Abstract
Purpose: Due to the poor prognosis of patients with ovarian cancer relapse (OCR), newer strategies are warranted to improve the therapeutic index. We performed a prospective phase I/II-study of regional abdominal hyperthermia (RHT) combined with systemic chemotherapy in OCR patients in order to evaluate outcome, efficacy and tolerance.
Materials and methods: OCR patients with an Eastern Cooperative Oncology Group status <2, without any thromboembolic disease or severe cardiovascular co-morbidities, and pre-treated with at least one systemic chemotherapy regimen due to epithelial ovarian cancer were enrolled into the present study. RHT was applied using a SIGMA 60 applicator and a Hybrid-System SIGMA-Eye/MRT composed of a 1.5T-MRT and a Sigma-Eye-applicator.
Results: Overall, 36 OCR patients were enrolled. The majority of the patients (>80%) were classified as platinum resistant. The most common chemotherapeutic agent applied was pegylated-liposomal-doxorubicin (47.2%) followed by carboplatin (16.6%) and topotecan (13.9%). One patient (2.8%) achieved a complete remission (CR), 12 patients (33.3%) yielded a partial remission (PR) and 16 patients (44.4%) developed a progressive disease (PD). In platinum-sensitive patients we observed higher response (57.1% versus 31%) and lower progression rates (28.6% versus 48.3%) than in platinum-resistant patients. Eleven patients (30.5%) discontinued treatment due to toxicity. The main toxicity was a haematological one with grade 3/4 anaemia, leucopenia and thrombocytopenia occurring in 13.9%, 5.6% and 8.3%, respectively. Median overall survival was 12 months (range: 1–48), while median progression-free survival was 5 months (range: 0.5–34).
Conclusions: Our results demonstrate the feasibility of RHT combined with systemic treatment. Prospective phase III trials are warranted to evaluate the benefit and efficacy in heavily pre-treated patients with OCR.
Introduction
Hyperthermia therapy, during which body tissue is exposed to high temperatures, has the effect of killing or weakening tumour cells by a concurrent limited effect on healthy cells. Tumour cells, with a disorganized and compact vascular structure, have difficulty dissipating heat. Hyperthermia may therefore cause their apoptosis by denaturation and coagulation of regulatory cellular proteins Citation[1–3]. Even if hyperthermia does not induce apoptosis of cancer cells, they may become more susceptible to certain chemotherapeutic agents, thereby increasing their therapeutic index. Former preclinical in vitro studies in various experimental settings were able to indicate that hyperthermia would enhance the cytotoxic effects of carboplatin, thus overcoming any drug resistance acquired Citation[4–6].
In an attempt to improve the unfavourable clinical outcome of relapsed ovarian cancer, hyperthermia has been applied in experimental settings for advanced stages of the disease with concomitant systemic chemotherapy. The applied chemotherapeutic agents were mainly platinum-based, such as cisplatin or carboplatin Citation[7–13]. Consistent with the preclinical models, most study results suggest that hyperthermia may overcome platinum resistance in advanced ovarian cancer, although there is still no consistent evidence that hyperthermia contributes to any clinical improvement beyond chemotherapy alone Citation[11]. Furthermore, many authors report a significant dose-limiting, mainly due to haematological toxicities Citation[11], Citation[13], Citation[14].
The primary aim of the present feasibility study was to evaluate the efficacy, feasibility and tolerance of regional abdominal hyperthermia in conjunction with platinum- and non-platinum-based agents in patients with platinum-resistant or platinum-sensitive ovarian cancer relapse. Despite the fact that no conclusive in vitro data exist which prove the enhancement of the efficacy of non-platinum agents such as pegylated liposomal doxorubicin or topotecan in combination with hyperthermia, in this pilot study we wanted to demonstrate that hyperthermia can also be safely combined with non-platinum cytotoxic agents applied in platinum resistant and heavily pre-treated patients with ovarian cancer. Various pre-clinical and early phase I studies indicate promising activity and show encouraging results of topotecan or pegylated liposomal doxorubicin in combination with hyperthermia in different solid tumours Citation[28–32].
