Abstract
This study presents results from a new optimisation technique that reduces HIFU treatment times by minimising individual heating and interpulse cooling times while adhering to normal tissue constraint limits at each sonication position. The potential clinical usefulness of this technique is demonstrated through its implementation in three dimentsional (3D) simulations of HIFU treatments for a range of tumour geometries, normal tissue constraint values, tissue perfusion levels and focal zone scanning path trajectories, all studied as a function of the applied power magnitude. When compared to typical open loop values the optimised treatment times were lower for all conditions studied, including when treatment-limiting normal tissue thermal build-up was present. While use of this technique guarantees minimum pulse heating and interpulse cooling times for each pulse, the total treatment time gains realised depend on the individual clinical treatment configuration. In combination with a judiciously selected scan path, use of the pulse time optimisation procedure reduced treatment times in a small, superficial tumour by 85%. In addition, in all cases studied the use of an increased applied power always decreased the treatment time, including cases when significant normal tissue thermal build-up was present. Importantly, the power maximisation and pulse time minimisation procedures can be applied independently of the optimisation of the focal zone's scan path, size and shape. Given the basic nature, universal applicability and ready clinical adaptability for use in real time model predictive control, the pulse time minimisation and power maximisation approaches have significant clinical promise for reducing HIFU treatment times.
Introduction
High intensity focused ultrasound (HIFU) can treat a large number of tumours safely and effectively. However, for many cases the associated long treatment times present a major impediment to clinical acceptance. Thus an important issue facing this treatment modality involves minimising the overall treatment time Citation[1] while both delivering the necessary thermal dose at each sequential focal zone position, and preventing normal tissue overheating. This is a difficult balancing act, especially for large malignant tumours embedded in sensitive tissues, and is most important in magnetic resonance guided HIFU (MRgHIFU) where many patients’ comfort level is compromised by the MRI environment itself. HIFU treatments are usually performed by sequentially heating individual sub-regions of the tumour by either mechanical movement of a transducer or electronically steering a phased array. A serious problem with treating sizeable tumours is the thermal build-up in the intervening normal tissue that lies between the transducer and the tumour Citation[2], Citation[3]. Consequently many studies have used a fixed delay time between heating pulses to limit the normal tissue temperature increase Citation[4–6]. While that approach is effective in avoiding thermal damage in normal tissues, it can create excessively long treatment times. In this paper we concentrate on two aspects of the trade-off between tumour and normal tissue heating. Specifically, we present an approach to optimise both the individual heating pulse and interpulse cooling times for each sonication pulse, and the associated applied power magnitude. Several prior studies have investigated individual components of this trade-off, but none have provided a comprehensive optimisation approach.
Several parametric studies have investigated the effects of applying different power magnitudes Citation[7–9] for cases where normal tissue thermal build-up was not important (e.g. situations with large acoustic windows), thus avoiding the difficulties associated with the tumour and normal tissue heating trade-off. As expected, those studies came to the conclusion that increasing the applied power magnitude shortened treatment times. In extending those studies, Fan and Hynynen Citation[6] investigated treatment configurations where thermal build-up in normal tissues was significant, and found that the avoidance of normal tissue damage required the implementation of interpulse cooling intervals, thus implying the opposite conclusion, namely that higher powers could lead to longer treatments. In a later study, McDannold et al. Citation[4] heated rows of focal spots in order to determine the interpulse cooling times that would allow sufficient cooling of the normal tissue between the focal zone and the transducer. Subsequently, for reasons of patient safety, HIFU investigators Citation[5] have used similar cooling periods when normal tissue constraints were present, and many studies have used fixed heating and interpulse cooling times. However, none have studied or identified the optimal conditions that would result in minimal heating pulse and interpulse cooling times for treatments consisting of multiple pulses with active normal tissue constraints.
The complete optimisation of HIFU treatments is a large, complex problem that involves the simultaneous optimisation of many variables Citation[6], Citation[10], Citation[11], including not only the heating and interpulse cooling periods and applied power level for each pulse, but also the focal zone's scanning size, shape and trajectory Citation[3], Citation[6], Citation[7], Citation[9], Citation[17–20]. The complexity of this optimisation problem has precluded exhaustive optimisation studies that could lead to definitive conclusions. This is especially true since the optimal values of these multiple variables may be interdependent, further complicating their simultaneous optimisation. In the current paper the problem is broken into smaller parts by first investigating the optimisation of the pulse heating and interpulse cooling times, and the applied power level. The results demonstrate that these variables can indeed be optimised, and that their optimisation can be decoupled from the optimisation of all other scan variables. This decoupling will allow future studies of the optimisation of the other scan parameters to proceed more rapidly.
Methods
The overall goal of these studies is to simulate multiple clinical situations to show the general applicability of the proposed technique. Thus, the presented algorithm has been applied to different 3D tumour geometries with varied tissue perfusions, using different scanning path trajectories and normal tissue safety limits, all studied for a range of applied power levels.
