Abstract
Purpose: Although induction heating cancer therapy (IHCT) using magnetic nanoparticles can be a promising approach to treatment-less multi-nodular cancers, the objective requirement for successful clinical application has not clearly been elucidated. We intended to define objective heat doses suitable for IHCT, especially focusing on the sizes of liver cancer nodules.
Materials and methods: Alternating magnetic fields were applied to three human pancreatic cancer cell lines, the intercellular space of those cell pellets were filled with magnetic nanoparticles, and confirmed the cytotoxic effect of IHCT. Subsequently, the temperatures of liver cancer nodules in IHCT were simulated using a computer software program and the required heat dose for various sized tumours were determined.
Results: Heating the cancer cells up to 50°C for 10 min was sufficient for complete cell killing and the heat dose of 1.7 W/gtumour is required for 10 mm tumour. Larger tumours require a smaller heat dose, e.g. 20 mm and 40 mm tumours require 0.7 W/gtumour and 0.6 W/gtumour, respectively, whereas smaller tumours require large amounts of heat, e.g. 5 mm and 1 mm tumours require 5.1 W/gtumour and 105 W/gtumour, respectively.
Conclusions: Integrating the presently available technologies, including high-quality magnetic nanoparticles (1000 W/gmaterial) and effective drug delivery systems (1–2 mgmaterial/gtumour), treatment of a 10 mm tumour seems possible. Since treatment of smaller tumours less than 5 mm require substantial heat dose, researchers involved in IHCT should target cancer nodules of 10 mm or more, and develop a heat delivery system providing a minimum of 1.7 W/gtumour.
Introduction
Multiple liver metastases, especially those from notorious pancreatic cancer, have been awaiting an advent of new treatment strategies Citation[1–3]. Thermal ablation, which involves heating the target cancer nodules to more than 46°C that results in irreversible cellular damage known as necrosis Citation[4], has been developed as one of the less invasive cancer treatment strategies Citation[5]. In order to deliver heat to cancer nodules, piercing needles wired to radiofrequency Citation[6] and microwave Citation[7] systems are widely used, mainly for liver tumours Citation[6]. Due to the technical difficulties of needle puncture Citation[8], Citation[9], however, this approach is not suitable in the case of patients with advanced-stage cancers, including multiple and/or micro-disseminated lesions. In order to overcome these limitations, the development of induction heating using magnetic nanoparticles has recently attracted attention Citation[10–12]. Magnetic nanoparticles selectively distributed to the cancer nodules generate heat when an external alternating magnetic field (AMF) is applied Citation[12]. This induction heating cancer therapy (IHCT), however, has not been clinically employed, probably because the exact goals have not been clearly defined.
In the clinical setting the temperature of target cancer nodules is determined by the balance between the amount of applied heat dose (W/gtumour) and heat loss to the surrounding tissue Citation[13]. The reason for past incomplete IHCT development may be explained mainly by the insufficient administered heat dose against heat loss, resulting in the entire target cancer tumours not being heated up to tissue lethal temperature. The heat dose administered in IHCT takes into account two factors: the heating power of magnetic material (W/gmaterial) that is represented by the specific absorption rate (SAR) Citation[10], and the amount of magnetic nanoparticles accumulated in the cancer nodule, which is represented by the weight of particles per unit weight of target tumour tissue (gmaterial/gtumour). The main issue that we have to address should be to set the objective heat dose values which overcome the amount of heat loss.
Heat loss is basically mediated by static thermal diffusion, however, the cooling effect of blood flow should also be taken into consideration in a clinical setting Citation[14–16]. It is true that the presence of blood flow acts as a heat radiator and disrupts heating the cancer nodules, we eliminate blood flow issue from our estimation since this obstacle may be overcome by employing a surgical technique known as Pringle's manoeuvre Citation[17] which temporally occludes hepatic vascular inflow. The efficacy of Pringle's manoeuvre in reducing heat loss has been clinically shown in radio-frequency ablation treatment Citation[18], Citation[19]. We therefore focused only on static thermal diffusion loss to surrounding tissues as a heat loss factor. Since the size of liver tumours are known to vary in the clinical field, physical condition required for IHCT should be different according to the tumour sizes.
We addressed here the principal question of how much heat dose is required for successful IHCT in liver metastases of various sizes. The lethal temperatures of three human pancreatic cancer cell lines of different heat sensitivity were experimentally defined using magnetic nanoparticles and a previously developed AMF generator Citation[20]. We then calculated the heat doses required for IHCT, especially focusing on the sizes of liver cancer nodules, by employing a computer-controlled heat simulator. Possibilities of achieving the estimated heat dose by integrating presently available technologies are discussed.
