Abstract
Purpose: The prognosis for locally advanced breast cancer (LABC) patients continues to be poor, with an estimated five-year survival of only 50–60%. Preclinical data demonstrates enhanced therapeutic efficacy with liposomal encapsulation of doxorubicin combined with hyperthermia (HT). Therefore this phase I/II study was designed to evaluate the safety and efficacy of a novel neoadjuvant combination treatment of paclitaxel, liposomal doxorubicin, and hyperthermia.
Materials and methods: Eligible patients received four cycles of neoadjuvant liposomal doxorubicin (30–75 mg/m2), paclitaxel (100–175 mg/m2), and hyperthermia. They subsequently underwent either a modified radical mastectomy or lumpectomy with axillary node dissection followed by radiation therapy and then eight cycles of CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy.
Results: Forty-seven patients with stage IIB-III LABC were enrolled and 43 patients were evaluable. Fourteen patients (33%) had inflammatory breast cancer. Combined (partial + complete) clinical response rate was 72% and combined pathological response rate was 60%. Four patients achieved a pathologically complete response. Sixteen patients were eligible for breast-conserving surgery. The cumulative equivalent minutes (CEM 43) at T90 (tenth percentile of temperature distribution) was significantly greater for those with a pathological response. Four-year disease-free survival was 63% (95% CI, 46%–76%) and the four-year overall survival was 75% (95% CI, 58–86%).
Conclusions: Neoadjuvant therapy using paclitaxel, liposomal doxorubicin and hyperthermia is a feasible and well tolerated treatment strategy in patients with LABC. The thermal dose parameter CEM 43 T90 was significantly correlated with attaining a pathological response.
Introduction
Locally advanced breast cancer accounts for approximately 5–10% of breast cancers diagnosed in the USA. Unlike early breast cancer, which has a five-year overall survival of about 80–90%, locally advanced breast cancer carries a relatively poor prognosis with a five-year overall survival of about 55% Citation[1–3]. Inflammatory breast cancer (IBC) carries a worse prognosis, with median overall survival from time of diagnosis of 2.5 years Citation[4] and a five-year survival rate of 15% Citation[5]. In the early 1980s, pre-operative (neoadjuvant) chemotherapy was introduced in an attempt to improve outcomes in this subgroup of patients. The proposed advantages of pre-operative therapy over conventional (post-operative) therapy include down-staging of the tumour (allowing a more conservative surgical procedure or even changing an inoperable tumour to one that could be resected) assessment of tumour response to the chemotherapeutic agents used, and early introduction of systemic therapy, given the high risk of occult micro-metastatic disease.
However, many questions remain about the optimal pre-operative treatment regimen. With the development of newer agents and the advent of targeted therapies, the optimal strategy for treatment of LABC remains to be elucidated. Hyperthermia has a number of tumour effects with therapeutic potential when combined with chemotherapy. When tumours are heated to temperatures greater than 41°C there is increased blood flow, increased drug delivery and direct tumour cell toxicity Citation[6]. The rationale behind using a combination of liposomes and hyperthermia to improve drug delivery to tumours is several-fold. Hyperthermia has been shown to increase extravasation of liposomes out of the tumour microvasculature and to increase overall drug accumulation in tumours Citation[7–10]. In addition, pre-clinical data has indicated that several cancer chemotherapeutic agents (including doxorubicin) in combination with hyperthermia have supra-additive cytotoxic effects Citation[11–13]. The therapeutic benefits from liposomes and hyperthermia individually, coupled with the potential advantages seen by their combination, make the use of the two modalities together an attractive method for drug delivery to tumours.
Thus, we undertook a phase I/II study to evaluate the safety/tolerability and determine the response of a novel combination treatment of paclitaxel, liposomal doxorubicin (Evacet™), and local hyperthermia in patients with locally advanced breast cancer in the pre-operative setting.
Materials and methods
Study design
This was a pilot phase I/II, open-label study of liposomal doxorubicin (Evacet™) and paclitaxel in combination with local breast hyperthermia for the neoadjuvant treatment of locally advanced breast cancer. Protocol-eligible patients were treated with the combination of Evacet™, paclitaxel and hyperthermia every three weeks for four cycles. After the neoadjuvant therapy, patients received appropriate surgical removal (as reassessed after neoadjuvant treatment) of their primary breast tumour as well as axillary lymph node dissection. Immediately after surgery, patients underwent radiation therapy followed by eight cycles of standard dose CMF therapy. If eligible (ER+ and/or PR+), patients received tamoxifen therapy for a total of five years ().