Our results will constitute the basis of future phase III trials as a possibility to establish the inclusion of hyperthermia as an adjunct to palliative chemotherapy in ovarian cancer patients.
Materials and methods
Study design
The present study was a pilot study with the primary end-point: feasibility of hyperthermia combined with systemic chemotherapy measured by the induced toxicity. The secondary end point was to describe the potential efficacy of the combined treatment defined by specific response criteria, cancer-related death and progression of the disease. All 36 patients were evaluated according to the intention to treat analyses (ITT) for both phase I (feasibility) and II (efficacy) objectives of the study. The initially planned number of patients was 40. All data were assessed in a prospective way. No dose increasing strategy in the phase I part of the study was followed.
The quality of hyperthermia was measured by the maximal total power achieved by the applicator used, as well as the maximum temperatures achieved in 179 heating sessions as measured vaginally and/or sublingually.
Patient selection
Thirty-six patients with relapse of epithelial ovarian cancer were recruited into the present study over a 5-year period (2002–2007). Patients were eligible if they had an Eastern Cooperative Oncology Group (ECOG) performance status Citation[15] of 0 or 1, an age above 18 years, no severe co-morbidities, no second malignancies, creatinine clearance ≥30 mL/min, adequate bone marrow function defined as white blood cell count (WBC) >1000/mL cells, and an absolute granulocyte count of ≥1.5 × 109/L cells and a platelet count of ≥100 × 109/L. All patients had at least one prior systemic chemotherapy pre-treatment due to ovarian cancer in their medical history. Exclusion criteria were any thromboembolic disease, central nervous system metastases, respiratory insufficiency, congestive heart failure and severe cardiovascular impairment. Patients were selected from the overall population of ovarian cancer of our hospital if they were in a good performance status (ECOG ≤1), had a good compliance, and mainly wished to be treated in a hyperthermic regime. Yearly we systemically treat approximately 100–120 patients. Another relevant point is that since we are a centre for clinical studies, the vast majority of our patients were enrolled in various other chemotherapy studies.
The study was approved by the Research Ethics Committee of the Charité (Application and Decision No. EA2/061/07). Participants gave their written informed consent before entering the study.
Patients were classified by platinum-resistant versus platinum-sensitive disease. Platinum resistance was defined either as a recurrence during six months after previous platinum-based chemotherapy or as a progression during a platinum-based regime Citation[8]. Patients were treated by various chemotherapeutic agents determined by the treating gynaecological oncologist in an interdisciplinary tumour board according to the current guidelines of the Ovarian Cancer Study Group (Arbeitsgemeinschaft Gynaekologische Onkologie, AGO-Ovar), as based on the pre-treatment regimes of the patients, their platinum-sensitivity status and their co-morbidities Citation[27]. The cytotoxic substances applied were pegylated liposomal doxorubicin, treosulfan, docetaxel, topotecan and carboplatin–as a monotherapy or in combination with gemcitabine. Details are presented in .
Table I. Cancer- and therapy-related characteristics of the 36 patients with ovarian cancer relapse after chemotherapy combined with regional abdominal hyperthermia.
RHT and supportive care
RHT was added to the systemic chemotherapy. The hyperthermia schedule was adapted according to each chemotherapy application regimen. The therapy was applied in a hospital setting (non-ambulatory). All patients were usually observed for at least 24 h after treatment before they were discharged. No routine application of the granulocyte colony stimulating factor (G-CSF) or of erythropoietin were allowed.
To apply RHT, a Sigma-60 (S-60) applicator with four antenna pairs and a sigma-Eye/MR (S-Eye/MR) applicator with 12 antenna pairs were used. The S-Eye/MR applicator is tuned in such a way as to be compatible with an MR-tomograph (Siemens Symphony 1.5,Tesla, Germany). A length of 4 cm was required as the minimum water distance between the antennae and the body surface. The RHT was performed according to a standard protocol. The applicator's mid plane was positioned on the patient's navel for abdominal heating. An optimum of 30 min was required to achieve a steady-state temperature of 41–42°C in a reference point under ideal conditions. The steady-state time (therapeutic time) was assessed after 60 min. A therapeutic time below 30 min was defined as a disrupted treatment.
