm2).
Results
HepaRG® cells reconstitute the TK-NOG mouse liver
According to the manufacturer’s protocol, the HepaRG® cells were seeded at low confluency in six-well culture plates (2 × 104 cells/cm2; Day 1) to induce transdifferentiation (Cerec et al., Citation2007). The cells reached 100% confluency within 1 week (Day 7), and the medium was then replaced with the differentiation medium, which contained 1.7% DMSO. The hepatocyte-like cells, which are similar to mono- or bi-nucleated human hepatocytes, appeared 21 days after seeding (). The gene expression of the following drug metabolism-related molecules, in the HepaRG® cell differentiation cultures, was analyzed on Days 1, 7, 21 and 35 by qPCR: 12 CYP450 and 2 phase II enzymes, 5 SLC and 4 ABC transporters, and 3 nuclear hormone receptors (). As previously reported, the gene expression levels of the major drug metabolism enzymes and transporters were markedly increased in differentiative stage (>Day 7) compared to proliferative stage (Day 1) of HepaRG® cells, except for SLCO1B3. We then intrasplenically transplanted fully differentiated HepaRG® cells (Day 35) into five TK-NOG mice to investigate the functionality of the transplanted HepaRG® cells in vivo (). Twelve weeks after transplantation, the engraftment of the HepaRG® cells was demonstrated by human-specific marker staining: four-fifths of recipients showed the formation of HLA-positive cell colonies, suggesting that their livers had been repopulated with the transplanted human cells. The HLA-positive engrafted cell colonies were categorized by morphological differences into either hepatocyte-like cell colonies that were organized as polygonal cells with characteristic round nucleus or biliary-like cell colonies that were organized as ductal epithelial cells (). Histological analysis of the recipient livers suggests that the HepaRG® cells differentiated into mature hepatocyte or biliary cell lineages in vivo. To confirm this result, we performed immunohistochemical analysis for hepatocyte or biliary cell markers. Consistent with morphological characteristics, hepatocyte-like colonies were stained with the hepatocyte marker human albumin and were not stained with the biliary marker CK-19 (, upper panel), whereas biliary-like colonies were found to be Alb-negative/CK-19-positive colonies (, lower panel).
Figure 1. In vitro differentiation of HepaRG® cells. (A) Phase-contrast photographs of HepaRG® cells at the proliferative stage (D1: low-density culture), and the differentiative stage (D7: confluent culture; D21 and D35: differentiation culture with 1.7% DMSO). D1, 7, 21 and 35 in HepaRG® stage indicate the number of days after seeding. Bar = 100 μm. (B) The relative expression of 26 human drug metabolism-related mRNAs on D1, 7, 21 or 35 in the HepaRG® cells was assessed by qPCR. Each bar represents the average of two independent determinations, and the standard error is shown.
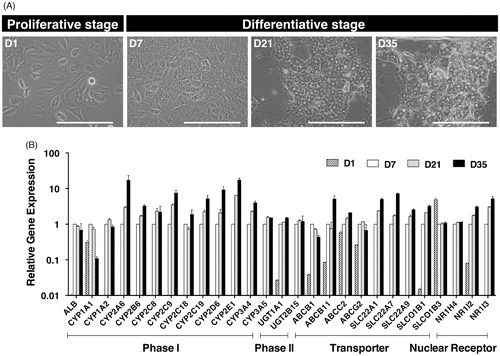
Figure 2. Reconstitution of human liver structures from differentiated HepaRG® cells in vivo. Hepatocyte-like colonies (upper panel) and biliary-like colonies (lower panel) in a TK-NOG mouse liver that was transplanted with differentiation D35 HepaRG® cells were subjected to immunohistochemical analysis. Serial liver sections were stained for H&E, HLA, hAlb and CK-19. Bar = 100 μm.
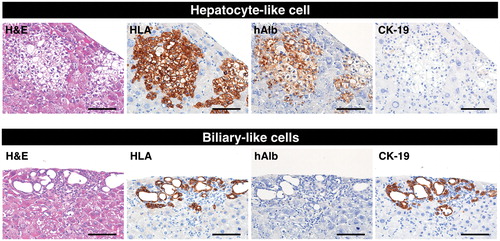
Table 1. Colony-forming ability of various differentiation stages of HepaRG® cells in TK-NOG livers.
