Abstract
Purpose: To evaluate the safety of delivering pre-operative regional hyperthermia (HT) plus an intensified chemo-radiotherapy (CRT) regimen in patients suffering from locally advanced rectal cancer.
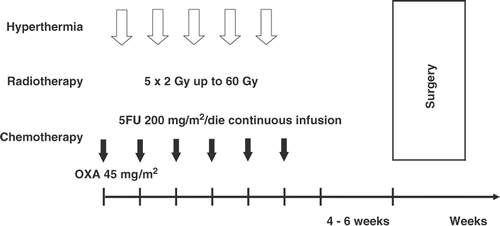
Methods: Between June 2000 and April 2006, 76 patients with locally advanced (cT3-4 N0/+) rectal adenocarcinoma were treated with HT plus CRT. HT was given once a week, to a total of five treatments, 1 to4 h after radiotherapy (50 Gy with 2-Gy fractions for 5 weeks, plus a 10-Gy boost on the tumour bed, with the same fractionation schedule). Chemotherapy consisted in 5FU 200 mg/m2 continuous infusion throughout the 6 weeks of irradiation and OXA 45 mg/m2 in a weekly bolus. Surgery followed 4 to 6 weeks after the completion of HT plus CRT.
Results: HT plus CRT was generally well tolerated. At pathologic examination, there was a pathologic complete response (pCR) (ypT0 ypN0) in 18 out of 76 patients (23.6%), a partial response (PR) in 34/76 ones (44.7%) and a stable disease (SD) in 20/76 (26.3%) ones; 4/76 patients (5.2%) had a progression disease (PD) (distant metastases) at the time of surgery. Good predictors of a longer disease-free survival (DFS) were in order ypN status (log-rank test: p = 0.0008), ypT status (p = 0.002) and pCR (p = 0.03).
Conclusion: Preoperative CRT combined with regional HT yielded acceptable toxicity. The rate of pCR was encouraging, although further studies are needed to prove the long-term efficacy of adding HT to CRT.
Introduction
In locally advanced mid-low rectal cancer, the major goal is to achieve a high rate of resectability, thus allowing sphincter-saving surgical procedures. Neoadjuvant CRT achieves a high rate of pCR Citation[1], Citation[2], nevertheless the impact of pCR on outcome is still a controversial issue Citation[3], Citation[4]; it seems, however, that patients with a complete or nearly complete response after CRT have a better local control and survival Citation[5–7]. In recent years, several investigators have tried new CRT regimens, either by associating 5FU with new available drugs (such as OXA Citation[8–10], raltitrexed Citation[11], or irinotecan Citation[12], Citation[13]), or administering capecitabine Citation[14], or capecitabine plus OXA Citation[15–17]. Many institutions have also tested the new biologic agents in various associations with chemotherapy and radiotherapy, using cetuximab Citation[18–20], or gefitinib Citation[21], or bevacizumab Citation[22], Citation[23]. These new intensive regimens have put into evidence the issue of how much they impact on patient quality of life Citation[24], Citation[25].
Another major question rises about the efficacy of adjuvant chemotherapy after preoperative CRT and surgery; a retrospective study by Fitkau et al. Citation[26] suggested that, for ypN0 patients, postoperative chemotherapy could be spared; for patients with ypN2 status, an intensification of the postoperative chemotherapy should be considered. In an EORTC trial, Collette et al. found no difference in DFS and OS comparing the patients who received postoperative chemotherapy or not Citation[27]. A letter by Fitkau outlines that it is not defined what parameter may be the best for the selection of the patients to postoperative chemotherapy following neoadjuvant CRT Citation[28].
Regional HT has already been tested in locally advanced rectal cancer in a preoperative setting in association with CRT Citation[29–32]. In vitro temperatures of 40°–43°C enhance the effect of radiotherapy (as they interfere with the repair of radiation-induced DNA damage) and of some cytotoxic drugs, such as alkylating agents, nitrosoureas, platinum compounds, and antibiotics. HT is able to increase the anti-proliferative effect of OXA in in vitro colon cancer cells with a G1/S arrest and a G1/G0 reduction Citation[33]. Besides, HT enhances the effect of radiotherapy in human colon cancer cells transplanted into nude mice by changing the expression of the apoptosis genes, such as p53, Bcl-2 and Bax Citation[34].
In this phase II trial we investigated the safety and efficacy of adding regional HT to CRT (5FU 200 mg/m2 continuous infusion for 6 weeks plus weekly OXA 45 mg/m2) as neoadjuvant treatment. Primary endpoints were acute and late toxicities; secondary end-points were percentage of pCR and rate of sphincter-sparing surgery.
Patients and methods
Patients
Between June 2000 and April 2006, 76 patients with biopsy-proven locally advanced (cT3-4 N0/+) rectal adenocarcinoma of the middle and lower rectum, located within 12 cm from the anal verge, were included in the study. Staging was performed by pelvic CT or MRI or by transrectal ultrasonography (TRUS). Inclusion criteria were a biopsy-proven rectal carcinoma, stage cT3-4 N0-1, adequate renal and liver function, normal white blood count and platelet count. Exclusion criteria were medical conditions that could make the patients unfit for surgery, chronic inflammatory bowel disease, tumour located above 12 cm from anal verge. Patient characteristics are shown in .