Optimisation procedure
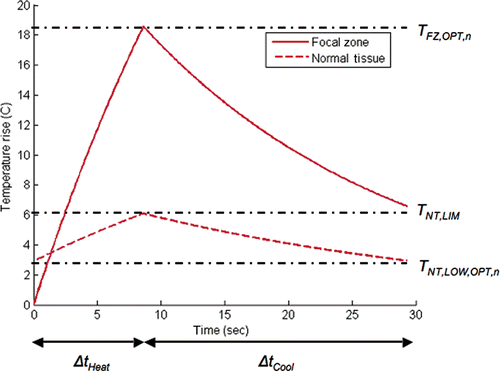
This procedure optimises the heating and interpulse cooling time of each individual ultrasound pulse in order to minimise the total treatment time (ttreat) as summed over all sequential pulses in the treatment:Here, ΔtHeat,n and ΔtCool,n are the pulse heating and interpulse cooling times for an ultrasound pulse applied at a fixed magnitude at focal zone position n. In order for a focal zone position to be optimally treated the following three conditions must be met. (1) The power must be applied for an optimal time ΔtHeat,n such that at the end of the heating period the temperature in the focal zone reaches its optimal value TFZ,OPT,n simultaneously with the normal tissue reaching its limiting value TNT,LIM (). (2) TFZ,OPT,n is determined such that the desired target dose is delivered to focal zone location n during the combined heating and interpulse cooling periods for that pulse Citation[16]. 3) After the power is turned off, the interpulse cooling period (ΔtCool,n) is just long enough so that conditions 1 and 2 can be met by the next heating pulse at focal spot position n + 1. These simple optimality conditions are independently and universally applicable to each ultrasound pulse within any given treatment configuration. Satisfying the above optimality conditions provides the shortest possible combination of pulse heating and interpulse cooling times for each pulse within any treatment configuration, thus meeting one of the necessary conditions needed to minimise the total treatment time. If the conditions are not met for every pulse, the total treatment time will be longer than optimal.
Figure 1. Optimal thermal response of a single pulse at a new focal spot location (and at the corresponding critical normal tissue location) when the normal tissue temperature is constrained. In this example the temperature in the focal spot being heated starts at body temperature, while the normal tissue temperature starts at an elevated level due to thermal build-up accumulated by the previous pulses. The normal tissue is allowed to cool to its optimal value between pulses.
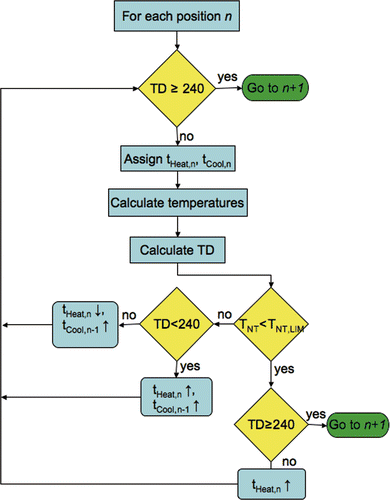
A flowchart of the HIFU treatment optimisation for a single focal spot position is shown in . The optimisation routine is iterative and uses an initial estimate of ΔtHeat of one second and ΔtCool of zero to determine the initial temperature and thermal dose distributions in the tissue region. Based on the calculated temperature and thermal dose values, ΔtHeat and ΔtCool are then adjusted until the optimal heating and interpulse cooling times are identified that deliver the desired dose at position n without violating the normal tissue constraints.
Figure 2. HIFU treatment optimisation algorithm used for calculating the heating and interpulse cooling times at a single focal spot location.
The size of the focal zone, as well as the spacing of the individual pulses and the trajectory of the scanning path determine the extent of SAR overlap among the multiple heating pulses applied throughout the course of the treatment. In this study, the optimisation algorithm accounts for prior dose accumulated during the treatment due to such overlap. For example, if a prior dose of 35 cumulative equivalent minutes (CEM) has been previously deposited at the nth treatment position by previous SAR overlap, then the dose target for the nth position is reduced to 205 CEM, and the associated TFZ,OPT,n is appropriately lowered. Future dose is unknown and is not accounted for in the current study since it will be difficult to predict clinically, and not accounting for it always results in overdosing in the tumour, thus providing a safety margin for the treatment.
