Abstract
Purpose: The management of head and neck cancer requires skilled integration of multiple modalities such as surgery, radiation, chemotherapy and hyperthermia. Chemoradiation can benefit from the addition of a proven modality such as hyperthermia in increasing survival, disease-free survival and quality of life without increasing the risk of complication.
The purpose of this retrospective study was to evaluate the feasibility and efficacy of hyperthermia with chemoradiation in advanced head and neck cancers.
Materials and methods: Between January 2004 and May 2008 40 patients with advanced head and neck cancers were allocated for hyperthermia with chemoradiotherapy. All patients underwent radiation on a telecobalt machine. A total dose of 70 Gy in 7 weeks with conventional fractionation was given with weekly chemotherapy of cisplatin 50 mg or paclitaxel 60 mg. Patients underwent hyperthermia on a radiofrequency machine at 8.2 MHz for 30 min at 41°–43°C with 10 min pre-cooling to 5°C.
Results: No patient had life-threatening complications. Only 38 out of 40 patients were eligible for assessment of immediate response as one patient died during treatment and the other did not complete treatment. Complete response was 76.23% (29 pts), and 23.68% (9 pts) had partial response. Overall survival by the Kaplan−Meir method was 75.69% at 1 year and 63.08% at 2 years.
No enhanced mucosal or thermal toxicities were documented as compared to our earlier experience with chemoradiation.
Conclusion: This retrospective analysis demonstrates the feasibility and efficacy of chemoradiation with hyperthermia in advanced head and neck cancer. The study is encouraging enough to start a randomised trial to compare chemoradiation with triple modality of treatment.
Introduction
The management of advanced head and neck cancer requires skilled integration of multiple modalities such as radiation, surgery, chemotherapy and hyperthermia. Chemoradiation has emerged over the last decade as a standard of care in certain anatomical subsets such as laryngeal and hypopharyngeal cancers. Adelstein et al. Citation1 have reported an improved survival following concurrent chemoradiation with cisplatin over radiation alone. In all 225 patients with unresectable cancer of the oral cavity, oropharynx, larynx, or hypopharynx were included in the series. Bonner et al. Citation2, in their study of cetuximab, a vascular endothelial growth factor inhibitor, have shown an improved local control and 3-year survival in unresectable squamous cell head and neck cancers. Chemoradiation can probably benefit from the addition of a proven modality such as hyperthermia in increasing survival, disease-free interval or quality of life.
Valdagni has demonstrated distinct survival benefits of hyperthermia with radiation in head and neck cancers [17, 18]. Our own unpublished data of 120 patients has shown an impressive 70% complete response immediately following hyperthermia and radiation. Effectiveness of hyperthermia with radiation has been conclusively demonstrated by Van der Zee et al. in pelvic cancers [19].
Certain cytotoxic drugs such as cisplatin have shown thermal sensitising effects in vitro while taxanes and anthracyclines have not shown any such potential Citation4, Citation7 However, a possible additive or potentiating effect of these drugs in vivo transcends mechanisms related to cellular changes alone. Hence in vitro results may not reflect reality in the clinic Citation3. The retrospective analysis reported here emanates from a single institution and author. Patients were treated and assessed over the last four years. This is a clinical audit and a study of the feasibility of adding hyperthermia to chemoradiation in treating head and neck cancers.
Materials and methods
Patients with head and neck cancer with stages III and IV have been treated with radical external radiotherapy, weekly chemotherapy and weekly hyperthermia since 2004. Patients underwent histological confirmation of epithelial cancer, endoscopy, complete blood chemistry and chest X-ray before beginning the trimodality treatment. Patients with metastatic disease, short and fat neck and Karnofsky index of less than 70 were not offered the trimodality treatment. Patients with confounding factors that precluded chemotherapy were not treated with trimodality treatment.
Radiotherapy
All patients underwent radiation on a telecobalt machine (Theratron 780C, Atomic Energy of Canada Ltd, Ontario, Canada) with parallel opposed compensated beams or multiple beams as per the requirement. A dose of 70 Gy in 7 weeks with conventional fractionation was planned for all patients. Patients were treated 5 days a week from Monday to Friday.