HepaRG®-derived hepatocyte-like cells have the characteristics of mature hepatocytes
We have demonstrated that fully differentiated HepaRG® cells (Day 35) have the potential to transdifferentiate into both hepatocyte and biliary cells in vivo through bipotent progenitors in TK-NOG mice; however, the chimerism of the livers reconstituted with HepaRG® cells was extremely low, and human albumin was undetectable in mouse plasma. HepaRG® cells at various differentiation conditions (Day 1, 7, 21 and 35) were intrasplenically injected into TK-NOG mice to identify a suitable differentiation stage for optimal reconstitution of the mouse liver. Twelve weeks after transplantation, successful engraftment was determined using ELISA to detect the serum level of human albumin and was confirmed by histological analysis of TK-NOG mice livers. Human albumin was detected in two animals (2 out of 8) that had received 7-day (6.9 μg/ml) and 21-day (14.2 μg/ml) HepaRG® cells (). The HepaRG-derived colonies, which were categorized by morphologic characteristics into hepatocyte-like and biliary-like, were counted according to the criteria described in the “Materials and methods” section (). Interestingly, undifferentiated (proliferative) HepaRG® cells (Day 1) only differentiated into biliary-like cells in vivo; we failed to identify the differentiation into hepatocyte-like cells. In contrast, differentiated HepaRG® cells corresponding to cultures at Days 7 and 21 days were successfully engrafted, and gave rise to both hepatocyte-like and biliary-like cells in TK-NOG mouse livers. However, the repopulation and expansion potentials were markedly decreased in the fully differentiated HepaRG® cells (Day 35). Consistent with the hAlb ELISA result, an increased number of hepatocyte-like colonies was observed in the livers of recipient animals; this finding was confirmed by the production of detectable levels of human albumin ().
Next, the hepatocytic functionality of the differentiated HepaRG® cells was evaluated by histochemistry and immunohistochemistry. We compared mature human hepatocyte characteristics, including glycogen accumulation and the expression of the CYP3A4 and MRP2 proteins, between hepatocyte-like colonies in mouse livers repopulated with differentiated HepaRG® cells (day 21) and hepatocyte colonies in mouse livers repopulated with the HEP187170 hepatocytes, respectively. Successful engraftment of the HEP187170 hepatocytes was determined 12 weeks after transplantation by detecting the serum level of human albumin using ELISA. Human albumin was detected in all the HEP187170 hepatocytes transplanted animals (eight out of eight) with varied concentration ().
Table 2. Engraftment of cryopreserved human hepatocytes in TK-NOG livers.
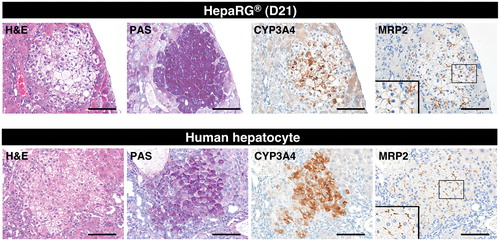
The human hepatocytes could be clearly distinguished from mouse hepatocytes by their pale cytoplasm in H&E stained sections and by glycogen accumulation, which was restricted to the cytoplasm of the human hepatocytes in PAS-stained sections (, lower panel); these findings are consistent with previous descriptions (Hasegawa et al., Citation2011). Hepatocyte-like cells exhibited H&E and PAS staining profiles similar to those of human hepatocytes (, upper panel). Furthermore, the immunohistochemical analysis of hepatocyte-like cells revealed that the expression of a major drug-metabolizing enzyme in the liver, CYP3A4, and a major organic anion transporter in bile excretion in the liver, MRP2, expression level of which were also similar to those of humanized livers repopulated by the HEP187170 hepatocytes (, upper and lower panel). These results imply that the hepatocyte-like cells that were differentiated in vivo could preserve similar drug-metabolizing activities to those of HepaRG® cells that were differentiated in vitro.
Figure 3. Expression of functional liver markers in reconstituted livers from TK-NOG mice. Hepatocyte-like colonies in a TK-NOG mouse liver that was transplanted with differentiation D21 HepaRG® cells (upper panel) and human hepatocyte colonies in a TK-NOG mouse liver that was transplanted with cryopreserved human hepatocytes (HEP187170; 26 years, female) (lower panel) were assessed for functionality by histochemical and immunohistochemical analyses. Serial liver sections were stained for H&E, PAS, CYP3A4 and MRP2. Bar = 100 μm.