Materials and methods
AMF generator
An AMF was generated by using a portable induction heating device developed at Kanazawa University, Japan Citation[20]. The maximum strength of the AMF is 150 Oe (15 mT) with a frequency of 114 kHz.
Magnetic nanoparticles
Ferucarbotran particles (Resovist®; Bayer Schering Pharma, Berlin) have been used as magnetic nanoparticles Citation[21]. The concentration of Resovist® is 27.9 mg Fe/mL or 0.5 mol Fe/L or 40 mg γ-Fe2O3/mL. In this study, we used a double-concentrated ferucarbotran (80 mg γ-Fe2O3/mL) solution that was provided by Meito Sangyo (Aichi, Japan), who is the supplier of ferucarbotran to Bayer Schering Pharma.
Mammalian cancer cell lines
We used three human pancreatic cancer cell lines (SUIT-2, BxPC-3, and AsPC-1) as representative of the pancreatic cancer that causes typical experimental liver metastasis Citation[22]. The SUIT-2 cells were generously provided by Dr. Takeshi Iwamura (Miyazaki Medical College, Miyazaki, Japan) Citation[23]. The BxPC-3 and AsPC-1 cell lines were obtained from the American Type Culture Collection (Bethesda, MD).
Measurement of heating potential of ferucarbotran represented by SAR
The heating potential of each material is physically represented by SAR and is calculated using the following equation:where C is the specific heat capacity of the sample (J/g/°C) and ΔT (°C)/Δt (s) is the initial 30 s slope of the temperature versus time curve Citation[10]. The specific heat capacity is unique for each material, for example, Cwater = 4.185 J/g/°C and Cferucarbotran =3.64 J/g/°C. Vs is the sample volume and m is the mass of iron oxide. Since the value of ΔTemp/Δtime of the ferucarbotran solution at 150 Oe, 114 kHz was 0.71°C/s, SAR value of the ferucarbotran was calculated as 32.3 W/gmaterial.
Induction heating of cell pellet with intercellular spaces filled with ferucarbotran
Considering the in vivo features of cancer nodules with distributed magnetic nanoparticles, we employed an in vitro tumour model of a cell pellet whose intercellular spaces were filled with five different concentrations of ferucarbotran. A total of 20 µL cell suspension containing 5 × 106 cells and 70 µL of five different concentrations of ferucarbotran (20, 27, 40, 60, and 80 mg γ-Fe2O3/mL) were mixed and centrifuged to form a cell pellet. The temperature of the cell pellets and irradiated AMF, was monitored with an optical fibre thermo sensor (FL-100, Anritsu Meter, Tokyo, Japan). The mean temperature of three independent experiments attained after 300 s of exposure to the magnetic field was defined as the equilibrium temperature.
Heat doses administered to heat a cell pellet in vitro to various equilibrium temperatures
In order to determine the actual heat doses administered to the in vitro tumour model we measured the amount of γ-Fe2O3 by our original method Citation[24]. Briefly, since the magnetic moment of γ-Fe2O3 per unit is constant (0.49/mg emu), the amount of γ-Fe2O3 surrounding intercellular space can be measured by detecting the magnetic moment by using a superconducting quantum interference device (SQUID) magnetometer. The values of the magnetic moments of γ-Fe2O3 were substituted into the following equation:
Cytotoxic effect of heat at different temperatures, generated by induction heating
We confirmed the cytotoxic effect of induction heating by using ferucarbotran; an AMF (100–150 Oe, 114 kHz) was applied to cell pellets from three different cell lines (SUIT-2, BxPC-3, and AsPC-1). Cell pellets were heated up to the target temperatures (39°C, 42°C, 46°C, 48°C, 51°C, and 60–65°C) and maintained at each temperature for 10 min. Each heated cell was plated in a 60-mm cell culture dish at the concentration of 1 × 105 cells/dish and cultured for 7 days. The control growth curve was determined by using the ferucarbotran-containing cells that were not subjected to an AMF. Viable cells, determined by the trypan blue dye exclusion method, were counted on days 1, 3, and 7. The data values and bars represent the mean and SD of three independent experiments.