Power and temperature data were acquired for the S-60 and S-Eye/MR. Temperature–time curves were registered for each patient in one reference point either via the vagina (orifice or stump after hysterectomy), in the rectum or in the anus praeter as well as sublingually (orally) in order to estimate the systemic temperature.
A limiting parameter of the treatment was a heat intolerance or haematological toxicity as described below. In cases of some intolerance or side effects induced by the heat, but without early termination of the therapy, the physician in charge was usually able to address the problem by reducing the total power, changing the phases of the channels, repositioning the patient, cooling the bolus water, using water bags, or administering medication–especially in the case of edge effects.
Toxicity evaluation
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (CTC; version 2.0) Citation[15], Citation[16]. Dose modifications (25% dose reduction of chemotherapy) were applied in the case of increased renal retention values during therapy (creatinine clearance <60 mL/min). In cases of febrile neutropenia or thrombocytopenia <100 ×109/L, treatment had to be delayed until the recovery of marrow function had been ascertained.
Response evaluation
Responses were classified according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria Citation[17]. CT scans of measurable and assessable sites were carried out within 4 weeks before the start of the treatment and repeated every 3 cycles or when clinically indicated. Responses were confirmed by CT scan approximately 4 weeks after determining the initial response. Measurement for CA 125 was performed in addition, to evaluate the response to therapy. The time to progression was measured from the start of the treatment until progression. Overall survival was defined as being the time between the start of the treatment and that of death or the last follow-up date. A follow-up of the patients after completion of treatment took place every 3 months via clinical examination, tumour marker values CA125, and transabdominal/transvaginal sonography. A CT or MRI scan was then performed in those cases where a tumour relapse had been suspected. Relapse was then defined according to the RECIST criteria Citation[17].
Statistical analysis
Analyses were conducted in an exploratory fashion. All results are presented in raw numbers, rates, or medians and ranges, mean ± standard deviation according to the underlying distribution. Binomial-exact 95%-confidence intervals (CI) are also reported. The analyses of time-to-event data in the case of recurrence and overall survival were calculated according to the Kaplan-Meier method and survival curves were compared using the log-rank test. For the calculation of recurrence-free and overall survival, the interval between start of chemotherapy and date of tumour progression, death or last contact was analysed. Statistical analyses were performed by SPSS 15 (SPSS, Chicago) and GraphPad Prism 5 statistical software® (GraphPad, La Jolla, CA).
Results
Response and survival
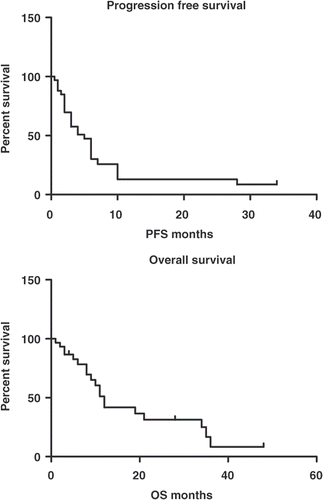
Overall, 36 patients with ovarian cancer relapse under palliative systemic chemotherapy combined with RHT were evaluated. All patients were assessable for the safety analysis and evaluable for response. The mean patient age was 53.32 years ±9.8 (SD); (range: 25–68). The mean follow-up period was 12.9 ± 12.63 SD months (range: 1–48 months). Seven patients (19.4%) were platinum sensitive, while 29 patients (80.6%) were platinum resistant. Overall, 20 patients (55.5%) had distant metastases in liver parenchyma (36.1%), pleura (16.7%) and abdominal wall (11.1%). The majority of the patients (47.2%) was under third-line chemotherapy and the most common chemotherapeutic agent applied was pegylated liposomal doxorubicin (47.2%) followed by topotecan in 13.9% of the patients. The initial Fédération Internationale de Gynécologie et d'Obstétrique (FIGO) stage of the majority of the patients (80.5%) was stage III. Detailed cancer-related patient characteristics are presented in . The median overall survival (OS) was 12 months (range: 1–48; 95%CI: 9.8–14.19), while the median progression-free survival (PFS) was 4 months (range: 0.5–34; 95%CI: 2.17–5.82). The Kaplan-Meier survival curves of OS and PFS are presented in . Only one patient showed a complete response to treatment and still continues RHT and pegylated liposomal doxorubicin over a period of 2.5 years every 4 weeks; within the last year, bevacizumab has been added. This patient was 22 years old, with a heavily pre-treated platinum resistant relapsed ovarian cancer. The combination of pegylated doxorubicin and hyperthermia achieved a complete response. However, due to the high risk of tumour progression the patient desired the addition of bevacizumab. Until now, we have not observed additional side effects. Last examination in June 2009 confirmed no evidence of disease.