Chemotherapy
Weekly chemotherapy was administered with brief infusion of chemotherapy on every Saturday. No radiation was administered on Saturday but hyperthermia with 8.2 MHz radiofrequency followed within an hour of chemotherapy.
An aliquot of 50 mg cisplatin or paclitaxel 60 mg was administered weekly as 1.5 h infusion with appropriate premedication.
Hyperthermia
Patients underwent hyperthermia on a radiofrequency machine at 8.2 MHz. All patients underwent 10 min of pre-cooling to 5°C. A pair of antennae was placed across the neck guided by visible tumour or anatomical landmarks. The radiofrequency input was started after impedance matching. The power varied from 400 to 800 kW. A gradual escalation of power was stopped when patients complained of discomfort or pain. Invasive thermometry with a thermistor probe was performed when feasible. Patients received hyperthermia for 30 min at 41°–43°C after pre-cooling for 10 min.
Patients were evaluated twice a week to assess acute toxicities and response. Patients who developed neutropenia were given colony-stimulating factor but continued on the protocol. However, hyperthermia was suspended if patients developed grade II or higher thermal burns.
It is a single institution study analysed retrospectively.
Patients and outcome evaluation
Immediate response was analysed as complete, partial or no response as per the World Health Organization guidelines. Patients were evaluated periodically to assess the disease status and toxicity at an interval of 3 months in the first year and 6 months in subsequent years. Toxicity in hyperthermia is assessed by Grade I-IV burns. Grade I–superficial burn with partial thickness, Grade II–deep burn with partial thickness, Grade III–full thickness burn, Grade IV–soft tissue necrosis. Patients who died and those who were lost to follow up were censored for survival analysis thereafter. The Kaplan–Meier survival analysis has been performed.
Results
Forty patients with head and neck cancer were treated over the last four years with concurrent chemoradiotherapy and hyperthermia. shows the clinical profile with anatomical subsites of this group. Most patients had oral and oropharyngeal cancers. Only two patients had a T2N0M0 lesion, while the rest of them had T3-T4 and N0-N3 metastatic neck nodes. shows stratification according to TNM staging (AJCC).
Table I. Clinical profile.
Table II. Stratification according to TNM staging (AJCC).
Only 38 patients were eligible for assessment of immediate response as one patient died during the treatment and the other did not complete the treatment. Death was not related to any treatment modality.
The total radiation dose delivered ranged from 60 to 70 Gy with a mean dose of 66.23 Gy (standard deviation-4.079).
Patients received hyperthermia for 30 min at 41°–43°C after pre-cooling of 10 min. Thermometry was done in 23 patients who had nodal metastasis. The maximum temperature never exceeded 43°C in any of the patients. The average temperature is 42.4°C after excluding one outlier at 39°C.
shows the number of hyperthermia sessions and chemotherapy cycles delivered for all patients. An overwhelming number of 37 patients received more than four hyperthermia sessions. Similarly 36 patients received more than three cycles (4–7) of chemotherapy. Those who received less than 3 cycles of chemotherapy did so because of chemotherapy related toxicity like nausea and vomiting. An equal number of patients received cisplatin and paclitaxel. While one patient died, one patient had incomplete treatment in the paclitaxel group. The follow up ranged from 4 to 68 months with a median duration of 9 months.
Table III. Details of frequency of hyperthermia and chemotherapy sessions with radiation therapy in 40 patients of head and neck cancer.
Table IV. Drug-wise initial response of patients.