Computer simulations of tempo-spatial change in temperature
All the simulations were performed using a finite element method software program, COMSOL Multiphysics ver. 3.5 (COMSOL; Burlington, MA). The density, thermal conductivity and specific heat at constant pressure of the magnetic particles were assumed to be equal to those of magnetite at 37°C and constant in spite of changes in temperature. Those for cells and dextran were assumed to be equal to those of pure water, since almost all contents of cells are water and dextran is highly hydrated in water. All calculations were executed under the assumption that the magnetic particles were distributed homogeneously in a tumour and cell pellet. The density, thermal conductivity and specific heat at constant pressure of the tumour and pellet containing the magnetic particles were calculated assuming that the values were ‘additive’ of those of water and magnetite at 37°C. Though those temperature dependent properties of water are known to show relatively small diversity at the heat range of 30°C to 70°C, internal database of COMSOL enables provision of much precise simulation for a tumour and cell pellet without magnetic particles by automatically adapting appropriate values at each target temperature.
The tempo-spatial change in temperature in cell pellets and a human liver containing a tumour with magnetic particles was calculated with COMSOL. The governing equation is as follows.where ρ is the density (kg/m3), Cp the molar specific heat at constant pressure (J/kg/°C), T the temperature (°C), t the time (s), κ the thermal conductivity (W/m/°C), Q the heat generation rate (W/m3), and ∇ means Laplacian.
A mesh consisting of triangular elements was generated automatically and optimally by COMSOL at a resolution as fine as was permitted by the computer's memory (approximately 16 GB). For every calculation we confirmed that there was less than 5% difference in the obtained value between the result obtained with the finest mesh and that with a one-step coarser mesh. The UMFPACK direct solver and adequate conditions needed for each calculation were automatically selected by COMSOL.
Simulation of small cell pellet in vitro
The tempo-spatial change in temperature of a 20 µl cell pellet in a centrifuge microtube was simulated at five different heat doses (0.7, 1.5, 2.1, 7.3, and 9.5 W/gtumour). In this case, the 2-dimentional axisymmetrical model was implemented following the actual shape of the microtube. A 100-µm thick polypropylene wall with a thermal conductivity of 0.12 W/m/°C and a 100-µm thick external air layer with 0.025 W/m/°C around the pellet were assumed. The fineness of mesh in COMSOL, i.e. total mesh number of the triangle elements, is represented by the degree of freedom as previously reported by Salloum et al. Citation[25]. The total numbers of the triangle elements automatically and optimally generated by COMSOL and degree of freedom were about 7,000 and 13,000, respectively. At t = 0, all parts of the model were at 37°C and the outer side of the air layer and the top of the microtube were fixed at 25°C.
Simulation of liver tumours in vivo
The tempo-spatial change in temperature of in vivo tumours with two different sizes (10 mm and 5 mm in diameter) was calculated at a heat dose of 1.7 W/gtumour. The assumption made in these calculations was that the human liver was 1200 mL in volume and had the shape of a triangular prism, with a rectangular base with short and long sides of 16.65 cm and 28.84 cm, respectively, and a height of 5 cm. The total number of the triangle elements automatically and optimally generated by COMSOL and degree of freedom were about 65,000 and 360,000, respectively Citation[25]. At t = 0, all parts of the model were at 37°C and the outer surface of the liver was fixed at 37°C.
Heat dose required for liver tumours of various diameters.
Generalized correlations between the size of liver tumours and the heat dose required for achieving the target temperature in the IHCT were calculated using COMSOL. The model and boundary conditions were the same as described above. We simulated the heat dose that can heat tumours 0.5, 1, 2, 4, 5, 10, 20, and 40 mm in diameter up to 42°C, 46°C, and 50°C at the periphery after AMF exposure for 300 s.
Results
Increase in the temperature of SUIT-2 cell pellet containing ferucarbotran in the intercellular spaces and subjected to an AMF.
The increase in the temperature of the SUIT-2 cell pellet filled with five different concentrations of ferucarbotran (20, 27, 40, 60, and 80 mg γ-Fe2O3/ml initial ferucarbotran concentration) and subjected to an AMF of 150 Oe at 114 kHz is shown in . Equilibrium temperatures for these concentrations were 38.3°, 46.5°, 51.6°, 64.3° and 69.7°C, respectively. The same experiment was conducted for different types of cell pellets (BxPC-3 and AsPC-1) and similar results were obtained (data not shown).