Table I. Patient characteristics (n = 76).
The local ethical committee approved the protocol and all patients gave written informed consent.
Treatment
Radiotherapy consisted of prone-position irradiation, using a four-field box technique with 6 MV photons. The target volume was meant to include the macroscopic tumour, the mesorectum and the internal iliac and presacral nodes up to the L5-S1 junction. The upper border of the radiation fields was at the L5-S1 interspace. The distal border was 5 cm below the distal margin of the macroscopic tumour or at the bottom of the obturator foramina. Lateral limits of the anteroposterior and posteroanterior fields were 1.5 cm lateral to the margins of the bony pelvis. Lateral fields encompassed the entire sacrum posteriorly and extended anteriorly to the anterior margin of the symphysis pubis. The dose delivered to the pelvis in this way was of 50 Gy in 25 daily fractions of 2 Gy, Monday to Friday over a period of 5 weeks; then a boost of 10 Gy with the same fractionation schedule was delivered to the tumour, identified by endoscopy.
Chemotherapy started simultaneously with radiotherapy. 5FU was infused continuously for all the duration of irradiation through an implantable subcutaneous i.v. access device using a portable elastomeric pump, at a dose of 200 mg/m2/day. OXA was given as a 2-hour i.v. infusion at 45 mg/m2 on the first day of each week of radiotherapy for a total of six courses ().
All patients received premedication with corticosteroids.
Hyperthermia
HT was delivered once weekly during CRT, 1-4 hours after radiotherapy, to a total of five treatments, using the BSD-2000 system with a Sigma-60 applicator. Endoluminal thermometry catheters were placed in the rectum, bladder, and vagina. Temperature position curves were recorded along the catheters at intervals of 5 to 10 minutes. No invasive thermometry was made, so the temperature was not registered directly in tumours but only along the catheters. The part of the catheter related to the tumour was specified through CT, TRUS, or endoscopy.
The aim was to continue the treatment for 60 minutes after tumour temperature had reached 40.5°C, or generally for a maximum total duration of 90 minutes. Temperature position curves were recorded along the catheter at intervals of 5 to 10 minutes. We determined index temperatures, Tmax T90 and Tmin, by taking into consideration all tumour-related measurement points during the therapeutic range of time. T90 is the temperature reached or exceeded by 90% of the tumour-related measurement points; Tmax and Tmin are the maximal and minimal temperatures achieved at each HT session, respectively.
Patients were instructed to refer any bad sensation suggestive of hot spots, such as a burning sensation, a feeling of pressure, or any pain. Any symptom mentioned by the patient that disappeared within one minute of power decrease was taken to show that the temperature was too high: in these cases, we took adjustments of treatment settings, such as changes in power output per channel, frequency or phase settings, or placement of an additional water bolus.
Toxicity scoring
Acute CRT toxicity was defined as the adverse effects occurring during the treatment or within 6 months from the end of it; late toxicity was defined as the one occurring after 6 months from the completion of treatment. Toxic effects were registered according to RTOG-EORTC scoring criteria Citation[35].
HT toxicity was registered according to the score system proposed by the Berlin group Citation[29] ().
Table II. HT toxicity score, from B. Rau et al. Citation[29].
Post-CRT restaging
After the completion of CRT, within the 4–6 weeks of interval before surgery, patients underwent a restaging procedure (endoscopy or TRUS) to assess the new clinical stage.
Surgery and postoperative treatment
Surgery was planned 4 to 6 weeks after the end of CRT. Any attempt was made to do a conservative surgery (TME) if at least a 2-cm distal margin of safety could be obtained. The anastomosis usually was created with the ‘double-stapling’ technique Citation[36]. If this could not be achieved, an abdomino-perineal excision was performed. In low-lying tumours, the choice was left to the discretion of the surgeon provided that a free distal margin more than 1 cm was maintained. Lymph node dissection up to the stem of the inferior mesenteric artery and including the total mesorectum was generally performed.
Adjuvant chemotherapy was performed in single cases, at the discretion of the medical oncologist, mostly in cases of SD or PD.
Assessment of pathologic response
Visible residual tumour or the corresponding fibrotic area as well as perirectal lymph nodes, distal and lateral resection margins and mesorectal tissue distal to the tumour were examined to assess the radicality of the surgical resection and to determine the pathological stage according to the AJCC-TNM classification. All surgical specimens underwent routine pathological processing, which included fixation in 10% formalin, painting of the circumferential margin (CRM), and serial slicing from the distal margin at 3 to 5 mm intervals. To determine the CRM, the lateral resection margin of the fresh specimen was inked and subsequently the specimen was fixed in formalin for 48 h; blocks of the tumour in relation to the inked CRM were collected; measurements of the margin were done microscopically. A specimen with tumour ≤1 mm from the inked margin was considered as having a positive CRM.
The median number of analysed lymph nodes per patient was 12 (range 10–14).
The pCR was defined as the absence of residual tumour cells in the surgical specimen regardless of the presence of mucine lakes.