Figure 1. Kaplan-Meier survival curves and data for overall and progression-free survival for the 36 patients with ovarian cancer relapse after chemotherapy combined with regional abdominal hyperthermia. Point 0, beginning of combination treatment; PFS, progression-free survival; OS, overall survival.
During the follow-up period, 22 (61.1%) of the 36 patients died and all patients (97.2%) but one experienced a new relapse or progression of the malignant disease. Sixteen patients (44.4%) presented a rapid progressive disease still being under combined treatment, which also led to discontinuation of treatment. Twelve patients (33.3%) presented a partial response according to imaging studies (CT or MRI). When dividing the patients into platinum resistant and platinum sensitive, we see that the latter had higher rates of overall response (31% versus 57.1%) and lower rates of rapid progressive disease under treatment (48.3% versus 28.6%).
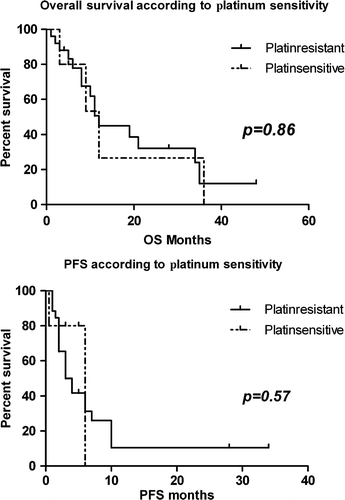
The median OS separately for platinum-sensitive and platinum-resistant patients was 12 months (95%CI: 4.53–19.46) and 12 months (95%CI: 3.32–20.67), respectively and so not significantly different (p = 0.86). The median PFS for platinum-sensitive and platinum-resistant patients was 6 months (95%CI: 2.54–7.26) and 3 months (95%CI: 1.38–4.62), respectively. These PFS values were also not significantly different (p = 0.57) between the two groups. The fact that no statistical significance could be reached between the two groups is probably attributable to the small number of patients; especially the platinum-sensitive ones. The equivalent Kaplan-Meyer survival curves for platinum-resistant and -sensitive patients are presented in .
Course of therapy and toxicity
Thirty-six patients received a total of 179 RHT treatment cycles. The median number of applied treatment cycles per patient was 4 (range: 1–22) and the average number per patient was 5.1 ± 4 (SD). Only 6 (16.7%) of the 36 patients completed all initially scheduled cycles of chemotherapy combined with RHT. In 11 patients (30.5%) treatment had to be discontinued due to haematological toxicity (10.3%), gastrointestinal symptoms such as severe nausea, vomiting or diarrhoea (3.4%), allergy or cutaneous exanthema (6.8%), and acute renal failure in one patient. One further patient discontinued therapy due to personal reasons. In all other patients, therapy was stopped due to rapid progressive disease with clinical symptoms such as mechanical ileus and ascites. In 10 patients (27.8%), isolated treatment cycles had to be delayed for 1 to 2 weeks on average due to thrombocytopenia or a reduced general condition of the patients. The reported toxicity was related to both RHT and systemic chemotherapy. Data are presented in .
Table II. Clinical course and response to therapy of the 36 patients with ovarian cancer relapse under chemotherapy combined with RHT.
The overwhelming majority of the heating sessions took 30 to 60 min to complete; generally they lasted for approximately 60 min (time at steady state temperature plateau). The mean maximum total power (W) achieved by the applicator, was 646.6 ± 70.58 W (range: 450–720; 95%CI: 615.6–665.6).