Simulation geometry
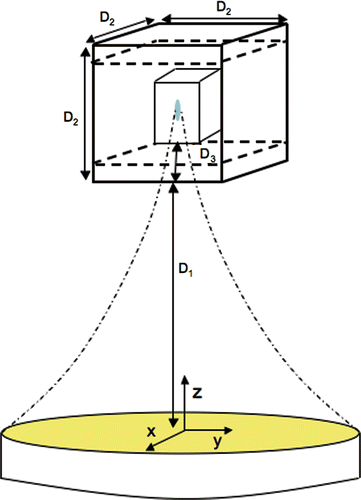
Two different tumour geometries were simulated. The first () was for a small, superficial tumour with a volume of 1 cm3 embedded in a tissue region with dimensions of 3.3 × 3.3 × 3.3 cm3. The second region () was for a larger, deeper tumour with a volume of 4.4 cm3 embedded within a tissue region of dimensions of 10 × 10 × 10 cm3. Both simulated regions were homogeneous with constant properties. An explicit finite-difference approximation of Equation 2 was implemented with a temporal resolution of 1 s and a spatial resolution of 1 × 1 × 1 mm3. This magnitude of resolution was found to be adequate in previous studies that involved various sizes of discrete blood vessels with sharp, localised temperature gradients Citation[17–20]. In addition, this optimisation algorithm was designed to be used in a MRgHIFU system, where temperature measurement resolution is larger than the present study. For example, the same model was used in a related experimental study Citation[21], with a temporal resolution of 6.2 s and 2 × 2 × 3 mm spatial resolution. The resulting model predictions showed excellent agreement with the experimental data.
Figure 3. Schematic of 3D simulation geometries. The distances for the 1 cm3 superficial tumour geometry are: D1 = 11.2 cm, D2 = 3.3 cm, D3 = 0.3 cm. The distances for the 4.4 cm3 tumour are D1 = 7.8 cm, D2 = 10 cm, D3 = 4.6 cm. The origin is located on the longitudinal axis of the transducer. Normal tissue constraint planes are shown (dashed lines), located 1 cm above and below the treated tumour volume in both cases.
Normal tissue constraint planes were specified 1 cm in front and behind the treatment volume for both simulated geometries. With the proximal constraint at this position, for the small superficial tumour there was a thin, 3-mm layer of normal tissue between the skin and the constraint plane, For the larger, deeper tumour the proximal face of the tumour region was at a depth of 4.6 cm from the skin, resulting in a much larger normal tissue region where thermal build-up can occur. Preliminary studies were performed that showed that the smallest focal zone produced by the phased array required a total of 45 sonication points to fill the small treatment volume thoroughly, while 243 points were required for the larger treatment volume. The spacing was very conservatively selected, ensuring that all points in the treatment volume were adequately treated. The focal zone‘s scanning locations were spaced 3 mm apart in all directions for the smaller tumour, and were arranged in a 3 × 5 pattern in each of three planes at different depths, with the planes located at z = 12.5, 12.8 and 13.1 cm from the transducer. The larger tumour used a 2 × 2 × 4 mm focal zone spacing arranged in a 9 × 9 × 3 pattern with the three scanning planes located at z = 12.6, 13 and 13.4 cm. For simplicity and consistency among the models, in all cases the thermal boundary condition on all six faces of the simulated tissue region was kept at 37°C, as was the initial condition.
Thermal model
The temperature responses were modelled with the 3D Pennes bio-heat transfer equation (BHTE) Citation[22]:where T is temperature (°C), ρ is the tissue density (kg/m3), Ct is the specific heat of tissue (J/kg°C), k is the thermal conductivity of the tissue (W/m°C), Qap is the applied power density deposited by an external applicator (W/m3), W is the Pennes perfusion parameter (kg/m3s), Cb is the specific heat of blood and Tb is the arterial blood temperature. Although this equation has its limitations, it has been shown to adequately predict the major thermal aspects of in vivo experimental temperatures in previous studies Citation[23–25].
Thermal dose Citation[26] characterises the time varying temperatures’ cumulative effects:where TD is the equivalent cumulative thermal dose at the reference temperature of 43°C, often given in CEM, Tt is the temperature during time Δt, and R is 0.25 for temperatures below 43°C and 0.5 for values above.
Power deposition
The power deposition pattern was simulated as being generated from an existing ultrasound phased array with 256 transducer elements arranged randomly on a spherical surface (Imasonics, Besancon, France). The transducer operates at a frequency of 1 MHz with a radius of curvature of 13 cm. The power deposition was simulated using the hybrid angular spectrum technique (HAS) Citation[27]. This technique is an extension of the traditional angular spectrum method Citation[28], Citation[29] that can simulate ultrasound beam propagation in both homogeneous and inhomogeneous tissue regions. It is a spatial-frequency domain technique Citation[30] in which the spatial pattern of the acoustic pressure is encoded into a spectrum of plane waves, each travelling at a unique angle. After accounting for propagation with a linear filter in the frequency domain, a fast Fourier transform algorithm gives the predicted focused pressure pattern in the space domain.