Pathologic PR was defined as presence of few poorly differentiated pleomorphic tumour cells (pTmic if cellular aggregations were less than 5 mm in diameter). The specimen was deemed as pathologic SD in presence of extensive areas of tumour infiltration through the rectal wall or the perirectal tissue.
Endpoints and statistical analysis
The primary endpoints of the study were acute and late toxic effects of neo-adjuvant treatment (CRT and HT).
Secondary endpoints were rate of pCR and rate of sphincter-sparing surgery.
Follow-up visits were scheduled 1 month after treatment, then once every 3 months during the first 2 years, and every 4 to 6 months after. Patients were submitted to digital rectal examination at every visit; to proctoscopy 3 months after surgery and then every 6 months for the first 2 years, then once per year; to abdomino-pelvic Computed Tomography (CT) 6 months after surgery, then every 6 months for the first 2 years, then once per year.
Overall survival (OS) and DFS were measured from the date of surgery. DFS was defined as the time from surgery to the onset of local recurrence or metastatic disease. Statistical evaluations were performed using the software Intercooled Stata, version 9.0. We applied the Kaplan-Meyer method; the long-rank test and the Chi-square test were also performed. Student's t-test was performed to investigate the correlation between thermometry data and pCR status.
Results
Feasibility and toxicity
All patients completed HT plus CRT except one patient who stopped chemotherapy due to grade 3 neutropenia. All patients underwent surgery.
CRT and HT-related acute toxicities are shown in .
Table III. CRT–related acute toxicity according to RTOG–EORTC scoring criteria Citation[35], observed in all patients (n = 76).
The only dose-limiting toxicity was a grade 3 neutropenia in 1 in 76 patients (1.3%), who stopped chemotherapy and went on with radiotherapy only. OXA-related neurotoxicity was found in 4 out of 76 patients (5.2%); it consisted in peripheral sensitive neuropathy and/or reversible grade 2 cold-related paresthesias/dysesthesias. Diarrhoea was present in 12 out of 76 patients (15.7%). No cases of dermatitis were observed.
HT-related toxicity (registered according to the Berlin scoring system Citation[29], shown in ) consisted in general discomfort (grade 0) due to bolus pressure in 7 of 76 patients (9.2%), local discomfort (hot-spot phenomena, grade 1) in 3 of 76 patients (4%), more severe discomfort which persisted after the end of the treatment (grade 2) in 2 out of 76 (2.6%). No patient had grade 3 toxicity. Subcutaneous burns (subcutaneous induration), grade 4 toxicity, occurred in 4 of 76 patients (5.2%) and disappeared spontaneously within two weeks. No patients developed skin burns. No one HT session had to be ended prematurely because of discomfort.
Late radiotherapy toxicity consisted in persistent diarrhoea in 2 patients (2.6%).
Surgical complications occurred in 3 of 76 patients (4%); one patient developed a perianal abscess 7 days after surgery and 5 weeks after CRT; it required a surgical procedure and then healed. Another one, 8 days after surgery and 5 weeks after CRT, had a fistula which required surgical intervention. Another patient, soon after the intervention performed 4 weeks after CRT, had a delayed wound healing, which recovered after 2 months from TME. No cases of intestinal obstruction or severe faecal incontinence were observed.
Hyperthermia results
All patients were submitted to 5 HT sessions, once weekly.
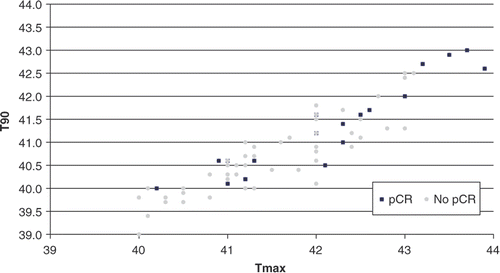
T90 and Tmax data for each patient, divided in pCR and no-pCR groups, are shown in . Mean T90 was 40.8°C (95% confidence interval [CI] 40.6–41.0°C); mean Tmax was 41.6°C (95% CI 41.4–41.8°C).
A correlation was found between higher T90 values and pCR status and it was statistically significant (Student's t-test: p = 0.002); a correlation (also statistically significant [Student's t-test: p = 0.004]) is also evident between Tmax values and pCR. Poor correlation existed between T90 and Tmax.
Among the study population, there was no significant difference in achieved temperatures between female and male patients, nor between old versus young ones, nor versus proximally or distally seated tumours.
Pathologic results
Among 76 patients 23 (30%) had metastatic lymph nodes at surgery (5 of 12 lymph nodes in one patient, 5 of 10 in two, 4 out of 14 in two, 4 out of 13 in two, 4 out of 11 in two, 3 out of 13 in one, 3 out of 12 in three, 2 out of 14 in one, 2 out of 13 in two, 2 out of 1 in two, 2 out of 11 in two, 1 out of 14 in one, 1 out of 12 in one, and 1 out of 11 in one).
Among the 56 out of 76 patients who underwent TME, 50 had negative CRM. In no patients was a macroscopic (R2) CRM noted. In six patients, a microscopic (R1) CRM was observed; in all these six cases TME specimens were intact, i.e. no specimen defects were noted.