Figure 1. Temperature of a cell pellet by induction heating. Cell pellets filling the intercellular space with five different concentrations of ferucarbotran were applied for an AMF. The equilibrium temperatures; 38.3°, 46.5°, 51.6°, 64.3°, and 69.7°C were obtained for the five different concentrations of 20♦, 27▾, 40▴, 60•, and 80▪ mg γ-Fe2O3/mL initial ferucarbotran concentration, respectively (A). The value between the equilibrium temperature and applied heat dose was plotted for three different cancer cell lines (SUIT-2▪, BxPC-3•, and AsPC-1▴) (B). Heat dose of 0.7, 1.5, 2.1, 7.3, and 9.5 W/gtumour, resulted in equilibrium temperatures of 38.3°, 46.5°, 51.6°, 64.3°, and 69.7°C, respectively.
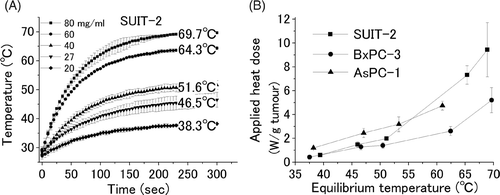
Heat doses used to heat the cell pellets in vitro to various equilibrium temperatures.
In the representative cell line SUIT-2, the amounts of γ-Fe2O3 in cell pellets containing five different concentrations of ferucarbotran were 21, 48, 64, 227, and 295 mg γ-Fe2O3/gtumour, respectively. Since the SAR of γ-Fe2O3 is 32.3 W/gmaterial, 21 mg γ-Fe2O3/gtumour corresponds to an applied heat dose of 0.7 (SD 0.1) W/gtumour resulting in an equilibrium temperature of 38.3°C. Similarly, 48, 64, 227, and 295 mg γ-Fe2O3/gtumour correspond to applied heat dose of 1.5 (SD 0.2), 2.1 (SD 0.04), 7.3 (SD 0.8), and 9.5 (SD 2.2) W/gtumour, respectively, providing 46.5°, 51.6°, 64.3°, and 69.7°C equilibrium temperature, respectively. The relation between the heat doses used to heat SUIT-2 (▪), BxPC-3(•), and AsPC-1 (▴) cell pellets in vitro to various equilibrium temperatures is shown in . Further, the heat dose required to increase the temperature of an in vitro tumour model surrounded by air up to 50°C is in the range 1.5–2.0 W/gtumour.
Simulation of thermal gradient in in vitro cell pellets
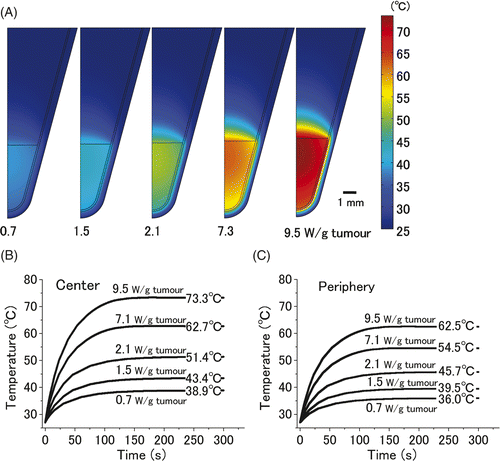
Thermal gradient simulations of 20 µL cell pellets are shown in . When a heat dose of 9.5 W/gtumour was administered to a cell pellet the temperature at the centre of the cell pellet increased to 73.3°C and the temperature at the periphery of the pellet gradually decreased to 62.5°C. Although the entire cell pellet was subjected to a homogenous AMF, we observed a temperature gradient between the centre and the periphery of the cell pellet. The time-dependent changes in the temperature at the centre and the periphery of the cell pellet are shown in . The equilibrium temperature at the centre of samples that were heated with doses of 0.7, 1.5, 2.1, 7.3, and 9.5 W/gtumour was 38.9°, 43.4°, 51.4°, 62.7°, and 73.3°C, respectively. These simulation data were quite comparable to actual experimental data (), indicating the validity of the COMSOL Multiphysics simulation results. The equilibrium temperatures at the periphery of the cell pellet were 36.0°, 39.5°, 45.7°, 54.5°, and 62.5°C, respectively.
Figure 2. Computer simulation of thermal gradient in a small in vitro cell pellet surrounded by air. Thermal gradients of the 20 µL cell pellet, which was heated with five different heat doses (0.7, 1.5, 2.1, 7.3, and 9.5 W/gtumour) for 300 s are shown (A). The time-dependent change in the temperature at the centre (B) and periphery of pellet (C). Equilibrium temperatures at the centre of the tumour on administration of the above-mentioned heat doses were 38.9°, 43.4°, 51.4°, 62.7°, and 73.3°C, respectively. Those at the periphery were 36.0°, 39.5°, 45.7°, 54.5°, and 62.5°C, respectively.