The values of the maximum temperatures achieved in 179 heating sessions were as follows: mean temperature as measured in the rectum or in the anus praeter was 41.72 ± 0.26°C (range: 41.3–42.1; 95%CI: 41.6–41.83). The mean maximum temperature sublingually was 37.4 ± 0.17°C.
We found no evidence for heat-specific acute, sub acute or chronic toxicities such as thermal burns or tissue damage. When evaluating the parameters TRISE(vaginal) and TRISE (rectal) according to Franckena et al. Citation[33], we measured on average values of 2.72 and 2.84 respectively. In a univariate analysis, TRISE was significant for the response rate (p = 0.03), though a multivariate analysis was not representative due to the small number of patients. Also significant for the response rate were platinum sensitivity and the absence of distant metastases (p < 0.01). TRISE was measured according to the formula:The toxicity profile of the combined treatment classified into CTC grade 1 to 2 and grade 3 to 4 is presented in detail in . Although a mild (grade 1 to 2) anaemia, thrombocytopenia and leucopenia was developed by 30.5%, 22.2% and 38.9% of the patients respectively, only 13.9%, 8.3% and 5.6% of the patients developed severe, grade 3 to 4, haematological disturbances. Other side effects such as nausea and vomiting, electrolyte disturbances and allergic reactions occurred in the majority of the cases to only a rather mild degree. In one patient severe hyponatraemia of 125 mmol/L occurred during the third treatment cycle and resulted in impaired consciousness.
Table III. Common and RHT-related toxicity.
Discussion
Over the last 25 years, five-year survival rates for patients with advanced epithelial ovarian cancer have improved from 36% in the mid 1970s to 45% by the year 2002 Citation[18]. However, since approximately 75% of women with epithelial ovarian cancer initially present with stage III or IV disease the vast majority of the patients (60–85%) will suffer from a tumour relapse Citation[19]. The selection of salvage therapy is commonly based upon whether women are ‘sensitive’ or ‘resistant’ to initial platinum-based treatment. Patients who respond to initial platinum-based therapy and have a significant relapse-free interval (more than six months) have a high probability of responding again to platinum-based treatment at the time of relapse. The length of the prior response is highly predictive of the upper limit of the duration of disease control that can be expected with second-line platinum-based chemotherapy Citation[21]. The length of the treatment-free interval is an important predictor of outcome. This was illustrated in a report that included 72 patients with measurable disease recurrence who had received at least two platinum-based regimens, and who had a treatment-free interval of at least four months. Treatment outcome was stratified according to the length of the treatment-free interval: With a treatment-free interval between 5 and 12 months, the response rate was 27%, and the pathological CR rate was 5%. With a treatment-free interval of 13 to 24 months, the response rate was 33%, and the pathological CR rate was 11%. With a treatment-free interval of longer than 24 months, the response rate was 59% and the pathological CR rate was 22% Citation[22].
Successful management of women who are platinum resistant requires the use of non-cross-resistant agents. Single agent therapy is usually chosen, since combination regimens are more toxic, but without a proven survival benefit compared to single agent therapy. Many cytotoxic agents are considered to be active, including paclitaxel, docetaxel, cyclophosphamid, pegylated liposomal doxorubicin, topotecan, gemcitabine, vinorelbine and treosulfan. The overall response rates vary between 25% for paclitaxel weekly Citation[23], 17% for pegylated liposomal doxorubicin Citation[24], 15% for topotecan Citation[25] and 13–21% for gemcitabine Citation[26]. Toxicity profiles also vary with a myelotoxicity profile (grade 3 or 4 neutropenia) as high as 12% for liposomal doxorubicin and 8% febrile neutropenia for topotecan.
In the present study we treated 29 platinum-resistant patients and 7 platinum-sensitive patients with recurrent epithelial ovarian carcinoma with systemic chemotherapy in combination with RHT. The thermal enhancement of platinum drugs is well established Citation[4–6], but since we also applied non-platinum agents in platinum resistant patients, we aimed to demonstrate the feasibility and efficacy of non-platinum agents in combination with hyperthermia.