Table I. Pathological response by patient characteristics.
Eligibility criteria
Patients with histologically confirmed adenocarcinoma of the breast with clinically estimated primary tumour size >2 cm and with clinical stage IIB (T2N1M0, T3N0M0), IIIA(T0N2M0, T1N2M0, T2N2M0, T3N1M0, T3N2M0), or IIIB (T4Any NM0) were included. Patients were required to be ≥18 years old with ECOG performance status of 0–1 and could not have had prior chemotherapy or radiotherapy. They were also required to have good cardiac function as documented by a left ventricular ejection fraction of >50% by MUGA or gated SPECT perfusion imaging. Patients were excluded if they had multifocal primary tumours, distant metastatic disease, or prior or concomitant malignancy. Patients with serious medical illness including, but not limited to, congestive heart failure, myocardial infarction or cerebral vascular accident within the last six months, life threatening cardiac arrhythmias, acute or chronic liver disease, or major surgery within the past three months were also excluded. Informed consent was obtained for all administered treatments as approved by the Institutional Review Board at Duke University.
Outcome assessment
The objectives of the study were to describe the safety and tolerability and examine the response rates of concurrent paclitaxel (Taxol), liposomal doxorubicin, and hyperthermia treatment of locally advanced breast cancer. The primary endpoint of this study was pathological response rate. Secondary endpoints included (1) the rate of breast conservation therapy (BCT) following neoadjuvant therapy, (2) clinical response rate, (3) correlative tumour physiology studies (extracellular pH, pO2, perfusion/vascularity (MRI-RPI), and tumour intracellular pH (MRS) and (4) disease-free and overall survival.
Clinical response to therapy was defined by physical exam and/or radiological studies. Complete, partial, and no clinical responses were defined as no evidence of tumour, reduction of tumour size by ≥50%, and no change in tumour size, respectively. Pathological response to therapy was defined by pathological evaluation of the surgical specimen. Complete, partial, and no pathological responses were defined as no evidence of invasive carcinoma, reduction in tumour size by ≥50% of the initial size estimate based on radiological studies, and no reduction in tumour size from initial size estimates based on radiological studies, respectively.
Neoadjuvant chemotherapy
The dose limiting toxicities for Evacet and paclitaxel as single agent therapies have been reported previously Citation[14], Citation[15]. For paclitaxel as a single agent, the maximally tolerated dose is 210 mg/m2 without growth factor support when given as a three-hour infusion. The main dose-limiting toxicity is prolonged neutropenia. For Evacet as a single agent, the maximum reported tolerated dose in previously treated patients is 150 mg/m2, and the dose-limiting toxicity is myelosuppression. Based on the single agent data and expected increase in toxicity when the agents are combined, doses of paclitaxel and Evacet were alternately increased until the maximum tolerated dose was reached at concentrations of 100–175 mg/m2 and 30–75 mg/m2, respectively. Criteria for dose limiting toxicity and dose modification were defined prior to the initiation of the study.
All patients were pre-medicated with dexamethasone, diphenhydramine, cimetidine, and ondansetron. Paclitaxel (100–175 mg/m2) and liposomal doxorubicin (Evacet) were given every 21 days for four cycles. After the maximum tolerated dose (MTD) for the combination of paclitaxel and liposomal doxorubicin was determined in the phase I portion of the trial, predetermined dose adjustments were only made for haematological toxicities.