Cytotoxic effect of heat generated by induction heating at different temperatures
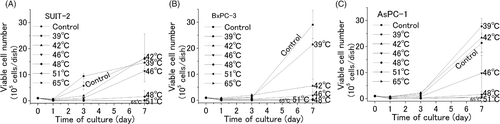
The number of viable cancer cells 1, 3, and 7 days after exposure to six different temperatures is shown in . The growth curves indicated the difference in heat sensitivity among the three cell lines. The BxPC-3 cells were relatively ‘sensitive cells’ because exposure to a temperature of 42°C induces obvious cellular damage and 46°C is more than enough to kill cells. In contrast, SUIT-2 and AsPC-1 cells are ‘heat tolerant cells’ because exposure to 46°C is not sufficient to kill them; more than half the number of cells that were considered in the control case were viable on day 7.
Figure 3. Cytotoxic effect of heat, generated by induction heating. An AMF (100–150 Oe, 114 kHz) was applied to three different cell pellets (SUIT-2, BxPC-3, and AsPC-1) containing ferucarbotran. After 10-min exposure to various temperatures (39°C, 42°C, 46°C, 48°C, 51°C, and 60–65°C), each cell pellet was cultured for 7 days. The numbers of viable cells 1, 3, and 7 days after incubation were counted. The values and bars are the mean and SD of three independent experiments.
Temperature simulation of a liver tumour in vivo
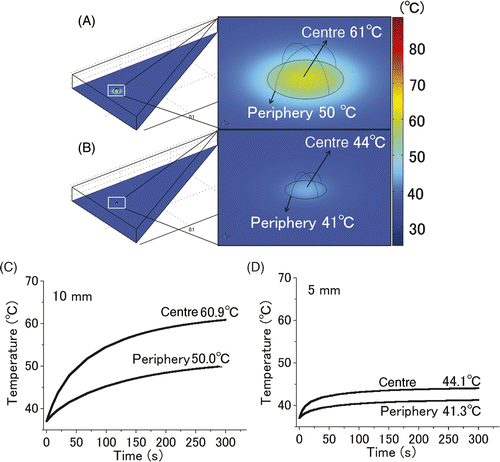
The spatiotemporal change in the temperature of liver tumours of different sizes (10 mm and 5 mm in diameter) is shown in . The image of the entire liver and an enlarged image of the tumour portion are shown in 4A and 4B. Time-dependent changes in the temperature at the centre and periphery of the tumour are shown in 4C and 4D. The temperatures at the centre and periphery of the 10 mm tumour were 61°C and 50°C (4A, 4C), respectively. In contrast, the temperatures at the centre and periphery of the 5 mm tumour were 44°C and 41°C (4B, 4D), respectively.
Figure 4. Computer simulation of the thermal gradient in in vivo liver tumour of different sizes. The temperature changes in the 10 mm (A, C) and 5 mm tumours (B, D) with a heat dose of 1.7 W/gtumour for 300 s were simulated. Images of the entire liver and an enlarged image of a part of the tumours (A, B). Time-dependent change in the temperatures at the centre and periphery of the 10 mm (C) and 5 mm (D) tumours. In the simulation of the 10-mm-diameter tumour, the centre and periphery of the 10-mm tumour were heated to 61°C and 50°C, respectively, however, those of the 5-mm tumour were heated only to 44°C and 41°C, respectively.
Heat dose required for liver tumours of various diameters
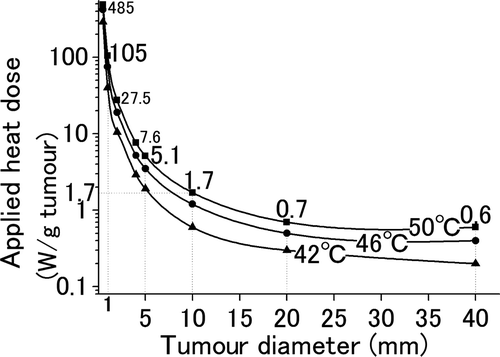
Generalized correlations between the size of liver tumours and the heat dose required for heating tumours up to 50°C, 46°C, and 42°C are shown in . To increase the temperature of the periphery of the tumour up to 50°C, the heat doses required for tumours with diameters of 40, 20, 10, 5 and 1 mm were 0.6, 0.7, 1.7, 5.1 and 105 W/gtumour, respectively.