Negligibly low tumorigenicity of HepaRG® cells
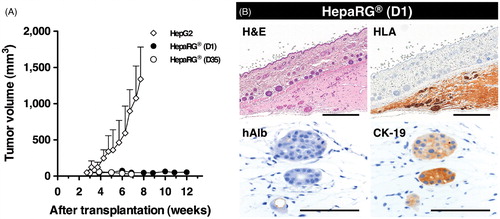
If tumorigenic abilities remained in the HepaRG® cells, the humanized liver, which was repopulated with the human hepatocyte-like HepaRG® cells, will likely become cancerous. Therefore, the tumorigenicity of the HepaRG® cells was assessed using severely immunocompromised NOG mice. Day 1 and Day 35 HepaRG® cells (1 × 106 cells) as well as hepatocellular carcinoma HepG2 cells (1 × 104 cells; positive control) were subcutaneously transplanted into NOG mice. Subcutaneously formed xenografts were observed 3 weeks after transplantation. HepG2 cells grew rapidly forming tumors of 1000 mm3 within 7 weeks, whereas both the Day 1 (proliferative stage) and Day 35 (fully differentiated stage) HepaRG® cell grafts did not increase in size throughout the observation period (12 and 7 weeks, respectively), even though the HepaRG® cells were introduced at a concentration 100 times greater than the control HepG2 cells (). Twelve weeks after transplantation, the HepaRG® cell grafts (Day 1) were analyzed by immunohistochemical staining (). The few HLA-positive surviving cells were observed in a gelatinous matrix. These remaining cells did not express albumin but expressed the biliary-lineage marker CK-19. From these results, it was hypothesized that the negligibly low tumorigenicity of the HepaRG® cells would not affect the generation of humanized-liver mice.
Figure 4. Tumorigenic potency of HepaRG® cells in NOG mice. (A) The growth potential of HepaRG® cells was evaluated in NOG mice. A total of 1 × 106 cells were subcutaneously transplanted into NOG mice. D1 and D35 in HepaRG® stage indicate the number of days after seeding. A total of 1 × 104 HepG2 cells were used as positive control for s.c. transplantation. (B) Histologic and immunohistochemical analyses of D1 HepaRG® xenografts in NOG mouse. Twelve weeks after transplantation, the xenografts were processed for H&E staining, HLA, hAlb and CK-19 staining. Bar = 500 μm, Bar = 50 μm.
Discussion
In this study, we report that the human hepatic cell line HepaRG® is a promising cell source for the steady generation of humanized-liver mice. Using differentiated HepaRG® cells, we have demonstrated in vivo the transdifferentiation of both hepatocyte-like and biliary-like cell populations into hepatocytic and biliary lineages through a common hepatic progenitor that actively proliferates. Previous studies have described in vitro differentiation from proliferative HepaRG® cells to mature hepatocyte-like cells that express liver-specific marker proteins or mRNAs, such as albumin and CYP3A4 (Cerec et al., Citation2007; Gripon et al., Citation2002). We also confirmed that the CYP mRNAs were undetectable in the proliferative stage in HepaRG® cells, whereas at the differentiation stage, they were abundantly expressed, mirroring the degree of cellular differentiation, with the exception of CYP1A1 (Aninat et al., Citation2006; Antherieu et al., Citation2010).
Most cytochrome P450 enzymes appear either at or near birth or between 2 and 4 weeks following birth; in contrast, CYP1A1 is expressed very early in development in rodents (Rich & Boobis, Citation1997) and humans (Yang et al., Citation1995). Because the differentiation stages of HepaRG® cells could be induced after exposure to beta-naphthoflavone (BNF), phenobarbital (PB) and rifampicin (RIF), to express both mRNA and CYP protein activities, they could be used in screens as a substitute for and/or in complement to primary hepatocytes for CYP induction studies (Aninat et al., Citation2006; Antherieu et al., Citation2010; Gerets et al., Citation2012). Because of the responses of the HepaRG® cells in these systems, they were subsequently evaluated in vivo to determine whether or not they could serve as an alternative to primary hepatocytes.
We transplanted four distinct differentiation stages of HepaRG® cells into the livers of TK-NOG mice. Although HepaRG® cells in any differentiation stage could engraft and proliferate within the recipient livers, the HepaRG® cells in the proliferative stage (D1) differentiated only to Alb-negative/CK-19-positive biliary-like cells in the recipient mouse livers. These results presented here have also indicated that the differentiation program from progenitors toward hepatocyte lineage will not occur in in vivo microenvironments. Because HepaRG® cells drastically change their morphological and molecular biological characteristics in vitro after reaching confluency, high-density culture conditions induce and preserve the differentiation status of the cells (Cerec et al., Citation2007). Using intrasplenic injection of HepaRG® cells in the proliferative stage, the injected cells were distributed sparsely within the recipient liver, and it was not possible to increase the cell density of the injected HepaRG® cells, resulting in lost opportunities to initiate differentiation to both hepatic lineages.