Hyperthermia
Hyperthermia was started within one hour of completing the Evacet infusion. The overall hyperthermia dose goal was to reach 41–41.5°C in greater than 90% of measured points for 60 min duration. Patients are heated prone in the Duke Breast Applicator System (DBAS), with the involved breast hanging in a water-filled cup that provides electromagnetic coupling and surface temperature control. The four channels of DBAS can be independently adjusted for phase and amplitude during the treatment to achieve desired steering and to optimise patient comfort. Operating frequencies range between 140 MHz and 156 MHz, depending on the cup used and the breast size, with power levels from 60 to 200 W. Due to the variation in the breast volume (from 200 cc to 700 cc), different cup sizes were used. Although minimum sampled temperatures give a crude indication of the temperature distribution, the CEM43T90, CEM43T50, the T90 and T50 were determined for each of the HT fractions from tumour points temperatures Citation[16]. Thermometry was performed following RTOG guidelines; however, the majority of patients had one catheter placed interstitially to map temperature every 0.5 cm Citation[17]. Patients were premedicated with lorazepam and/or narcotic pain medication, and local anaesthesia with Lidocaine HCl (1% solution buffered with 0.1 mEq sodium bicarbonate/mL lidocaine) was used for the placement of sterile, blind-ended interstitial thermometry catheters. CT scan was used to verify the appropriate placement of the catheters. To monitor normal tissue and surface temperatures, skin surface probes were placed on the adjacent skin and on any scars near the hyperthermia field. The maximum allowable temperatures in adjacent normal tissues and tumour were 43°C and 48°C, respectively. During treatment, applied power was adjusted as needed to reach the targeted temperatures while maintaining patient comfort.
Thermal dose calculation
The formulation for thermal dose used in this study has been used extensively and has been previously described Citation[18–23]. Briefly, using the Arrhenius relationship, all time–temperature data are converted to an equivalent number of minutes at 43°C, where CEM 43°C is cumulative equivalent minutes at 43°C (the temperature most commonly used for normalisation), t is time of treatment, T is average temperature during intervals of heating, and R is a constant derived from in vitro studies Citation[18]. When the temperature is higher than 43°C, r = 0.5, and when the temperature is lower than 43°C, r = 0.25. The sum of CEM 43°C at temperatures exceeding the temperatures at 90% and 50% of the measured locations over the entire treatment duration were calculated. The ranges for the average over four treatments of these thermal metrics are from 1.5 to 159.3 (average of 11.5 min) for CEM43T90 and from 37.7 to 41.8 (average of 39.7°C) for T90.
Surgery
After the completion of neoadjuvant treatment, patients were reassessed by physical exam and radiological evaluation for the appropriate surgical intervention. The final surgical therapy (mastectomy or breast conservation therapy) was selected by the patient in conjunction with the recommendations of the surgeon.
Post-operative radiation therapy
All patients received post-surgical radiation therapy immediately following recovery. Patients were simulated in the treatment position in a customised foam mould. A computed tomography (CT) scan taken in the treatment position was used to determine the appropriate fields. Tangential fields comprehensively encompassed all breast tissue or the chest wall. Internal mammary lymph nodes (IMN) were included as part of the tangential fields or as a separate, matched field. The supraclavicular nodes were also treated using a separate field. The axillary nodes were generally not included in the supraclavicular field given that the patient underwent axillary dissection at the time of surgery.
For patients who underwent mastectomy, radiation was given at 2 Gy per day to a total of 50 Gy to the chest wall and internal mammary nodes with a scar boost of 10 Gy. If the patient underwent breast conserving surgery, radiation was given to a total dose of 46 Gy to the whole breast and internal mammary nodes with a tumour bed boost of 16 Gy, with the same dose fractionation scheme. The supraclavicular nodes received 46 Gy.
Adjuvant chemotherapy
If residual invasive disease was detected in the surgical specimen, the patient also received standard dose CMF chemotherapy every 21 days for a total of eight cycles. Chemotherapy began four weeks after the completion of radiation treatment. The doses of cyclophosphamide, methotrexate, and fluorouracil were 600 mg/m2, 40 mg/m2, and 600 mg/m2, respectively. Patients with a complete pathological response did not receive any subsequent chemotherapy. Patients with oestrogen receptor and/or progesterone receptor positive breast tumours received tamoxifen for a total of five years or until disease recurrence.
Statistical analysis
The purpose of this phase I/II study is to find a safe and tolerable dose of Evacet combined with paclitaxel and hyperthermia, and once defined, to examine the response rates of the treatment in LABC. The primary endpoints are response rate (CR + PR). Secondary endpoints include overall survival, disease-free survival and treatment-related toxicities. Point estimates for response rates, overall survival, disease-free and the related 95% CIs are reported. T-tests are used to compare the mean of two groups for hyperthermia parameters. Fisher's exact tests are used to compare the two groups for binary endpoints. Log-rank tests were used to compare the two groups for time-to-event endpoints. Kaplan-Meier time-to-event curves are presented.