Figure 5. Required heat dose for liver tumours with various diameters. The relationship between the tumour size and the heat dose administered for 300 s to heat the tumours to 42°C (▴), 46°C (•), and 50°C (▪) at their periphery was simulated. In order to increase the temperature of the tumour periphery up to 50°C, tumours with diameters 40, 20, 10, 5, and 1 mm require heat doses of 0.6, 0.7, 1.7, 5.1, and 105 W/gtumour, respectively.
Discussion and conclusion
The biomedical significance of our study is that it presents minimal heat dose that should be considered for the successful implementation of IHCT. Large liver tumours, more than 10 mm in diameter, may be treated by applying a 1.5–2 W/gtumour heat dose, which can be achieved by integrating present technologies; however, treatment of smaller tumours require substantial heat dose.
Defining the required temperature for cancer cell killing is the first issue we have to fix in starting development of thermal cancer therapy in liver metastases. Since heat sensitivity of clinical cancers is known to be variable, we addressed the lethal temperature of three pancreatic cancer cells that are representative of notorious liver metastasis-producing cancer. We settled the treatment time at 10 min, since long irradiation of AMF to living body tissue causes adverse events Citation[26] and application of temporary blood flow interrupting surgical technique (Pringle's manoeuvre) is usually applicable for 10-15 min Citation[17]. Our results demonstrated that maintaining a temperature of 46°C for 10 min was insufficient and resulted in the reproliferation of cancer cells, while a temperature of 50°C maintained for 10 min resulted in complete cell death regardless of the heat sensitivity of each cell type (). A standard value which should be employed here is the equivalent minute of 43°C (EM43) Citation[27] which is intended to equate various experimental conditions to 43°C exposure. When EM43 exceeds 240 min, cytotoxic effects are known to be sufficient Citation[28]. Our deduced parameter, 50°C for 10 min, corresponds to EM43 = 1280 min, and 46°C for 10 min corresponds only to EM43 = 80 min, reconfirming the legitimacy of the value. We are confident, therefore, that exposing the entire tumour from the centre to the periphery to a temperature of 50°C for 10 min is a necessary and sufficient condition for IHCT.
Before using the software COMSOL for present heat simulation, we confirmed its reliability by comparing the data obtained by using it with the experimental data. The value of the heat dose is the basic input parameter for COMSOL. Although we added the same concentration of ferucarbotran for the case of the three cell lines, the final concentration of ferucarbotran was different. SUIT-2 was used as the representative cell line to examine the amount of γ-Fe2O3 (see result) that provides a heat dose of 0.7, 1.5, 2.1, 7.1, and 9.5 W/gtumour () for an AMF of 150 Oe (at 114 kHz). When we entered these values into COMSOL, the simulated temperatures at the centres of the cell pellets () were observed to be well correlated with those detected experimentally (). This result indicates that COMSOL can be used in future studies for simulation of temperatures in vivo.
A major factor that affects static heat loss is the ratio of the relative surface area of the tumour to the tumour volume. The liver tumour simulations () indicated that a large tumour (10 mm) was sufficiently heated both at the centre (61°C) and periphery (50°C), however, a small tumour (5 mm) was not sufficiently heated, even at the centre (44°C), and periphery (41°C). A small sphere possesses a large surface area per unit volume, resulting in increased heat loss to the surrounding tissue. This phenomenon has already been validated by Rabin et al., addressing whether or not intercellular hyperthermia using magnetic nanoparticles is possible using physical equations Citation[29–31]. They demonstrated that when single cells (10–100 µm in diameter) containing nanoparticles are heated by radiating AMF, they would never be heated as long as they are surrounded by a large cellular structure free of nanoparticles. Assuming the diameter of cells become larger than 1 mm, they could reach the threshold for hyperthermic conditions Citation[29]. In addition to Rabin's findings that smaller tumours (less than 1 mm in diameter) are impossibly heated, we indicate a concrete heat dose value which is required for IHCT.
Our findings may be condensed into , which demonstrates generalized correlations between the size of liver tumours and the heat dose required for increasing the temperature of the tumours to 50°C. Though a 10-mm liver tumour requires 1.7 W/gtumour, larger tumours require a smaller heat dose, e.g. 20 mm and 40 mm tumours require 0.7 W/gtumour and 0.6 W/gtumour, respectively. In contrast, small tumours require large amounts of heat, e.g. 5 mm and 1 mm tumours require 5.1 W/gtumour and 105 W/gtumour, respectively. We then addressed whether a heat dose in the range of 0.6–105W/gtumour can be provided using present technologies.