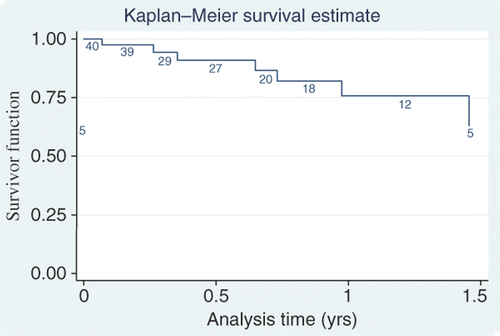
Of the 38 patients, complete response (CR) was documented in 29 patients (76.32%) and nine patients (23.68%) had partial response (PR). Regarding the 29 CR patients, 13 (34%) were treated with cisplatin, and 16 (42%) with paclitaxel (P: 0.479). Of the nine PR patients, seven (18%) were in the cisplatin group and two (5%) in the paclitaxel group. One patient died and one patient had incomplete treatment in the paclitaxel group. shows Kaplan-Meier survival curve. Overall survival by the Kaplan–Meir method is 75.69% at 1 year (95% confidence interval (CI) 51.88–88.85) and at 2 years it was 63.08% (with 95% CI 30.98–83.43). Out of 40 (92.5%) patients, 37 received four or more (4–7) hyperthermia sessions, while 36 out of 40 (90%) patients received four or more (4–7) chemotherapy sessions. Thus it can be surmised an overwhelming number of patients complied with the trimodality protocol. Seven patients were lost to follow up at various intervals.
No enhanced mucosal or thermal toxicities were documented as compared to our earlier experience with chemoradiation. Two patients had sustained grade I burns in a small area that corresponded to the edge of the antennae. Treatment was not interrupted in any patient due to thermal burns. They healed eventually with the application of nadoxin, an antibiotic, and analgesic drugs.
Discussion
Chemoradiation has emerged as a standard of care in the management of head and neck cancer as well as lung and cervical cancers. Adelstein et al. have demonstrated a survival benefit in those patients of unresectable head and neck cancer who received conventional radiation and cisplatin over patients treated with radiation alone. ‘The survival improved from 27% to37% at 3 years. Increased toxicity was seen due to chemotherapy Citation1.
Chemoradiation has been shown to improve loco-regional control and overall survival in many subsites of squamous cell carcinoma of the head and neck. The overall survival reported by Bonner et al. is only 55% at 3 years with cetuximab, and Brizel et al. have also demonstrated a similar overall survival of 55% at 3 years following concurrent chemoradiation Citation5, Citation6. There is a scope and an urgent need for further improvement in survival.
Chemoradiation leads to increased acute mucosal and skin toxicity. Evidence for increased chronic toxicity is also accumulating. Besides, two meta-analyses published recently have questioned the impact of chemoradiation in advanced stage tumours Citation9, Citation13.
A large number of tumours not sensitive to cytotoxic drugs remain incurable even after chemoradiation; yet another reason for these resistant tumours is tissue hypoxia. Heat is an effective radiation sensitiser. Hypoxic cells are also susceptible to cell death following hyperthermia, unlike after radiation and chemotherapy. Martin Franckena et al. have suggested that it would be more beneficial to add hyperthermia to radiation than chemotherapy to radiation in higher stage cancer Citation14.
Failure to improve survival any further could be due to the unbridled repopulation of resistant clones of cells, hypoxia, and poor drug delivery due to inadequate blood perfusion. Hyperthermia can overcome hypoxia, increase perfusion at lower temperatures and act on those cells that are resistant to radiation. This, then, is the raison d’être for adding hyperthermia to chemoradiation.
Thermal enhancement following administration of chemotherapy may be due to increased uptake of drug, increase in perfusion, amplification of DNA damage or inhibition of DNA damage repair, and/or it may be a combination of the above mechanisms of action Citation2, Citation10, Citation11. Chemoradiation with hyperthermia is an area that needs exploration in the clinics. Not all drugs have shown thermal enhancement in vitro. Alkylating agents, platinum analogues, bleomycin and mitomycin are considered of value while vinca alkaloids, taxanes, topotecan, 5-FU and methotrexate have not shown adequate thermal satisfaction. Arcangeli et al. Citation4 demonstrated an advantage of combining adriamycin and bleomycin with hyperthermia in which a total of 29 patients with head and neck cancer with nodes showed a favourable outcome. This study showed enhanced effectiveness of adriamycin and bleomycin combined with local hyperthermia in neck node metastasis from head and neck cancer. Kohno et al. have also demonstrated the effectiveness of combining bleomycin and mitomycin with hyperthermia in malignancies in a randomised study involving 65 patients Citation11.