Among the 20 out of 76 patients submitted to Miles procedure, no one showed positive CRM. So in summary we had 6 out of 76 R1 resections (7.9%) and no R2 resections.
Rate of pCR
Pathologic downstaging is shown in .
Table IV. Clinical stage at diagnosis and subsequent pathological stage after neo–adjuvant HT plus CRT and surgery for all patients (n = 76).
A pCR (ypT0 ypN0) was found in 18 out of 76 patients (23.6%) and they all underwent TME. After 2 years of follow-up, none of them developed local recurrences; only one patient developed distant metastases 31 months after surgery.
A PR was observed in 34 out of 76 cases (44.7%). Two patients developed a local recurrence plus distant metastases (lung in one case, liver in the other); they both underwent adjuvant chemotherapy with a FOLFOX scheme. Two other patients developed lung metastases and did not undergo chemotherapy due to the poor general conditions.
A SD was observed in 20 out of 76 cases (26.3%). Eighteen of them were initially cT3 N+, and two were cT3 N0. Ten among these 20 patients underwent adjuvant chemotherapy with a FOLFOX scheme. Two patients (not submitted to adjuvant chemotherapy) developed lung metastases after one year of follow-up.
There were 4 out of 76 cases of PD (5.2%): in two patients initially staged as cT3 N0 M0, liver metastases were found at surgery and a TME was performed due to the good down-staging (ypTmic ypN0); another two patients initially staged as cT3 N0 had a TME intervention and were found to be ypT3 ypN+; after 6 and 12 months from surgery respectively they developed liver metastases.
Type of surgery
Of 76 patients, 56 (73.7%) underwent TME. A Miles intervention was performed in the remaining 20 out of 76 patients (26.3%) in low-lying tumours or when at least a 2-cm distal margin of safety could not be obtained. Six out of 76 patients had a tumour initially sited at 5 cm from the anal verge (2 cm from dentate line); two of them had a down-staging at post-CRT examination, while the remaining four did not, nevertheless the surgeon decided to submit them all to a Miles intervention, as a free distal margin more than 1 cm could not be maintained.
Follow up
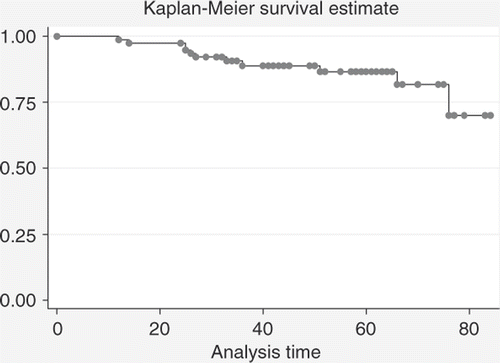
Median follow-up was 51 months (range 24–83). No patients were lost at follow-up. Five-year survival rate estimates +/ − standard deviations were 86.5% +/−4.3% for OS; 74.5%+/−5% for DFS; 94.6% +/−2.6% for local recurrence-free survival; 73.2% +/−5.4% for metastases-free survival. Kaplan-Meyer OS estimates are shown in ; DFS estimates are shown in .
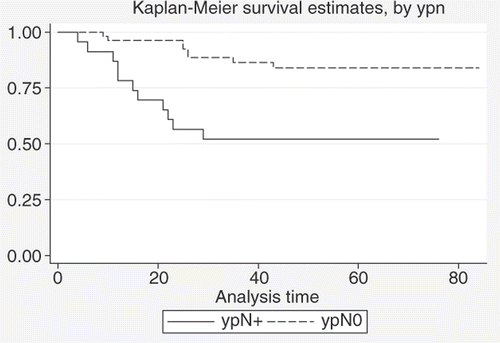
In a comparison is given between estimated DFS rates for patients with ypN0 and ypN+ status; a clear advantage is seen for ypN0 (estimated DFS at 5 years+/ − standard deviation is 84.0%+/− 5.2% and 52.1%+/− 10.4% for ypN0 and ypN+ patients, respectively) and it is statistically significant (log-rank test: p = 0.0008).
Figure 5. Comparison between DFS estimates for patients with ypN0 status (n = 53) and patients with ypN+ status (n = 23) (log-rank test: p = 0.0008).
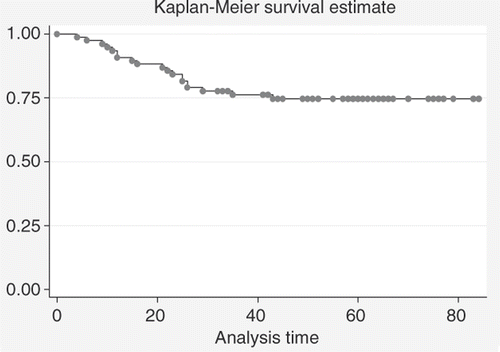
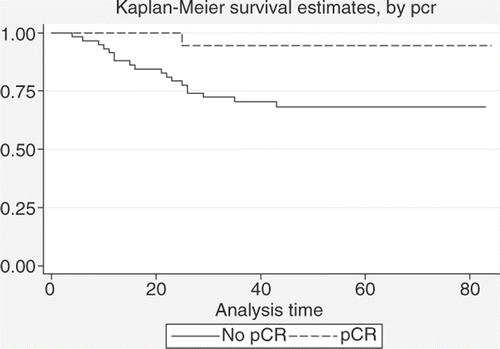
In we give a comparison between estimated DFS rates for pCR versus no pCR; also here a pCR yields a better DFS (94.4%+/− 5.4% and 68.3%+/− 6.2% estimated 5-year DFS+/− standard deviation for patients who achieved pCR and patients who did not), with statistical significance (p = 0.03).