The heat dose administered in IHCT depends on two parameters: the heating potential of each nanoparticle (represented by SAR) and the concentration of nanoparticles accumulated at target sites. For example, in order to provide a heat dose of 1.7 W/gtumour for targeting a 10-mm tumour, the accumulation of 52.6 mg ferucarbotran, the SAR of which is 32.3 W/gmaterial, is required (1.7 W/32.3 W/gmaterial = 0.0526 gmaterial). Since a dose equivalent to 52.6 mg ferucarbotran is contained in 1.3 mL commercially distributed Resovist® solution, it is not realistic to accumulate 52.6 mg ferucarbotran in 1 g of tumour by any drug delivery system. We conclude that successful clinical implementation of IHCT is difficult as long as nanoparticles with a low SAR such as Resovist® are used.
Researchers from the field of applied physics should contribute to IHCT development by providing high heat power magnetic nanoparticles. The SAR values of magnetic fluids should be improved by increasing the amplitude of the AMF, increasing the frequency of the AMF, and/or modifying the material properties of magnetic nanoparticles Citation[10]. Obtaining high value SAR by applying a high-frequency AMF was shown by Levy et al. who obtained a SAR value of 950 W/gmaterial for γ-Fe2O3 at 339 Oe and 700 kHz Citation[32]. However, the guidelines of the International Commission on Non-Ionizing Radiation Protection state that exposure to electromagnetic fields at frequencies above about 100 kHz causes significant increase in the temperature even in organs or tissues with no magnetic materials Citation[33]. Since the maximum strength of the AMF that can be applied to animals and humans is around 1300 Oe × 150 kHz (reported by Ivkov et al. Citation[26]), the development of materials that show high SAR within this AMF strength is desirable. Although the heat dose of 32.3 W/gmaterial (at 150 Oe and 114 kHz, our experiment) and the SAR of 123 W/gmaterial (at 88 Oe and 63 kHz, reported by Wang et al. Citation[34]) were achieved in the safe range of the AMF strength, it seems to be difficult to achieve SAR values as high as 950 W/gmaterial within the range by using γ-Fe2O3. Therefore, using non-ferucarbotran materials, for example ferromagnetic materials, is another option to obtain high SAR values Citation[35]. Since heat generation by ferromagnetic materials is mainly influenced by the strength of the magnetic field rather than its frequency, ferromagnetic materials have the potential to show a high SAR at clinically applicable low-frequency AMFs. We have been developing new ferromagnetic materials for achieving a heat dose of 450 W/gmaterial for a low-frequency AMF with a moderate strength (440 Oe, 120 kHz) (unpublished data). We assumed that the combination of newly developed magnetic nanoparticles and a clinically adapted AMF generator would be able to provide 800–1000 W/gmaterial, and this could be considered as the goal value of SAR in IHCT development.
Another factor requiring detailed study is the method of increasing the concentration of magnetic particles in the target cancerous tissue; this topic should be solved by researchers in the biomedical field. Nanoparticles or macromolecules larger than 10–100 nm are known to be predominantly precipitated in the cancerous tissues due to a unique physiological mechanism called enhanced permeability and retention effect Citation[36]. The precipitation of macromolecules was enhanced eight times more (8% injection dose/gtumour) than that of micromolecules due to this effect Citation[37]. Although attempts to increase the concentration of accumulated nanoparticles by conjugating them with cancer-targeting antibodies have resulted in an increase by a factor of 1.5–2 (13.7 ± 2.1% injection dose/gtissue), the amount accumulated is only 0.2 mgmaterial/gtumour Citation[12]. Since antibody conjugation has limited effect on the nanoparticles concentration Citation[38] it is clear that drug delivery systems that can precipitate at most 1–2 mgmagnetic nanoparticle/gtumour should be achieved using the currently available techniques.