Due to tissue inhomogeneities, scattering, and hardware imperfections, previous investigators Citation[31] have empirically found that the experimentally determined focal zone diameter is approximately twice as large as that predicted by simulations for uniform tissue properties. Therefore, an adjustment factor was used to increase the simulated focal zone in the x-y plane by a factor of two, producing a half-intensity focal zone diameter and length of 2 mm and 10 mm, respectively, emulating the sizes that we have seen when using this phased array to experimentally heat homogeneous agar phantoms. The resulting power deposition pattern, a distribution that is a reasonable approximation to the actual distributions to be expected for the treatment configurations that will utilise this transducer, is shown in . This power deposition pattern was electronically steered in the z-direction to obtain the SAR distributions for each of the three x-y treatment planes. These three power deposition patterns were translated at their fixed depths to obtain the SAR distribution for each of the sonication positions throughout the treatment volumes. In all results presented in this study, the power density value reported is the peak focal zone power density value. The peak power density values used in this paper are below the conservative mechanical index limits set by the FDA for diagnostic ultrasound transducers Citation[32]. The values for the homogeneous and constant thermal and acoustic properties used Citation[33], Citation[34] are given in .
Figure 4. Simulated power density (W/m3) contours for the 1-cm3 tumour volume. Shown are the contours in a y-z plane through the transducer's longitudinal axis when the transducer's geometric focus is located in the middle treatment plane. FWHM dimensions are 2 mm × 10 mm.
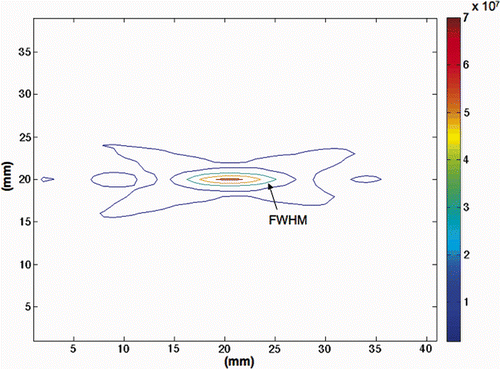
Table I. Thermal and acoustic parameters used in the simulations.
Scanning path trajectory
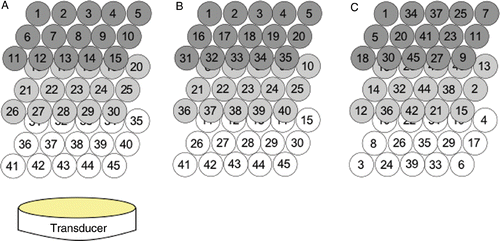
It has been shown in past studies Citation[6], Citation[11] that treatment time has a dependence on the path followed by the focal zone. Therefore, three different paths were simulated to illustrate the applicability of the pulse time optimisation approach to different scan path choices. The first two paths have been used by other investigators Citation[35], Citation[36]: a back-middle-front plane raster scan pattern that followed raster scans in the three successive x-y planes at different z-direction depths; and a scan pattern that followed raster scans in three successive y-z planes. The third pattern (the alternating path) attempted to minimise normal tissue heating due to normal tissue SAR overlap among sequential pulses. This was done by keeping a large distance between sequential treatment locations by both alternating between focal planes, and among positions within those planes. The alternating pattern used was found empirically as one that gave a significant reduction in total treatment time when compared to the two raster scans. shows all three scanning paths for the 1 cm3 tumour volume. The larger tumour volume followed similar patterns. The spacing between adjacent focal zone locations was very conservatively selected to ensure that the entire tumour volume was treated to the target thermal dose value.
Figure 5. Scanning sequences used in the 1-cm3 superficial tumour simulations. Each sequence heated 45 focal spots in a 3 × 5 × 3 pattern: (A) x-y raster pattern, (B) y-z raster pattern, (C) alternating pattern with reduced normal tissue SAR overlap. The 4.4-cm3 tumour used similar patterns with 243 points.
Results
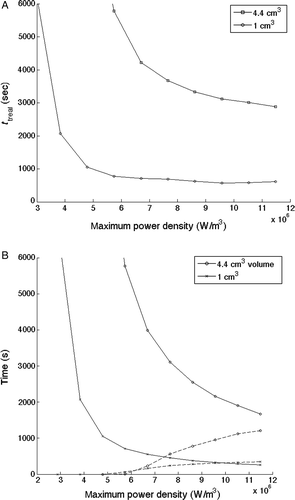
The effect of power magnitude on total treatment time is shown in . Treatment time is seen to decrease as applied power density increases for both the 1 cm3 and 4.4 cm3 tumour geometries for a perfusion value of 5 kg/m3 s while using the alternating scanning path trajectory and a normal tissue constraint value of 6°C above the basal temperature (37°C). The associated cumulative heating and cooling times are seen in . As the applied power density increases the heating time decreases, while the cooling time increases due to the activation of the normal tissue constraint (TNT,LIM).
Figure 6. (A) Optimised total treatment times (ttreat) for both tumour sizes as a function of the applied power magnitude for a tissue perfusion value of 5 kg/m3s, an alternating scanning path, and a normal tissue constraint limit of 6°C above basal temperature. (B) Corresponding cumulative heating time (solid lines) and cooling time (dashed lines) for the two tumour sizes.