We report high rates of progressive disease; all patients but one experienced a new relapse during the follow-up period with a mean time to progression of 8.62 months. This may be attributed to the fact that the majority of our patients were heavily pre-treated in a highly palliative situation and had already suffered from two or three recurrences of the malignant disease. However, despite the previous polychemotherapy regimes of most of our patients, we do report acceptable overall toxicity rates of the combined treatment. Severe episodes of haematological toxicity that led to discontinuation of therapy constituted only 11%. We report no severe incidences of cardiovascular complications during treatment, which may be accredited to the fact that patients with serious cardiovascular co-morbidity were precluded from the study.
Even if 30.5% of the patients discontinued the treatment due to toxicity (11% due to haematological toxicity), one has to consider that our patient collective was mainly platinum resistant and thus in a highly palliative situation, with 75% of the patients being already in the third or fourth chemotherapy line. These patients were heavily pre-treated and therefore had limited therapeutic reserves. When comparing our results to the toxicity profile of pegylated liposomal doxorubicin alone, no unusual higher toxicity rates are noticed. In a recent review by Strother et al. about pegylated liposomal doxorubicin in ovarian cancer Citation[34], considerable grade 3-4 hematologic toxicity (mainly neutropenia), ranges from 12% to 46%.
In a comparable study by Cho et al. on 45 patients with peritoneal carcinomatosis treated with systemic chemotherapy and RHT, the authors found no evidence of heat-specific subacute or chronic toxicities such as thermal burns or tissue damage Citation[12]. Furthermore, we can confirm their conclusion that the observed toxicities with RHT are comparable to known side effects of chemotherapy without hyperthermia. In contrast to our results, Atmaca et al. report in a recently published evaluation of 47 patients with whole body hyperthermia an overall cardiac toxicity rate of 49% (47% of mild degree) Citation[11]. This large difference may be attributed to the fact that our patient collective was treated by regional abdominal hyperthermia versus the whole body hyperthermia applied in the other study.
In most hyperthermia analyses of ovarian cancer patients, chemotherapy-induced myelosuppression was the major toxic effect Citation[8]. Rates of 50% to 65% of haematological toxicity of all grades are described. Our results are clearly more encouraging, with haematological toxicity being less than 40% and the majority of the cases being of mild grade 1 or 2. However, the actual toxicities of combined hyperthermia and chemotherapy, also depending on the dosage of the chemotherapeutic agents, have to be prospectively evaluated in large phase III trials. Also, this difference in toxicity profile may be assigned to the extent of applied hyperthermia (partial abdominal versus whole body). Nevertheless, we cannot report any lower overall response rates, even if the main body of our patient collective was platinum resistant. The overall response rate of most series ranges between 35 and 38%, which is equivalent to our results Citation[8], Citation[11], Citation[20].
Also congruent with previous reports of Franckena et al. Citation[33], we found a significant relationship between the thermal parameter TRISE (Custom made thermal dose parameter based on ALT50 and duration of heating) and the response rate.
In a large observational study from our institution analysing the efficacy of pegylated liposomal doxorubicin without hyperthermia under routine clinical conditions on 183 ovarian cancer patients, rather equivalent survival dates are demonstrated with the ones of the present study: median PFS and OS were 5.8 months (95% CI 5.1–6.6 months) and 16.6 months (95% CI 13.9–22.6 months), respectively Citation[35].
Since the vast majority (47.1% liver and abdominal wall) of the distant metastases reported were located in the abdominal cavity, they were also affected by the regional abdominal hyperthermia. Furthermore, since the chemotherapy was applied systemically and not only intraperitoneally, the cytotoxic effects were also systemic and potentially efficacious also against distant metastatic sites.
Our analysis shows that hyperthermia can safely be combined with platinum and non-platinum agents in heavily pre-treated patients with relapsed ovarian cancer. We could observe individual responses, so further prospective studies are warranted to investigate the efficacy of this combination therapy. Regional abdominal hyperthermia seems to have a relatively equivalent efficacy with whole abdominal hyperthermia by a lower toxicity profile. The statistically similar survival of platinum-resistant and platinum-sensitive patients, is probably attributable to the small number of all patients; especially the platinum-sensitive ones. Keeping in mind that there is currently no evidence that hyperthermia contributes to any clinical improvement beyond chemotherapy alone, we believe that further trials are warranted to prove the efficacy of RHT treatments in an effort to overcome drug resistance and to increase response to palliative salvage chemotherapy. The toxicity profile of combined treatment regimens in this poor prognostic group of immunosuppressed and myelosuppressed patients should always be considered and counterbalanced in respect to their benefits. The parameter ‘quality of life’ should also be purposed as a relevant study objective.