Figure 6. Comparison between DFS estimates for patients with pCR (n = 18) and patients with no pCR (n = 58) after neoadjuvant treatment (log-rank test: p = 0.03).
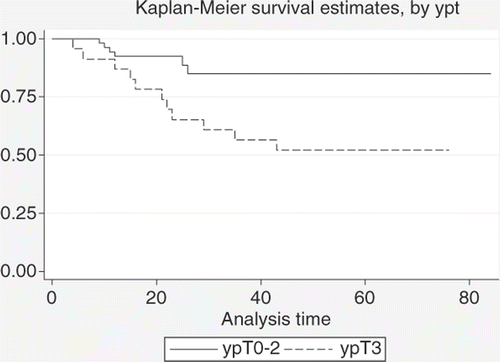
Finally, a comparison between ypT0-2 and ypT3 patients is given in , which shows an advantage for patients who achieved a ypT0-2 status (84.9%+/−4.9% and 52.2%+/−10.4% estimated 5-year DFS+/−standard deviation for ypT0-2 and ypT3 patients, respectively) (p = 0.002).
Figure 7. Comparison between DFS estimates for patients with ypT0-2 status (n = 53) and patients with ypT3 status (n = 23) after neoadjuvant treatment (log-rank test: p = 0.002).
No patient missed the follow-up procedures. During the observation period, 11 out of 76 patients died (14.5%). Four patients (5.3%) developed a local recurrence, three of them together with distant metastases. Another 19 out of 76 patients (25%) had distant metastases during the follow-up period, so the whole number of patients with distant metastases was 22 out of 76 (29%).
Discussion
The rates of pCR after neoadjuvant treatment range from 15% to 25% in various series examined by Rödel in a recent review Citation[13]. Larger series using CRT without HT have obtained rates of pCR of 8% (n = 415 patients) Citation[37], 11.4% (n = 375 patients) Citation[38], 16% (n = 123 patients) Citation[39]. Another review Citation[40] investigates the administration of neoadjuvant CRT with intensified chemotherapy, with the rationale that it can target potential micrometastases; pCR rates were 13–28% with 5FU or capecitabine plus OXA and radiotherapy doses of 45–50.4 Gy, 16–37% with 5FU or capecitabine plus irinotecan and the same doses of radiotherapy, 0–25% with 5FU or capecitabine plus molecularly targeted drugs (Cetuximab or Bevacizumab).
The rate of pCR (ypT0 ypN0) in our series (23.6%) was comparable to the ones of other series without HT; Rödel Citation[20] achieved a 9% pCR with Xeloda-OXA (XELOX) and radiotherapy without cetuximab, but one should be cautious because this trial is not randomised. The pCR rates with XELOX and radiotherapy without cetuximab seem to be higher (nearly 15%) in two phase II trials Citation[9],Citation[16]. In the EORTC Trial Citation[27], pCR (pT0) was observed in 5.3% and 13.7% of patients in the radiotherapy and CRT arms, respectively; no significant difference was observed in terms of metastasis incidence at 5 years between the two arms (radiotherapy 38.2%; CRT 33.7%); in our series the incidence of metastases was 29% (22/76) in all our patients (follow-up period 24–83 months). However, the pCR rate is an early surrogate endpoint that may not necessarily translate into improved long-term outcomes Citation[12]; in our series, among the 18 patients who had a pCR, no one developed a pelvic recurrence and only one patient had distant metastases. A French multi-centric phase II trial is ongoing, evaluating the effect of XELOX plus cetuximab plus radiotherapy followed by post-operative XELOX plus cetuximab in patients with locally advanced rectal cancer with synchronous operable metastases according to tumour molecular status.
Down-staging in our series was also a good predictor of outcome, as ypN0 and ypT0–2 patients had better DFS estimates than ypN+ and ypT3 ones; the data were statistically significant at log-rank test. These findings fit quite well with the ones of Rödel et al. Citation[7], who had (among 385 patients) a DFS at 5 years of 86% for patients with tumour regression grade (TRG) 4 (pCR), 75% for TRG of 2–3 (PR) and 63% for TRG of 0-1 (SD); these differences were not statistically significant (p = 0.11). Also in our series, like in the one of Rödel Citation[7], ypN positive was the strongest prognostic factor (log-rank test: p = 0.0008 in our series). Also, CRM status is a good predictor of prognosis Citation[41]; in our series, all but six patients submitted to TME had negative CRM at pathological examination.