Results
Between 2001 and 2003, a total of 47 patients were enrolled onto this study (21 and 26 patients in phase I and II, respectively). Patient characteristics for the 43 evaluable patients are listed in . The median age was 49 years (range 27–75), and enrolled patients were predominantly Caucasian (75%). Menopausal status was nearly equivalent in both phases of the study (44% pre-menopausal, 56% post-menopausal). At enrolment patients had a median tumour size of 6 cm (range 3–12 cm; n = 38) on MRI or ultrasound (US), and 33 (77%) had axillary lymph node involvement. Fourteen patients (33%) had inflammatory breast cancer diagnosed clinically and confirmed pathologically. Of the patients, 86% were ER and/or PR positive, and 26% were found to over-express HER2-neu. Including those with IBC, 40% of patients were considered inoperable, and only five (12%) patients were deemed eligible for BCT prior to initiating neoadjuvant therapy. The baseline median cardiac LVEF was 58% (range: 50–80%).
In phase I, the MTD was determined to be 175 mg/m2 for paclitaxel and 75 mg/m2 for Evacet. While 44 of 47 patients (94%) received all four cycles in either the phase I or phase II component, only 43 of the 44 also completed the entire course of hyperthermia and thus were evaluable for response. Upon completing neoadjuvant chemotherapy/hyperthermia, the median tumour size on MRI or US was 3 cm (range: 0–8 cm). Clinically, the combined response rate was 72% (95% CI, 58.6% to 85.4%; CR 28%; PR 44%). The median cardiac LVEF of 59% (range: 38–75%) was essentially unchanged after chemotherapy.
The most commonly reported toxicities during neoadjuvant treatment are listed in . Common toxicities included grade 2 alopecia (93% of patients), grade 4 neutropenia (66%), grade 1 fatigue (68%), grade 1 nausea (51%), and grade 1 sensory neuropathy (46%). Other reported adverse effects were grade 1 constipation (34%), grade 2 stomatitis (32%), and grade 2 arthralgia (29%). Side effects specifically related to hyperthermia were relatively uncommon, with only four (9%) patients experiencing a thermal burn (skin blisters and fat necrosis) one patient of whom developed a third degree burn.
Table II. Acute adverse events.
Nineteen of 44 patients were deemed inoperable at the initial assessment. Fourteen of these patients had inflammatory disease. Only five patients were candidates for breast conserving surgery. Eight patients elected to have BCS although 16 were eligible after reassessment following neoadjuvant treatment. At surgery, 32 patients (73%) were found to have axillary lymph node involvement. Using standard pathological procedures, the breast specimens were examined for residual tumour. A complete pathological response was seen in four patients (9%, 95% CI, 0.6%–18%). The combined pathological response was 60% (95% CI, 45.4%–74.6%, CR 9% and PR 51%).
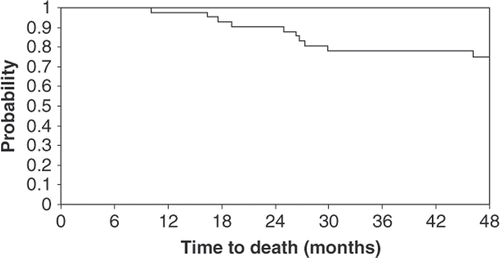
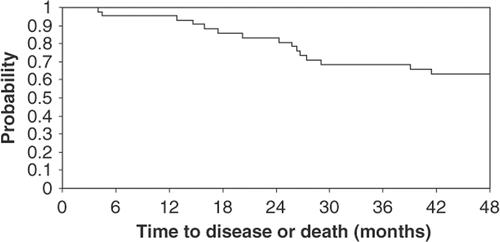
No patients progressed during neoadjuvant therapy. At the time of this report, a total of 16 patients had relapsed (48%). Four patients relapsed after surgery but prior to or during radiation therapy. Patterns of recurrence are detailed in . Ten patients (23%) died due to disease progression. With a median follow-up period of 54.8 months, four-year disease-free survival was 63% (95% CI, 46%–76%) and the four-year overall survival was 75% (95% CI, 58%–86%). The median survival time was not reached. The Kaplan-Meier time-to-event curves are presented in and .