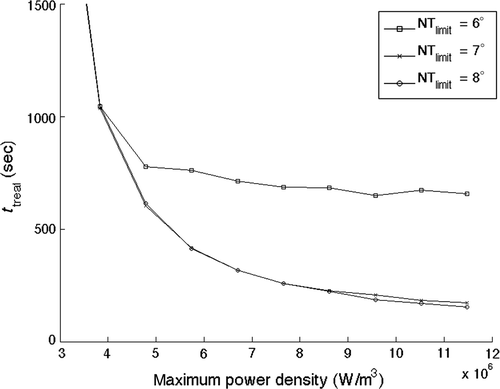
The effects of increasing the applied power on the total treatment time when different normal tissue constraints are present are shown in for the alternating path treating the 1-cm3 tumour. The 7° and 8°C normal tissue constraint limits show a smooth, monotonically decreasing total treatment time as the applied power level increases. The 6°C limit case results follow that same curve until the normal tissue constraints are activated at the ∼5 × 106 W/m3 applied power (as seen by the finite values of the cooling time that arise at that power level in ). At that point, the 6°C case results diverge, but still show a monotonically decreasing total treatment time with increasing applied power, although the treatment time decreases at a much lower rate than do the 7° and 8°C curves. Thus the results are seen to be quite sensitive to the constraint values. At the largest applied power density studied, the total treatment time is reduced by 75% when the temperature constraint is raised from 6° to 8°C. This large reduction is due to the complete elimination of interpulse cooling for the 8°C normal tissue constraint limit.
Figure 7. Optimised treatment time results for normal tissue temperature constraint limits of 6°C, 7°C and 8°C above the basal value versus total applied power for the 1-cm3 superficial tumour, a perfusion value of 0.5 kg/m3s, and the alternating scanning path trajectory.
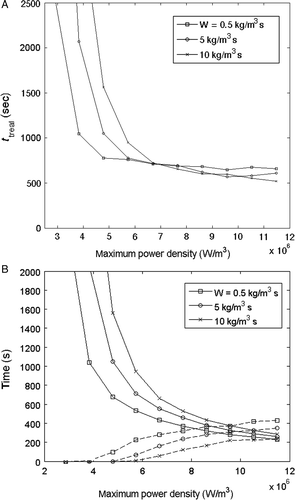
To further demonstrate the applicability of this time optimisation technique to additional treatment configurations, simulations were run to determine the effects of perfusion at values of 0.5, 5 and 10 kg/m3 s for the alternating path. The results, seen in , show the same trend of decreasing treatment time with increasing power density at all three perfusion rates. Again, the time gains associated with increasing power are very large before the normal tissue constraints are activated, and much less after that point. In addition, higher perfusions require longer heating times and shorter interpulse cooling intervals, and such increased perfusions also shift the need for interpulse cooling towards higher applied power values.
Figure 8. Comparison of optimised total treatment time for perfusion rates of 0.5, 5 and 10 kg/m3s for the alternating path in the 1-cm3 tumour geometry: (A) total treatment time, (B) cumulative heating time (solid lines) and cooling time (dashed lines).
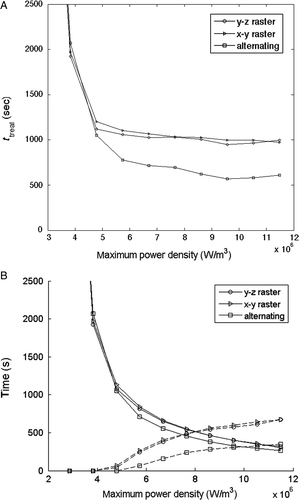
Finally, to illustrate its applicability to treatment configurations involving different scan paths, shows the optimised total treatment time as a function of the applied power level at a perfusion level of 5 kg/m3 s for the three different scan paths with the 1 cm3 tumour geometry. The associated cumulative heating and cooling times are shown in . Again, a monotonically decreasing optimised treatment time occurs with increasing applied power density for all paths. Also shown is the strong dependence of total treatment time on path, with the alternating path having an approximately 40% reduction in ttreat when compared to both raster paths. This decrease arises primarily from the reduced need for interpulse cooling times due to the reduction in thermal build-up since there is less normal tissue SAR overlap for this scan path.
Figure 9. (A) Optimised total treatment time and (B) cumulative heating time (solid lines) and cooling time (dashed lines) versus focal zone power density for optimised HIFU treatments with a perfusion value of 5 kg/m3s for the three scanning paths in applied to the 1-cm3 tumour geometry.
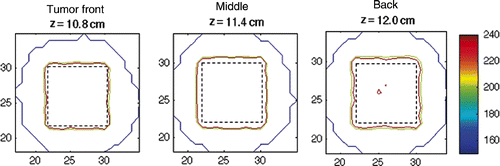
A typical dose distribution, representative of all simulation results in this study, is shown in . The 2D thermal dose profiles at varying distances from the transducer face, and within the tumour volume, are displayed. All points enclosed within the tumour boundaries were treated to a minimum of 240 CEM.