The benefits of the addition of chemotherapy to radiotherapy in a neoadjuvant setting are definitively demonstrated by a Cochrane metanalysis Citation[42]. 5FU, when added to radiation, appears to enhance apoptotic response significantly in p53 mutant cells, which often exhibit increased radiation resistance. While the mechanism of 5FU and radiation interaction is not fully understood, the absence of a G1/S block in mutant p53 cells following radiation may allow cells to progress to S phase where 5FU may be incorporated into the DNA resulting in cell killing. Besides, weekly OXA administration potentially allows optimised inhibition of radiation-induced, sub-lethal DNA damage repair.
The rationale for addition of HT to 5FU has been reviewed in detail. Combination of low-dose long-exposure 5FU prior to 2 h of heating at 42°C lead to a ten-fold reduction in cell survival compared to either treatment alone; cell cycle analysis with flow cytometry demonstrates that the effect is likely due to an accumulation of cells in S-phase, which are known to be particularly sensitive to heat cytotoxicity Citation[43],Citation[44]. These results provide a rationale for using 5FU as a continuous infusion in combination with HT. The effects of HT at various temperatures on intracellular uptake of 5FU and its conversion to active metabolites were also examined Citation[45]; it was found that temperatures ranging from 39° to 42°C are effective in increasing the rate of intracellular uptake of 5FU (two- to five-fold increase in intracellular concentrations compared to 37°C); the greatest enhancement is seen at 39°C; rates of production of active metabolites are also enhanced by heating, again with the greatest effect at 39°C.
The efficacy of HT in the treatment of rectal cancer is still a matter of debate. The only randomised phase III trial is the Rotterdam one, which compared radiotherapy versus radiotherapy plus HT in 143 patients with rectal cancer, mostly with unresectable tumours Citation[46]; the results were defined disappointing by the authors, and according to them this was due to the low doses of radiotherapy used (mean dose 56 Gy).
One important question about this study is whether intraluminal thermometry was sufficient or an invasive thermometry was needed. A reason for using invasive thermometry is the possibility of improving the temperature distribution or minimizing side effects by varying the adjustment parameters of the heating system. Nevertheless, invasive thermometry is expensive and represents a burden for the patient, with possible side effects or even complications, such as infections. Many surgeons are also sceptical about penetrating the tumour, particularly during preoperative treatment. Besides, in regional hyperthermia of the pelvis, various non-invasive endoluminal temperature measuring tracks are available, such as rectum, vagina, bladder, and urethra. In a paper published by the Berlin group Citation[47], for pelvic tumours, the authors pointed out that invasive measurements can be replaced by minimally-invasive or non-invasive techniques, which provide equivalent or even more complete information.
We found a correlation (statistically significant) between some thermal parameters (T90 and Tmax) and response to neoadjuvant treatment; for rectal cancer, this correlation has already been proved by the study of B. Rau et al. Citation[29]. This relationship is also established for superficial tumours Citation[48] and prostate cancer Citation[49].
The percentage of sphincter preservation in our series was 73.7% (56 out of 76). Comparing this data with other series, in the German Rectal Cancer Trial (CAO/ARO/AIO-94) Citation[37] the rate of sphincter-sparing surgery was 69% (286 out of 415 patients); in the Lyon R0-04 phase II trial Citation[9], it was 62.5 (25 out of 40 patients); finally, in the EORTC Radiotherapy Group Trial 22921 Citation[50], sphincter-sparing resection was performed in 255 patients who were assigned to preoperative radiotherapy (50.5%) and in 267 patients assigned to preoperative CRT (52.8%). About the studies with HT, in the one of Rau Citation[29], conservative surgery was performed in 86.5% of patients (32 out of 36).
The results of our study were encouraging in terms of rate of pCR and conservative surgery. The good results of our study may be due not only to HT but also to the relatively high dose of radiotherapy delivered (60 Gy/30 fractions). The most important studies with preoperative CRT delivered a dose of 45 Gy/25 fractions Citation[9], Citation[27], or 50.4 Gy/28 fractions Citation[37]. Despite the fact that some institutions are investigating dose-escalating chemotherapy schedules Citation[8] or new biologic agents Citation[18–23], regional HT in a preoperative setting should be considered in the new rectal-cancer trials.