The results from the assessment of tumour pH and oxygenation, gene expression profile for response to treatment, MRI, MRS, and RPI imaging are reported elsewhere Citation[24–26]. The mean T90 and T50 values for those patients achieving clinical response were 39.6°C and 40.9°C, respectively. The mean T90 and T50 values for patients with a pathological response were 39.8°C and 41.1°C, respectively (). For clinical non-responders, the values were 39.7°C and 41.1°C, compared with 39.4°C and 40.7°C for pathological non-responders, respectively. There were no statistical differences in the CEM 43 T5 or CEM 43 T90 between the clinical responders and clinical non-responders. However, there was a trend towards greater CEM 43°C T50 between pathological responders and non-responders (mean 103.7 versus 50.8, t-test p = 0.079). The CEM 43 T90 was significantly greater for those with a pathological response (mean 28.6 min versus 10.3 min, t-test p = 0.038). Only stage statistically impacted overall survival (log-rank p = 0.01).
Table III. Pathological response by thermal parameters.
Table IV. Patterns of recurrence.
Discussion
Despite increased awareness and screening and improvements in the early diagnosis of breast cancer, many patients still present with locally advanced disease. These patients, particularly those with inflammatory disease, continue to have a poor prognosis. Thus, a multidisciplinary, neoadjuvant approach has been employed to improve outcomes.
There are multiple neoadjuvant regimens suggested for use in this clinical setting: (1) early exposure of micro-metastatic, occult disease to chemotherapy, (2) using an in vivo indicator of response, and (3) down-staging advanced disease from inoperable to operable or from required mastectomy to breast conservation therapy. Extrapolated from promising adjuvant chemotherapy trials, anthracycline-based chemotherapy has been applied in the neoadjuvant setting. Although multiple studies Citation[27], Citation[28] showed no difference in overall survival or disease-free survival between adjuvant and neoadjuvant chemotherapy, these studies demonstrated that patients who received neoadjuvant chemotherapy have better local control rates and are more likely to conserve their breasts. Furthermore, those who achieved complete pathological responses appeared to have improved overall survival.
The rate of pathological complete responses (pCR) in patients with T1-3 breast cancers with anthracycline-based chemotherapy is only about 13% (range 4–29%) Citation[28]. Thus, taxanes have been added to neoadjuvant regimens to achieve better responses. At least seven trials have demonstrated improved pCR, but no differences in overall survival have been reported. The pCR in taxane-based chemotherapy is about 26% (range 11–31%) (NSABP B-27) in patients with T1-3 breast cancers. In more advanced breast cancers (i.e. T4) the response rates are likely to be even lower.
Currently, there is no consensus regarding the optimal neoadjuvant regimen for LABC. Additional trials are needed to determine the optimal regimen and agents to improve the pCR rates of neoadjuvant therapies. To this end, we undertook a phase I/II study to investigate the safety and efficacy of a novel neoadjuvant combination of paclitaxel, liposomal doxorubicin, and hyperthermia in locally advanced breast cancers. Paclitaxel has been shown to have activity in the metastatic, adjuvant, and neoadjuvant settings when added to standard doxorubicin-containing chemotherapy in node-positive breast cancer (NSABP B-28; SWOG-S9623; CALGB 9741). However, doxorubicin is often associated with significant side effects, (particularly cardiotoxicity). Liposomal encapsulation of a chemotherapeutic agent (in this instance, doxorubicin) can increase the therapeutic ratio of these agents. Liposomes are able to circulate longer than free drug and have a greater likelihood of escaping through relatively large endothelial gaps found in tumour microvessels Citation[29], Citation[30]. Liposomal doxorubicin (LD) has been used for the treatment of metastatic breast cancer, metastatic ovarian cancer and AIDS-related Kaposi's sarcoma Citation[31–33]. Furthermore, these studies have shown that LD conveys significantly less cardiotoxicity and gastrointestinal toxicity Citation[34], Citation[35].