Declaration of interest: No conflict of interest to declare.
References
- Cohen JD, Robins HI. Thermal enhancement of tetraplatin and carboplatin in human leukaemic cells. Int J Hypertherm 1990; 6: 1013–1017
- Cohen JD, Robins HI, Javid MJ. Sensitization of C6 glioma to carboplatin cytotoxicity by hyperthermia and thymidine. J Neurooncol 1990; 9: 1–8
- Ohno S, Siddik ZH, Baba H, Stephens LC, Strebel FR, Wondergem J, Khokhar AR, Bull JM. Effect of carboplatin combined with whole body hyperthermia on normal tissue and tumor in rats. Cancer Res 1991; 51: 2994–3000
- Page RL, McEntee MC, Williams PL, George SL, Price GS, Novotney CA, Hauck ML, Riviere JE, Dewhirst MW, Thrall DE. Effect of whole body hyperthermia on carboplatin disposition and toxicity in dogs. Int J Hypertherm 1994; 10: 807–816
- Tapazoglou E, Cohen JD, Schmitt CL, Khatana A, Sapareto SA, Robins HI. Whole body hyperthermia and carboplatin: Cytotoxicity for murine leukaemia and normal marrow. Br J Cancer 1991; 64: 528–530
- Repasky EA, Tims E, Pritchard M, Burd R. Characterization of mild whole-body hyperthermia protocols using human breast, ovarian, and colon tumors grown in severe combined immunodeficient mice. Infect Dis Obstet Gynecol 1999; 7: 91–97
- Formenti SC, Shrivastava PN, Sapozink M, Jozsef G, Chan KK, Jeffers S, Morrow PC, Muggia FM. Abdomino-pelvic hyperthermia and intraperitoneal carboplatin in epithelial ovarian cancer: Feasibility, tolerance and pharmacology. Int J Radiat Oncol Biol Phys 1996; 35: 993–1001
- Douwes F, Bogovic J, Douwes O, Migeod F, Grote C. Whole-body hyperthermia in combination with platinum-containing drugs in patients with recurrent ovarian cancer. Int J Clin Oncol 2004; 9: 85–91
- Cohen JD, Robins HI. Whole body hyperthermia and intraperitoneal carboplatin in residual ovarian cancer. Adv Exp Med Biol 1990; 267: 197–202
- Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, Ahn WS, Namkoong SE. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol 2007; 106: 193–200
- Atmaca A, Al-Batran SE, Neumann A, Kolassa Y, Jäger D, Knuth A, Jäger E. Whole-body hyperthermia (WBH) in combination with carboplatin in patients with recurrent ovarian cancer–A phase II study. Gynecol Oncol 2009; 112: 384–388
- Cho CH, Wust P, Hildebrandt B, Issels RD, Sehouli J, Kerner T, Deja M, Budach V, Gellermann J. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: Evaluation of two annular-phased-array applicators. Int J Hyperthermia 2008; 24: 399–408
- Westermann AM, Grosen EA, Katschinski DM, Jäger D, Rietbroek R, Schink JC, Tiggelaar CL, Jäger E, Zum Vörde sive Vörding P, Neuman A, et al. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer. Eur J Cancer 2001; 37: 1111–1117
- Ceelen WP, Peeters M, Houtmeyers P, Breusegem C, De Somer F, Pattyn P. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann Surg Oncol 2008; 15: 535–541
- Minna JD, Higgins GA, Glatstein. EJ. Cancer of the lung. Cancer: Principles and Practice of Oncology, V De Vita, S Hellmann, S Rosenberg. Lippincott, Philadelphia 1984; 536
- National Cancer Institute. Cancer Therapy Evaluation Program: Common toxicity criteria. Accessed 10 January 2009 from: http://ctep.cancer.gov/reporting/ctc.html. August 9, 2006.