Conclusion
Preoperative CRT combined with regional HT resulted in acceptable toxicity. Although pCR rates seem encouraging, the efficacy of adding HT to the neoadjuvant CRT on long-term outcome (especially metastatic disease) is still to be determined in larger studies.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Grann A, Feng C, Wong D, Saltz L, Paty PP, Guillem JG, Cohen AM, Minsky BD. Preoperative combined modality therapy for clinically resectable uT3 rectal adenocarcinoma. Int J Radiat Oncol Biol Phys 2001; 49: 987–995
- Luna-Perez P, Rodriguez-Ramirez S, Hernandez-Pacheco F, Gutierrez De La Barrera M, Fernandez R, Labastida S. Anal sphincter preservation in locally advanced low rectal adenocarcinoma after preoperative chemoradiation therapy and coloanal anastomosis. J Surg Oncol 2003; 82: 3–9
- Pucciarelli S, Toppan P, Friso ML, Russo V, Pasetto L, Urso E, Marino F, Ambrosi A, Lise M. Complete pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis Colon Rectum 2004; 47: 1798–1807
- Capirci C, Valentini V, Cionini L, De Paoli A, Rödel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008; 72: 99–107
- Theodoropulos G, Padmanabhan A, Kerner BA, Taylor CW, Aguila PS, Khaduja KS. T-level downstaging and complete pathological response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum 2002; 45: 895–899
- Mohiuddin M, Hayne M, Regine WF, Hanna N, Hagihara PF, McGrath P, Marks GM. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent cancers. Int J Radiat Oncol Biol Phys 2000; 48: 1075–1080
- Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005; 23: 8688–8696
- Aschele C, Friso ML, Pucciarelli S, Lonardi S, Sartor L, Fabris G, Urso ED, Del Bianco P, Sotti G, Lise M, et al. A phase I - II study of weekly oxaliplatin, 5-fluorouracil continuous infusion and preoperative radiotherapy in locally advanced rectal cancer. Ann Oncol 2005; 16: 1140–1146
- Gérard JP, Chapet O, Nemoz C, Romestaing P, Mornex F, Coquard R, Barbet N, Atlan D, Adeleine P, Freyer G. Preoperative concurrent chemoradiotherapy in locally advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: The Lyon R0-04 phase II trial. J Clin Oncol 2003; 21: 1119–1124
- Carraro S, Roca EL, Cartelli C, Rafailovici L, Castillo Odena S, Wasserman E, Gualdrini U, Huertas E, Barugel M, Ballarino G, et al. Radiochemotherapy with short daily infusion of low-dose oxaliplatin, leucovorin, and 5-FU in T3 - T4 unresectable rectal cancer: A phase II IATTGI study. Int J Radiat Oncol Biol Phys 2002; 54: 397–402
- Gambacorta MA, Valentini V, Coco C, Morganti AG, Smaniotto D, Miccichè F, Mantini G, Barbaro B, Garcia-Vargas JE, Magistrelli P, et al. Chemoradiation with raltitrexed and oxaliplatin in preoperative treatment of stage II-III resectable rectal cancer: Phase I and II studies. Int J Radiat Oncol Biol Phys 2004; 60: 139–148
- Klautke G, Feyerherd P, Ludwig K, Prall F, Foitzik T, Fietkau R. Intensified concurrent chemoradiotherapy with 5-fluorouracil and irinotecan as neoadjuvant treatment in patients with locally advanced rectal cancer. Br J Cancer 2005; 92: 1215–1220
- Rödel C, Sauer R. Integration of novel agents into combined-modality treatment for rectal cancer patients. Strahlenther Onkol 2007; 183: 227–235
- Dunst J, Reese T, Sutter T, Zühlke H, Hinke A, Kölling-Schlebusch K, Frings S. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol 2002; 20: 3983–3991
- Rödel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol 2003; 21: 3098–3104
- Rödel C, Liersch T, Hermann RM, Arnold D, Reese T, Hipp M, Fürst A, Schwella N, Bieker M, Hellmich G, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 2007; 25: 110–117
- Carlomagno C, Farella A, Bucci L, D'Armiento FP, Pesce G, Pepe S, Cannella L, Pacelli R, De Stefano A, Solla R, et al. Neo-adjuvant treatment of rectal cancer with capecitabine and oxaliplatin in combination with radiotherapy: A phase II study. Ann Oncol 2009; 20: 906–912
- Horisberger K, Treschl A, Mai S, Barreto-Miranda M, Kienle P, Ströbel P, Erben P, Woernle C, Dinter D, Kähler G, et al. Cetuximab in Combination with Capecitabine, Irinotecan, and Radiotherapy for Patients with Locally Advanced Rectal Cancer: Results of a Phase II MARGIT Trial. Int J Radiat Oncol Biol Phys 2009; 74: 1487–1493
- Bertolini F, Chiara S, Bengala C, Antognoni P, Dealis C, Zironi S, Malavasi N, Scolaro T, Depenni R, Jovic G, et al. Neoadjuvant treatment with single-agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy: A phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2009; 73: 466–472
- Rödel C, Arnold D, Hipp M, Liersch T, Dellas K, Iesalnieks I, Hermann RM, Lordick F, Hinke A, Hohenberger W, et al. Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys. 2008; 70: 1081–1086
- Valentini V, De Paoli A, Gambacorta MA, Mantini G, Ratto C, Vecchio FM, Barbaro B, Innocente R, Rossi C, Boz G, et al. Infusional 5-fluorouracil and ZD1839 (Gefitinib-Iressa) in combination with preoperative radiotherapy in patients with locally advanced rectal cancer: A phase I and II Trial (1839IL/0092). Int J Radiat Oncol Biol Phys 2008; 72: 644–649