Hyperthermia can further increase the therapeutic ratio by enhancing the permeability of tumour blood vessels to liposomes. In pre-clinical studies it has been demonstrated that the rate of liposomal extravasation is enhanced 4–8 fold for temperatures in the target range of this trial Citation[10]. In cats with soft tissue sarcomas, hyperthermia enhanced radiolabelled liposomal uptake by 4–16-fold compared to normothermia Citation[36]. Hyperthermia also increases oxygen levels within the tumour, which is critical to the effectiveness of radiation and chemotherapy Citation[37–40].
Our study demonstrates for the first time the efficacy and safety of a liposomal chemotherapy agent in combination with hyperthermia in LABC. Although the pCR response rate was only 9%, the combined pathological response rate was 61%. These results are encouraging, particularly given that our patient population had mostly T3 or T4 tumours, with 32% of the enrolled patients having inflammatory cancer (T4d). The reported 26% pCR rates with taxane-based chemotherapy in past studies were in more favourable patients with T1-3 tumours. Furthermore, despite the more advanced disease in our study population, the five-year DFS rate was similar to the 89% reported for stage II breast cancer Citation[41].
Previous studies have shown that the presence of a complete pathological response after neoadjuvant chemotherapy improves the likelihood of local benefit and may impact overall prognosis Citation[42]. Our study showed for the first time that optimally delivered hyperthermia is a feasible and tolerable treatment modality when combined with liposomal doxorubicin and paclitaxel. The mean CEM 43 T90 in responders to the neoadjuvant treatment was significantly higher than non-responders, 28.6 and 10.3 respectively (t-test p = 0.038). The importance of CEM 43 T90 as a measurement of hyperthermia ‘dose’ has been previously shown only in combination with radiotherapy. Jones et al. have previously demonstrated that significantly increased local control can be achieved in superficial tumours if at least 10 CEM 43 T90 was achieved in combination with radiotherapy Citation[23]. Similarly, our studies showed that improved pathological responses were achieved with higher CEM 43 T90.
Conclusions
Our study shows for the first time that neoadjuvant therapy with paclitaxel, liposomal doxorubicin and hyperthermia is a feasible and well tolerated treatment strategy in patients with LABC, including inflammatory cancer. The pathological response rates were significantly associated with achieving a sufficient thermal dose as measured by CEM 43 T90. In turn, as other studies have shown, pathological response rates were important in having a favourable overall outcome.
Declaration of interest: This work was supported by NIH PO1CA42745.
References
- Swain SM, Sorace RA, Bagley CS, Danforth DN, Jr, Bader J, Wesley MN, Steinberg SM, Lippman ME. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res 1987; 47: 3889–3894
- Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: A determinant of outcome. J Am Coll Surg 1995; 180: 297–306
- Honkoop AH, van Diest PJ, de Jong JS, Linn SC, Giaccone G, Hoekman K, Wagstaff J, Pinedo HM. Prognostic role of clinical, pathological and biological characteristics in patients with locally advanced breast cancer. Br J Cancer 1998; 77: 621–626
- Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: Clinical syndrome and molecular determinants. Breast Cancer Res 2000; 2: 423–429
- Lerebours F, Bieche I, Lidereau R. Update on inflammatory breast cancer. Breast Cancer Res 2005; 7: 52–58
- Hahn GM. Hyperthermia and Cancer. Plenum Press, New York 1982
- Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys 1996; 36: 1177–1187
- Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia 1999; 15: 345–370
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: Synergism between hyperthermia (42–43°) and adriamycin (of bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci USA 1975; 72: 937–940
- Herman TS. Effect of temperature on the cytotoxicity of vindesine, amsacrine, and mitoxantrone. Cancer Treat Rep 1983; 67: 1019–1022
- Herman TS. Temperature dependence of adriamycin, cis-diamminedichloroplatinum, bleomycin, and 1,3-bis(2-chloroethyl)-1-nitrosourea cytotoxicity in vitro. Cancer Res 1983; 43: 517–520