- Padhani AR, Ollivier L. The RECIST criteria: Implications for diagnostic radiologists. Br J Radiol 2001; 74: 983–986
- Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: Changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol 2003; 189: 1120–1127
- Roland PY, Barnes MN, Niwas S, Robertson MW, Alvarez R, Austin JM, Kilgore LC, Partridge EE. Response to salvage treatment in recurrent ovarian cancer treated initially with paclitaxel and platinum-based combination regimens. Gynecol Oncol 1998; 68: 178–182
- Katschinski DM, Wiedemann GJ, Longo W, d'Oleire FR, Spriggs D, Robins HI. Whole body hyperthermia cytokine induction: A review, and unifying hypothesis for myeloprotection in the setting of cytotoxic therapy. Cytokine Growth Factor Rev 1999; 10: 93–97
- Markman M, Markman J, Webster K, Zanotti K, Kulp B, Peterson G, Belinson J. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: Implications for patient management and clinical trial design. J Clin Oncol 2004; 22: 3120–3125
- Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL, Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991; 9: 389–393
- Markman M, Hall J, Spitz D, Weiner S, Carson L, Van Le L, Baker M. Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refractory ovarian cancer. J Clin Oncol 2002; 20: 2365–2369
- Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia A, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: Antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol 1997; 15: 987–993
- Bookman MA, Malmstrom H, Bolis G, Gordon A, Lissoni A, Krebs JB, Fields SZ. Topotecan for the treatment of advanced epithelial ovarian cancer: An open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol 1998; 16: 3345–3352
- Lund B, Hansen OP, Theilade K, Hansen M, Neijt JP. Phase II study of gemcitabine (2′,2′-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst 1994; 86: 1530–1533
- Chekerov R, Denkert C, Boehmer D, Suesse A, Widing A, Ruhmland B, Giese A, Mustea A, Lichtenegger W, Sehouli J. Online tumor conference in the clinical management of gynecological cancer: Experience from a pilot study in Germany. Int J Gyn Canc 2008; 18: 1–7
- Kouloulias VE, Koukourakis GV, Kouvaris I, Petridis AK, Gouliamos AD. The efficacy of caelyx and hyperthermia for anticancer treatment. Recent Patents Anti-Cancer Drug Disc 2007; 2: 246–250
- Hermisson M, Weller M. Hyperthermia enhanced chemosensitivity of human malignant glioma cells. Anticancer Res 2000; 20: 1819–1823
- Harrison LE, Bryan M, Pliner L, Saunders T. Phase I trial of pegylated liposomal doxorubicin with hyperthermic intraperitoneal chemotherapy in patients undergoing cytoreduction for advanced intra-abdominal malignancy. Ann Surg Oncol 2008; 15: 1407–1413
- Hahn CA, Jones EL, Blivin JL, Sanders LL, Yu D, Dewhirst MW, Secord AA, Prosnitz LR. Prospective assessment of quality of life in ovarian cancer patients receiving whole abdomen hyperthermia and liposomal doxorubicin. Int J Hyperthermia 2005; 21: 349–357
- Dvorak J, Zoul Z, Melichar B, Petera J, Vesely P, Vosmik M, Dolezel M. Liposomal doxorubicin combined with regional hyperthermia: Reducing systemic toxicity and improving locoregional efficacy in the treatment of solid tumors. J Chemother 2004; 16: 34–36
- Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, van Rhoon GC, van der Zee J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer 2009; 45: 1969–1978
- Strother R, Matei D. Pegylated liposomal doxorubicin in ovarian cancer. Ther Clin Risk Manag 2009; 5: 639–650
- Sehouli J, Camara O, Schmidt M, Mahner S, Seipelt G, Otremba B, Schmalfeldt B, Tesch H, Lorenz-Schlüter C, Oskay-Ozcelik G. North-Eastern German Society of Gynecological Oncology. Pegylated liposomal doxorubicin (CAELYX) in patients with advanced ovarian cancer: Results of a German multicenter observational study. Cancer Chemother Pharmacol 2009; 64: 585–591