In previous studies, the HepaRG® cells displayed a BrdU labeling index of ∼90% on Day 2, 50% on Day 7 at confluency, and close to 0 on Day 28, indicating complete inhibition of the proliferative activity in the fully differentiated HepaRG® cells (Parent et al., Citation2004). Therefore, the use of fully differentiated HepaRG® cells (D35) might have dramatically decreased the numbers of hepatocyte-like and biliary-like cell colonies formed compared to when the other differentiation stages (D7 and D21) were used to repopulate the recipient mouse livers. This correlates well with the occurrence of aging characteristics in cells after 3 weeks of culture (Pernelle et al., Citation2011). Many hepatocyte-like colonies were observed in the reconstituted TK-NOG livers that were transplanted with D7 or D21 HepaRG® cells, but the livers also contained biliary-like cell colonies. When the HEP187170 hepatocytes were intrasplenically transplanted into TK-NOG mice liver, we did not observe biliary-like cell colonies within several hundred transplantation experiments (data not shown).
In this study, we demonstrated the successful engraftment of differentiated HepaRG® cells into recipient mouse livers, which shows some features of mature hepatic functions, although the chimerism of the livers reconstituted with HepaRG® cells was extremely low (<5%) compared to that of livers reconstituted with the HEP187170 hepatocytes. To achieve stable generation of humanized-liver mice with HepaRG® cells, the purification of HepaRG® cells, which are committed to hepatic lineage differentiation, will be required. Recent whole-genome gene expression profiling revealed ∼3000 differentially expressed genes (>2-fold) between undifferentiated and differentiated HepaRG® cells (Hart et al., Citation2010; Rogue et al., Citation2012). The key molecules that are involved in committed differentiation to the hepatic lineage during HepaRG® differentiation might be found from these comparative analyses.
It is well known that the highly engraftable cancer cell line could be established from low metastatic cancer cells by serial passage in appropriate sites of immunodeficient mice. Therefore, another strategy to increase the liver reconstitution with the HepaRG® cells might be a serial transplantation. Small amount of engrafting HepaRG® cells are isolated from the TK-NOG mice liver, separated from mice cells by fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS), and transplanted into another TK-NOG liver. There are two possible mechanisms for allowing the proliferation of low engraftable cells within the mice liver, (i) adaptation: the HepaRG® cells adapt to the microenvironment of the mice liver and (ii) selection: the specific clone are selected in the microenvironment of the mice liver from heterogeneous HepaRG® population. It’s not known the exact mechanism, but there is a possibility to establish the HepaRG® sub-line that could be highly engraftable to TK-NOG livers. Another way to achieve high level of replacement with HepaRG® cells might be administration of additional doses of GCV after HepaRG® cells have repopulated the liver. This strategy can be developed to ablate any residual mouse hepatic cells after human cell reconstitution.
Using uPA/SCID mice with damaged livers as recipients, mouse liver repopulation by HepaRG® cells arose by cells creeping into the parenchyma without clustering (Cerec et al., Citation2007). In contrast, most human hepatocyte-like cells (pale cytoplasm in H&E staining or human albumin-positive) derived from differentiated HepaRG® cells were present as small foci that appeared to grow by clonal expansion within the TK-NOG recipient livers. This growth characteristic is quite similar to that of HEP187170 hepatocytes (). In agreement with these data, HepaRG-derived hepatocyte-like cells in the recipient mouse livers have many morphologic and functional similarities with that of the HEP187170 hepatocytes. The current data show that the hepatocyte-like cells derived from differentiated HepaRG® cells expressed liver functional markers such as gene expression and protein expression of CYP3A4 and MRP2 in vivo and displayed glycogen storage. These results suggest that the hepatocyte-like cells within the recipient mouse livers have acquired a limited, normalized human hepatocyte function.