- Schiller JH, Storer B, Tutsch K, Arzoomanian R, Alberti D, Feierabend C, Spriggs D. Phase I trial of three-hour infusion of paclitaxel with or without granulocyte colony-stimulating factor in patients with advanced cancer. J Clin Oncol 1994; 12: 241–248
- Shapiro CL, Ervin T, Welles L, Azarnia N, Keating J, Hayes DF. Phase II trial of high-dose liposome-encapsulated doxorubicin with granulocyte colony-stimulating factor in metastatic breast cancer. TLC D-99 Study Group. J Clin Oncol 1999; 17: 1435–1441
- Wu L, McGough RJ, Arabe OA, Samulski TV. An RF phased array applicator designed for hyperthermia breast cancer treatments. Phys Med Biol 2006; 51: 1–20
- Dewhirst MW, Phillips TL, Samulski TV, Stauffer P, Shrivastava P, Paliwal B, Pajak T, Gillim M, Sapozink M, Myerson R, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys 1990; 18: 1249–1259
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, George SL. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993; 25: 289–297
- Hand JW, Machin D, Vernon CC, Whaley JB. Analysis of thermal parameters obtained during phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia 1997; 13: 343–364
- Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, Hand J, Vernon C, van Rhoon G, van der Zee J, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys 1997; 39: 371–380
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003; 19: 267–294
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Vujaskovic Z, Rosen EL, Blackwell KL, Jones EL, Brizel DM, Prosnitz LR, Samulski TV, Dewhirst MW. Ultrasound guided pO2 measurement of breast cancer reoxygenation after neoadjuvant chemotherapy and hyperthermia treatment. Int J Hyperthermia 2003; 19: 498–506
- Dressman HK, Hans C, Bild A, Olson JA, Rosen E, Marcom PK, Liotcheva VB, Jones EL, Vujaskovic Z, Marks J, et al. Gene expression profiles of multiple breast cancer phenotypes and response to neoadjuvant chemotherapy. Clin Cancer Res 2006; 12: 819–826
- Craciunescu OI, Blackwell KL, Jones EL, MacFall JR, Yu D, Vujaskovic Z, Wong TZ, Liotcheva VB, Rosen EL, Prosnitz LR, et al. DCE-MRI parameters have potential to predict response of locally advanced breast cancer patients to neoadjuvant chemotherapy and hyperthermia: A pilot study. Int J Hyperthermia 2009; 25(6)405–415
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst 2005; 97: 188–194
- Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007; 94: 1189–1200
- Wu NZ, Da D, Rudoll TL, Needham D, Whorton AR, Dewhirst MW. Increased microvascular permeability contributes to preferential accumulation of stealth liposomes in tumor tissue. Cancer Res 1993; 53: 3765–3770
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA 1998; 95: 4607–4612
- Angioli R, Palaia I, Calcagno M, Manci N, Zullo MA, Bellati F, Perniola G, de Vivo A, Benedetti Panici P. Liposome-encapsulated doxorubicin citrate in previously treated recurrent/metastatic gynecological malignancies. Int J Gynecol Cancer 2007; 17: 88–93
- Udhrain A, Skubitz KM, Northfelt DW. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi's sarcoma. Int J Nanomedicine 2007; 2: 345–352
- O’Brien ME. Single-agent treatment with pegylated liposomal doxorubicin for metastatic breast cancer. Anticancer Drugs 2008; 19: 1–7
- Krown SE, Northfelt DW, Osoba D, Stewart JS. Use of liposomal anthracyclines in Kaposi's sarcoma. Semin Oncol 2004; 31: 36–52
- Rahman AM, Yusuf SW, Ewer MS. Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int J Nanomedicine 2007; 2: 567–583
- Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PE, Dewhirst MW, Needham D, Thrall DE. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res 2000; 6: 3748–3755
- Griffin RJ, Okajima K, Barrios B, Song CW. Mild temperature hyperthermia combined with carbogen breathing increases tumor partial pressure of oxygen (pO2) and radiosensitivity. Cancer Res 1996; 56: 5590–5593
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 1996; 12: 367–373
- Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, MacFall JR, Brizel DM, Meyer RE, Prescott DM, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 2000; 46: 179–185
- Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia 2006; 22: 365–373
- Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, Buzdar AU, Booser DJ, Valero V, Bondy M, et al. Residual risk of breast cancer recurrence five years after adjuvant therapy. J Natl Cancer Inst 2008; 100: 1179–1183
- Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer 2004; 91: 2012–2017