Discussion
Heating and interpulse cooling time minimisation
The optimised total treatment times presented represent the shortest possible heating pulse times and interpulse cooling times that could be obtained for each treatment configuration studied. Any open loop protocols with the same treatment configuration, but using fixed heating and cooling times to avoid normal tissue constraint violations, would be longer than optimal. For example, if a typical open loop heating time of 15 seconds and cooling time of 80 seconds Citation[37] were used for the presented 45-pulse treatment, the total open loop treatment time would be approximately 71 minutes for the 1 cm3 treatment volume. By comparison, for a power just into the flat region of (∼5 × 106 W/m3), the optimised results would require a 17 min treatment if either of the two commonly used raster scan trajectories were followed, a reduction to only 25% of the open loop value. Furthermore, when the new optimisation approach is combined with the alternating path trajectory (which takes into account normal tissue heating due to SAR overlap), the treatment time is reduced to only 10 minutes, or 15% of the open loop time. Clinically, the exact amount of time gained by the use of the pulse time optimisation approach will depend on the individual treatment configuration.
While the ablation rates reported in this study for the optimised treatment with the alternating scan path at high power levels are much larger than the typical open loop values as described previously, they are generally lower than those found elsewhere in the literature, where most studies have been performed with no active, normal tissue constraints Citation[7], Citation[14], Citation[46–48]. This difference is to be expected since, to the authors’ knowledge, the present study is the first that has an optimisation routine that calculates the heating and cooling times for each sonication pulse based on the avoidance of constraint violations for the normal tissues.
The normal tissue constraint location and magnitude used in this work (43°C at 1 cm away from the tumour edge) is conservative, and much more stringent than a normal tissue dose limitation Citation[13] since dose limits can allow many small high-temperature fluctuations to occur, thus significantly shortening the treatment time. Indeed, the maximum thermal dose accumulated at the normal tissue constraint for both the 7° and 8°C limit was less than 1 CEM, indicating if a thermal dose threshold were used that the treatment time gains could be increased even further than those presented in the current study.
Power maximisation to reduce treatment time
In addition to the treatment time, gains obtained from the time optimisation procedure, the current results show that increasing the applied power level for all treatment configurations studied always reduces the total treatment time. The associated time gains per unit of applied power are large at lower power levels when the normal tissue constraints are not activated, and become significantly smaller at larger powers. When an increased power is used, the reduction in heating time is larger than the subsequent time lost due to the need for additional normal tissue cooling time, a result previously not shown. These results thus complement and extend previous studies that showed how higher powers reduce treatment times when no normal tissue constraints are active. Therefore, in terms of minimising total treatment time, when single pulses are used to treat each focal zone position, using higher applied power levels is always better, and any treatment strategy that uses lower applied powers to obtain some other treatment gain will be less than optimal in terms of total treatment time.
Effects of tissue properties
As seen in , the treatment time reductions attained using this optimisation algorithm hold for all simulated perfusion values. This study used a simple, homogeneous, uniform perfusion, while in reality the perfusion rates will differ between the tumour and surrounding normal tissue. In terms of ttreat, a worst-case scenario would have a high perfusion rate in the tumour, and a lower perfusion rate in the normal tissues. However, the literature reports that the opposite is likely true. Perfusion in the tumour decreases due to the destruction of microvasculature Citation[43], while the perfusion rate in normal tissues has been found to increase between 5- and 10-fold at temperatures of 42°–44°C Citation[44], Citation[45]. Such differences in perfusion could be used to deliver the dose more quickly and efficiently when taken into account by an on-line implementation of the pulse time optimisation procedure.
The literature also shows that the tissue attenuation coefficient increases in ablated tissue Citation[46–48]. While all the above authors conclude that the attenuation magnitude changes with tissue heating, the magnitude of that change and the timing of its occurrence following each heating pulse are uncertain and vary depending on the treatment conditions (e.g., transducer frequency and maximum temperature). For this study, operating at a transducer frequency of 1 MHz and achieving a maximum peak temperature of 57°C, the attenuation changes would be less than ∼25%. While any attenuation increase would have some effect on the optimal heating and interpulse cooling times for each treatment configuration, it would not change the basic conclusions of the present study.
Further improvements
Since future dose delivery due to SAR overlap was not included in this study, significant overdosing occurred in the tumour tissue. From the treatment efficacy viewpoint, overdosing in the treatment volume region is not a problem, and indeed can be seen as a benefit. However, when viewed from the treatment time viewpoint, overdosing is a sign of inefficiency, because of the additional heating time used and the related interpulse cooling time required. While the problem of predicting the amount of future thermal dose is a difficult one, the results presented in the current study show that a significant amount of overdosing is present for all three paths studied. Thus, eliminating overdosing has the potential to offer additional time savings and make treatments even more clinically practical.