A trimodality approach for the treatment of head and neck cancer was earlier published by Amichetti in a small cohort of patients Citation2. This study shows the feasibility of implementing trimodality treatment in head and neck cancers. No prohibitive toxicities were observed. Initial response in patients treated with paclitaxel and cisplatin is an interesting observation as taxanes have not shown thermal sensitisation in vitro. But Anna Cividalli et al. demonstrated in their study that hyperthermia enhanced the effectiveness of paclitaxel in vivo and evaluation, in terms of cure shows a very high enhancement ratio Citation3. It is hypothesised that increased perfusion must have led to a higher cellular concentration with lethal effects Citation3. Cisplatin and paclitaxel are some of the molecules used in chemoradiation of head and neck cancer. Cisplatin has been shown to be a thermal sensitiser, while the effects of paclitaxel as a thermal sensitiser are equivocal. Addition of hyperthermia to chemoradiation with paclitaxel or cisplatin is assumed to show an incremental response.
This study documents feasibility and effectiveness of trimodality of treatment with paclitaxel, cisplatin, hyperthermia and radiation. Long-term follow up and randomised trials will help assess the exact benefit of trimodality treatment over chemoradiation alone.
Conclusion
This retrospective analysis demonstrates the feasibility of chemoradiation with hyperthermia in advanced head and neck cancer. Head and neck cancer with very high tumour burden have a gloomy survival prospect. The study is encouraging enough to start a randomised trial to compare chemoradiation with chemohyperthermia and radiation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adelstein DJ, Li Y, Adams GI, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–98
- Bonner JA, Harari PM, Giralt. Radiotherapy plus cetuximab for squamous cell carcinoma of head and neck cancer. N Eng J Med 2006; 354: 567–578
- Valdagni R, Amicheti M. Report of long-term follow up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymphnode in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1994; 28: 163–169
- Valdagni R, Liu FF, Kapp DS. Important prognostic factors influencing outcome of combined radiation and hyperthermia. Int J Radiat Biol Phys 1988; 15: 959–972
- Van der Zee J, Gonzales GD, Van Rohen GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective randomised, multicentre trial. Dutch Deep Hyperthermic Group. Lancet 2000; 355: 1119–1125
- Arcangeli G, Cividdalli A, Mauro F, Nervi C, Pavin G. Enhanced effectiveness of adriamycin and bleomycin combined with local hyperthermia in neck node metastasis from head and neck cancers. Tumori 1975; 65: 481–486
- Dahl O. Mechanism of thermal enhancement of chemotherapeutic cytotoxicity. Hyperthermia and Oncology, M Urano, E Douple. VSP, Utrecht 1994; 29
- Cividalli A, Cruciani G, Livdi E, et al. Hyperthermia enhances the response of paclitaxel and radiation in a mouse adenocarcinoma. Int J Radiat Oncol Biol Phys 1999; 44: 407–412
- Brizel DM, Albers ME, Fisher SR. Hyperfractionated radiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Eng J Med 1998; 338: 1798–1804
- Green JA, Kirwan JM, Timey JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systemic review and meta analysis. Lancet 2001; 358: 781–786
- Lukka H, Hirte H, Fyles A, et al. Concurrent cisplatinum based chemotherapy plus radiotherapy for cervical cancer–A meta analysis. Clin Oncol 2009; 14: 203–212
- Franckena M, Ludy C, Lutgens PC, et al. Radiotherapy and hyperthermia for treatment of primary locally advanced cervix cancer: Results in 378 patients. Int J Radiat Oncol Biol Phys 2009; 73: 242–250
- Amichetti M, Graiff C, Fellin G, et al. Cisplatin, hyperthermia, and radiation (trimodal therapy) in patients with locally advanced head and neck tumours: A phase I-II study. Int J Radiat Oncol Biol Phys 1993; 26: 801–807
- Knsumato T, Holden SA, Ara G. Hyperthermia and platinum complexes; Time between treatments and synergy in vitro and in vivo. Int J Hyperthermia 1995; 11: 575–586
- Kohno F, Kaneshige E, Fujiwara K. Thermochemotherapy for gynaecological malignancy. Hyperthermic Oncology, J Overgaard. Taylor and Francis, London and Philadelphia 1984; 753–756
- Kaplan EL, Meir. Non parametric estimation from incomplete observation. J Am Stat Assoc 1958; 53: 457–481