- Czito BG, Bendell JC, Willett CG, Morse MA, Blobe GC, Tyler DS, Thomas J, Ludwig KA, Mantyh CR, Ashton J, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int J Radiat Oncol Biol Phys 2007; 68: 472–478
- Willett CG, Duda DG, Tomaso di, Boucher E, Czito Y, Vujaskovic BG, Vlahovic Z, Bendell G, Cohen J, Hurwitz KS, et al. Complete pathological response to bevacizumab and chemoradiation in advanced rectal cancer. Nat Clin Pract Oncol 2007; 4: 316–321
- Urso E, Serpentini S, Pucciarelli S, De Salvo GL, Friso ML, Fabris G, Lonardi S, Ferraro B, Bruttocao A, Aschele C, et al. Complications, functional outcome and quality of life after intensive preoperative chemoradiotherapy for rectal cancer. Eur J Surg Oncol 2006; 32: 1201–1208
- Schulze T, Wust P, Gellermann J, Hildebrandt B, Riess H, Felix R, Rau B. Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia 2006; 22: 301–318
- Fietkau R, Barten M, Klautke G, Klar E, Ludwig K, Thomas H, Brinckmann W, Friedrich A, Prall F, Hartung G, Küchenmeister U, Kundt G. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum. 2006; 49: 1284–1292
- Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G, European Organisation for Research and Treatment of Cancer Radiation Oncology Group. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007; 25: 4379–4386
- Fietkau R, Klautke G. Adjuvant chemotherapy following neoadjuvant therapy of rectal cancer: The type of neoadjuvant therapy (chemoradiotherapy or radiotherapy) may be important for selection of patients. J Clin Oncol. 2008; 26: 507–508
- Rau B, Wust P, Hohenberger P, Löffel J, Hünerbein M, Below C, Gellermann J, Speidel A, Vogl T, Riess H, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: A phase II clinical trial. Ann Surg 1998; 227: 380–389
- Wust P, Rau B, Gellerman J, Pegios W, Löffel J, Riess H, Felix R, Schlag PM. Radiochemotherapy and hyperthermia in the treatment of rectal cancer. Recent Results Cancer Res 1998; 146: 175–191
- Anscher MS, Lee C, Hurwitz H, Tyler D, Prosnitz LR, Jowell P, Rosner G, Samulski T, Dewhirst MW. A pilot study of preoperative continuous infusion 5-fluorouracil, external microwave hyperthermia, and external beam radiotherapy for treatment of locally advanced, unresectable, or recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2000; 47: 719–724
- Fritzmann J, Hünerbein M, Slisow W, Gellermann J, Wust P, Rau B. Influence of preoperative (hyperthermic) radiochemotherapy on manometric anal sphincter function in locally advanced rectal cancer. Strahlenther Onkol 2004; 180: 281–288
- Elias DM, Sideris L. Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg Oncol Clin N Am 2003; 12: 755–769
- Liang H, Zhan HJ, Wang BG, Pan Y, Hao XS. Change in expression of apoptosis genes after hyperthermia, chemotherapy and radiotherapy in human colon cancer transplanted into nude mice. World J Gastroenterol 2007; 13: 4365–4371
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31: 1341–1346
- Knight CD, Griffen FD. An improved technique for low anterior resection of the rectum using the EEA stapler. Surgery 1980; 88: 710–714
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740
- Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006; 24: 4620–4625
- Pucciarelli S, Gagliardi G, Maretto I, Lonardi S, Friso ML, Urso E, Toppan P, Nitti D. Long-term oncologic results and complications after preoperative chemoradiotherapy for rectal cancer: A single-institution experience after a median follow-up of 95 months. Ann Surg Oncol 2009; 16: 893–899
- Klautke G, Fietkau R. Intensified neoadjuvant radiochemotherapy for locally advanced rectal cancer: A review. Int J Colorectal Dis. 2007; 22: 457–465
- Gosens MJ, Klaassen RA, Tan-Go I, Rutten HJ, Martijn H, van den Brule AJ, Nieuwenhuijzen GA, van Krieken JH, Nagtegaal ID. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res. 2007; 13: 6617–6623
- Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev Jan 21, 2009, 1: CD006041
- Kido Y, Kuwano H, Maehara Y, Mori M, Matsuoka H, Sugimachi K. Increased cytotoxicity of low-dose, long-duration exposure to 5-fluorouracil of V-79 cells with hyperthermia. Cancer Chemother Pharmacol 1991; 28: 251–254
- Matsuoka H, Sugimachi K, Abe R, Ueo H, Akiyoshi T. Enhancement of cytotoxicity by hyperthermia after a long-term culture with 5-fluorouracil in transformed cells. Anticancer Res 1992; 12: 1621–1625
- Maeta M, Sawata T, Kaibara N. Effects of hyperthermia on the metabolism of 5-fluorouracil in vitro. Int J Hyperthermia 1993; 9: 105–113
- van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia. 2006; 22: 255–262
- Leopold KA, Dewhirst MW, Samulski TV, Dodge RK, George SL, Blivin JL, Prosnitz LR, Oleson JR. Cumulative minutes with T90 greater than Tempindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys 1993; 25: 841–847
- Tilly W, Gellermann J, Graf R, Hildebrandt B, Weissbach L, Budach V, Felix R, Wust P. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol. 2005; 181: 35–41
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–1123