The current results indicate that the use of optimised heating and cooling times, and larger applied power values can result in significant treatment time gains for all treatment configurations. In order to implement those optimisation approaches in a clinically practical manner, one must be able to both measure or estimate the values of the temperatures in the tumour and intervening normal tissues online, and to then rapidly find the optimal values of the cooling and heating times for each heating pulse. Blankespoor et al. Citation[49] have begun developing and implementing a real time, on-line MRgHIFU version of this approach. The initial prototype's experimental evaluation Citation[50] demonstrated that the large theoretical time gains predicted by the current simulations can be practically realised in real-time.
Conclusions
A new procedure has been developed and shown through simulations to significantly reduce HIFU treatment time through optimising individual pulse heating and cooling times. The results also show that increasing the applied power level monotonically decreases total treatment time for all presented scenarios, even when significant, treatment limiting normal tissue heating is present, and that the concommitant use of a judicicous choice of scan path can also result in significant additional time gains. Treatment time reductions of up to 85% are shown when compared to treatments that use typical fixed heating and cooling times. Use of the optimal heating and interpulse cooling time procedure, and maximising the applied power level for each heating pulse, provide two necessary, but not sufficient, conditions needed to minimise the total treatment time. These two treatment time reduction procedures are universally applicable to all treatment configurations and are decoupled from the steps involved in optimising the other scan parameters. Further work is needed to develop comprehensive approaches to optimising HIFU treatments’ other treatment configuration parameters, including the size and shape of the focal zone, as well as the trajectory.
Acknowledgements
This work was partially supported by NIH grant NIH-1-R01-CA134599, Siemens Medical Solutions, the Focused Ultrasound Foundation, a University of Utah Synergy Grant and the Ben B. and Iris M. Margolis Foundation. We appreciate the help of Dennis Parker and Joshua Coon on the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, Middleton MR. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a western population. Br J Cancer 2005; 93: 890–895
- Moros EG, Roemer RB, Hynynen K. Pre-focal plane high-temperature regions induced by scanning focused ultrasound beams. Int J Hyperthermia 1990; 6: 351–366
- McDannold K, Hynynen D, Wolf G, Jolesz F. MRI evaluation of thermal ablation of tumors with focused ultrasound. J Magn Reson Imaging 1998; 8: 91–100
- McDannold N, Jolesz FA, Hynynen K. Determination of the optimal delay between sonifications during focused ultrasound surgery in rabbits by using MR imaging to monitor thermal build up in vivo. Radiology 1999; 211: 419–426
- Damianou C, Hynynen K. Focal spacing and near-field heating during pulsed high temperature ultrasound therapy. Ultrasound Med Biol 1993; 19: 777–787
- Fan X, Hynynen K. Ultrasound surgery using multiple sonications–treatment time considerations. Ultrasound Med Biol 1996; 22: 471–482
- Lin WL, Liang TC, Yen JY, Liu HL, Chen YY. Optimization of power deposition and a heating strategy for external ultrasound thermal therapy. Med Phys 2001; 28: 2172–2181
- Liu H-L, Chen Y-Y, Yen J-Y, Lin W-L. Treatment time reduction for large thermal lesions by using a multiple 1D ultrasound phased array system. Phys Med Biol 2003; 48: 1173–1190
- Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am 1994; 95: 1641–1649
- Roemer R, Payne AH. Minimization of HIFU dose delivery time. Int Soc Therapeutic Ultrasound, Korea 2007; 1
- Malinen M, Huttunen T, Kaipio JP, Hynynen K. Scanning path optimization for ultrasound surgery. Phys Med Biol 2005; 50: 3473–3490
- Billard BE, Hynynen K, Roemer RB. Effects of physical parameters on high temperature ultrasound hyperthermia. Ultrasound Med Biol 1990; 16: 409–420
- Wu X, Sherar M. Theoretical evaluation of moderately focused spherical transducers and multi-focus acoustic lens/transducer systems for ultrasound thermal therapy. Phys Med Biol 2002; 47: 1603–1621
- Daum DR, Hynynen K. A 256-element ultrasonic phased array system for the treatment of large volumes of deep seated tissue. IEEE Trans Ultrason Ferroelec Freq Control 1999; 46: 1254–1268
- Wan H, Aarsvold J, O'Donnell M, Cain CA. Ultrasound surgery: Comparison of strategies using phased array systems. IEEE Trans Ultrason 1996; 43: 1085–1097
- Cheng KS, Roemer RB. Closed-form solution for the thermal dose delivered during single pulse thermal therapies. Int J Hyperthermia 2005; 21: 215–230