The NOG (T, B and NK cell-defective) mice (Ito et al., Citation2002) are more sensitive for detecting the tumorigenicity of HeLa S3 cells and are faster than traditional animal models using nude or SCID mice (Machida et al., Citation2009). Cerec et al. (Citation2007) reported that HepaRG® cells failed to give rise to tumors when injected subcutaneously into nude mice. Nevertheless, using the NOG mice as host animals for tumorigenicity testing in the current study showed that HepaRG® cells were not able to form proliferative colonies after 12 weeks (). The morphologic characteristics observed in sections of the patient’s liver from which the HepaRG® cells originated included bile duct-like structures that expressed both CK-18 and CK-19 (Parent et al., Citation2004) and were quite similar to those of the HepaRG® xenograft.
In conclusion, differentiated HepaRG® cells transplanted into the livers of TK-NOG mice can form two morphologically different colonies, which consisted of either hepatocyte-like cells or biliary-like cells. Furthermore, HepaRG® cells, which formed hepatocyte-like colonies in the recipient mouse liver, expressed several mature hepatocyte markers in addition to human serum albumin. These results indicated that HepaRG® cells are a possible cell source for the steady generation of humanized-liver mice.
Declaration of interest
Y.H., K.K., H.Y., M.N., F.B. and H.S. declare no competing financial interests. C.G.G. has an equity interest in Biopredic International.
Part of this work was supported by Grant-in-Aid for Scientific Research (24700439 to Y.H.) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, JCIA’s LRI program (to H.Y.), and The Food Safety Commission of Japan (1103 to H.S.).
Supplementary Material
Download PDF (21 KB)Acknowledgements
We would like to thank Y. Ando, M. Kuronuma, T. Ogura and K. Hioki for outstanding technical assistance with the animal experiments and Drs M. Ito and Y. Ohnishi for helpful discussions. We also would like to thank Dr D. Steen for English proofreading.
References
- Aninat C, Piton A, Glaise D, et al. (2006). Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos 34:75–83
- Antherieu S, Chesne C, Li R, et al. (2010). Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos 38:516–25
- Azuma H, Paulk N, Ranade A, et al. (2007). Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol 25:903–10
- Cerec V, Glaise D, Garnier D, et al. (2007). Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology 45:957–67
- Dandri M, Burda MR, Torok E, et al. (2001). Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 33:981–8
- Gerets HH, Tilmant K, Gerin B, et al. (2012). Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol 28:69–87
- Gripon P, Rumin S, Urban S, et al. (2002). Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA 99:15655–60
- Guillouzo A, Corlu A, Aninat C, et al. (2007). The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact 168:66–73
- Hart SN, Li Y, Nakamoto K, et al. (2010). A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos 38:988–94
- Hasegawa M, Kawai K, Mitsui T, et al. (2011). The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem Biophys Res Commun 405:405–10
- Ito M, Hiramatsu H, Kobayashi K, et al. (2002). NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–82
- Jiang L, Li JG, Lan L, et al. (2010). Human hepatoma HepaRG cell line engraftment in severe combined immunodeficient x beige mice using mouse-specific anti-Fas antibody. Transplant Proc 42:3773–8
- Kanebratt KP, Andersson TB. (2008). Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab Dispos 36:1444–52
- Machida K, Suemizu H, Kawai K, et al. (2009). Higher susceptibility of NOG mice to xenotransplanted tumors. J Toxicol Sci 34:123–7
- Mercer DF, Schiller DE, Elliott JF, et al. (2001). Hepatitis C virus replication in mice with chimeric human livers. Nat Med 7:927–33
- Nibourg GA, Chamuleau RA, van Gulik TM, Hoekstra R. (2012). Proliferative human cell sources applied as biocomponent in bioartificial livers: a review. Expert Opin Biol Ther 12:905–21
- Parent R, Marion MJ, Furio L, et al. (2004). Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology 126:1147–56
- Pernelle K, Le Guevel R, Glaise D, et al. (2011). Automated detection of hepatotoxic compounds in human hepatocytes using HepaRG cells and image-based analysis of mitochondrial dysfunction with JC-1 dye. Toxicol Appl Pharmacol 254:256–66
- Rich KJ, Boobis AR. (1997). Expression and inducibility of P450 enzymes during liver ontogeny. Microsc Res Tech 39:424–35
- Rogue A, Lambert C, Spire C, et al. (2012). Interindividual variability in gene expression profiles in human hepatocytes and comparison with HepaRG cells. Drug Metab Dispos 40:151–8
- Suemizu H, Hasegawa M, Kawai K, et al. (2008). Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem Biophys Res Commun 377:248–52
- Yang HY, Namkung MJ, Juchau MR. (1995). Expression of functional cytochrome P4501A1 in human embryonic hepatic tissues during organogenesis. Biochem Pharmacol 49:717–26