- Kotte A, van Leeuwen G, de Bree J, et al. A description of discrete vessel segments in thermal modelling of tissues. Phys Med Biol 1996; 41: 865–884
- Kotte AN, van Leeuwen GM, Lagendijk JJ. Modelling the thermal impact of a discrete vessel tree. Phys Med Biol 1999; 44: 57–74
- van Leeuwen G, Hand JW, Lagendijk JJW, Azzopardi D, Edwards A. Numerical modeling of temperature distributions within the neonatal head. Pediatric Res 2000; 48: 351–356
- van Leeuwen G, Kotte A, Raaymakers B, Lagendijk JJW. Temperature simulations in tissue with a realistic computer generated vessel network. Phys Med Biol 2000; 45: 1035–1049
- Todd N, Payne A, Parker D. 3-D MR temperature imaging with model predictive filtering reconstruction. Proceedings of the 17th Annual ISMRM Scientific Meeting and Exhibition. Curran Associates, Honolulu, Hawaii 2009; 445
- Pennes H. Analysis of tissue and arterial blood temperatures in the resting human forearm. Appl Physiol 1948; 1: 93–122
- Moros EG, Dutton AW, Roemer RB, Burton M, Hynynen K. Experimental evaluation of two simple thermal models using hyperthermia in muscle in vivo. Int J Hyperthermia 1993; 9: 581–598
- Rawnsley R, Roemer RB, Dutton AW. The simulation of discrete vessel effects in experimental hyperthermia. ASME J Biomech Eng 1994; 116: 256–262
- Roemer R. Thermal dosimetry and treatment planning. Thermal Dosimetry, M Gautherie. Springer, Berlin 1990; 119–214
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Vyas U, Christensen DA. Hybrid angular spectrum method for ultrasound beam propagation. Paper presented at the Third Annual Mountain West Biomedical Engineering Conference. The Canyons, Utah September, 2007
- Christopher PT, Parker KJ. New approaches to the linear propagation of acoustic fields. J Acoust Soc Amer 1991; 90: 507–521
- Ratcliffe JA. Some aspects of diffraction theory and their application to the ionosphere. Rep Prog Phys 1956; 19: 188–267
- Goodman JW. Introduction to Fourier Optics, third edn. Roberts. 2004
- Mahoney K, Fjield T, McDannold N, Clement GT, Hynynen K. Comparison of modelled and observed in vivo temperature elevations induced by focused ultrasound: Implications for treatment planning. Phys Med Biol 2001; 46: 1785–1798
- Food and Drug Administration (FDA). Information for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers, 2009. Co: Roberts and Company Publishers; Accessed 1 October 2009 from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm089001.htm
- Chato JC, Lee RC. The future of biothermal engineering. Heat and Mass Transfer in Living Systems., RK Diller. New York Academy of Sciences, New York 1998; 1–20
- Christensen DA. Ultrasonic Bioinstrumentation. John Wiley, NJ 1988
- Wu F, Chen WZ, Bai J, et al. Pathological changes in human malignant carcinoma treated with high‐intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1099–1106
- McDannold N, Tempany CM, Fennessy FM, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology 2006; 240: 263–272
- Gorny KR, Hangiandreou NJ, Hesley GK, et al. MR guided focused ultrasound: Technical acceptance measures for a clinical system. Phys Med Biol 2006; 51: 3155–3173
- Wu F, Wang ZB, Chen WZ, J, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med Biol 2004; 30: 245–260
- Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: Initial experience. Radiology 2005; 236: 1034–1040
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005; 235: 659–667
- Mougenot C, Salomir R, Palussiere J, Grenier N, Moonen CT. Automatic spatial and temporal temperature control for MR-guided focused ultrasound using fast 3D MR thermometry and multispiral trajectory of the focal point. Magn Reson Med 2004; 52: 1005–1015
- Palussiere J, Salomir R, Le Bail B, et al. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magn Reson Med 2003; 49: 89–98
- Yang R, Reilly CR, Rescorla FJ, et al. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg 1991; 126: 1002–1009, discussion 1009–1010
- Song CW, Rhee JG, Levitt SH. Blood flow in normal tissues and tumors during hyperthermia. J Nat Cancer Inst 1980; 64: 119–124
- Dudar TE, Jain RK. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res 1984; 44: 605–612
- Kolios MC, Sherar MD, Hunt JW. Temperature dependent tissue properties and ultrasound lesion. Adv Heat Mass Transfer Biotech 1999; 44: 113–118
- Worthington AE, Sherar MD. Changes in ultrasound properties of porcine kidney tissue during heating. Ultrasound Med Biol 2001; 27: 673–682
- Damianou CA, Sanghvi NT, Fry FJ. Maass-Moreno R. Dependence of ultrasonic attenuation and absorption in dog soft tissues on temperature and thermal dose. J Acoust Soc Am 1997; 102: 628–634
- Blankespoor A, Payne AH, Moellmer J, et al. Model predictive control of phased array HIFU treatments. Int Soc Therapeutic Ultrasound, Korea 2007; 1
- Blankespoor A, Payne A, Todd N, et al. Model predictive control of HIFU treatments in 3D for treatment time reduction. 8th International Symposium on Therapeutic Ultrasound. AIP, Ultrasound Minneapolis, MN 2008; 215–219