The main theoretical merit of IHCT may be to control micro-disseminated multiple cancer nodules, which cannot be treated with needle-puncture ablation systems. However, smaller tumours (less than 1 mm in diameter) are revealed to be impossibly heated by IHCT as path-breaker's report Citation[29]. Our data have added simple and concrete heat dose value which require for IHCT, demonstrating that treating small (1–5 mm in diameter) cancer nodules with IHCT is unrealistic since the required heat dose of 5.1–105 W/gtumour cannot be provided to date. For the time being, researchers involved in IHCT should target cancer nodules with a diameter of 10 mm or more, and develop heat delivery systems capable of providing a heat dose of 1.7 W/gtumour at least. It should be noted here that this 1.7 W/gtumour value is the minimal estimate without considering cooling effect of blood flow, and two to three times larger heat dose would be required in a clinical setting if blood flow interruption technique has not been incorporated Citation[16], Citation[39]. To achieve this goal of 1.7 W/gtumour, researchers in applied physics should develop magnetic nanoparticle systems capable of delivering 1000 W/gmaterial under conditions safe to humans, while researchers in the biomedical field should work on establishing a drug delivery system that can accumulate 1–2 mg of magnetic nanoparticles per gram of tumour.
Acknowledgements
The authors are grateful to Dr. Osamu Matsui and Dr. Shigeyuki Takamatsu (Kanazawa University) for valuable discussions, and to Dr. Takeshi Iwamura (Miyazaki Medical College) for providing the SUIT-2 cells.
Declaration of interest: This work was supported in part by a grant-in-aid for Cancer Research (21 TokubetuShitei-1) from the Ministry of Health, Labour and Welfare, and in part by a Grant-in-aid for Scientific Research (KAKENHI, 21591743) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Yamada H, Hirano S, Tanaka E, Shichinohe T, Kondo S. Surgical treatment of liver metastases from pancreatic cancer. HPB (Oxford) 2006; 8: 85–88
- Fujii M, Miyake H, Sasaki K, Takagi T, Takamura K, Tashiro S. Arterial infusion chemotherapy for the patient of unresectable pancreatic carcinoma with multiple liver metastases: A case report. J Med Invest 2003; 50: 199–202
- Katsumata K, Tomioka H, Sumi T, Yamasaki T, Takagi M, Kato F, Suzuki Y, Aoki T, Koyanagi Y. Liver metastasis of pancreatic cancer managed by intra-arterial infusion chemotherapy combined with degradable starch microspheres. Int J Clin Oncol 2003; 8: 110–112
- Larson TR, Bostwick DG, Corica A. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology 1996; 47: 463–469
- Timmerman RD, Bizekis CS, Pass HI, Fong Y, Dupuy DE, Dawson LA, Lu D. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin 2009; 59: 145–170
- Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann Surg 1999; 230: 1–8
- Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994; 74: 817–825
- White TJ, Roy-Choudhury SH, Breen DJ, Cast J, Maraveyas A, Smyth EF, Hartley JE, Monson JR. Percutaneous radiofrequency ablation of colorectal hepatic metastases–initial experience. An adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Dig Surg 2004; 21: 314–320
- Tranberg KG. Percutaneous ablation of liver tumours. Best Pract Res Clin Gastroenterology 2004; 18: 125–145
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993; 9: 51–68
- Minamimura T, Sato H, Kasaoka S, Saito T, Ishizawa S, Takemori S, Tazawa K, Tsukada K. Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite-incorporated microspheres in rats. Int J Oncol 2000; 16: 1153–1158
- DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Gruettner C, Adamson GN, Ivkov R. Development of tumor targeting bioprobes ((111)In-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapy. Clin Cancer Res 2005; 11: S7087–7092
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. Am J Roentgenol 2000; 174: 323–331
- Chinn SB, Lee FT, Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC, III, Warner TF, Mahvi DM. Effect of vascular occlusion on radiofrequency ablation of the liver: Results in a porcine model. Am J Roentgenol 2001; 176: 789–795
- Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer 2002; 95: 2353–2360
- Ahmed M, Liu Z, Humphries S, Goldberg SN. Computer modelling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumour ablation. Int J Hyperthermia 2008; 24: 577–588
- Pringle JH. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908; 48: 541–549
- Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: Effects of blood flow and treatment time on lesion size. Ann Surg 1998; 227: 559–565
- Percivale A, Stella M, Barabino G, Pasqualini M, Pellicci R. Radiofrequency thermal ablation of hepatocellular carcinoma: Our five year experience. Ann Ital Chir 2004; 75: 635–642
- Takamatsu S, Matsui O, Gabata T, Kobayashi S, Okuda M, Ougi T, Ikehata Y, Nagano I, Nagae H. Selective induction hyperthermia following transcatheter arterial embolization with a mixture of nano-sized magnetic particles (ferucarbotran) and embolic materials: feasibility study in rabbits. Radiat Med 2008; 26: 179–187
- Reimer P, Balzer T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur Radiol 2003; 13: 1266–1276
- Enomoto T, Oda T, Aoyagi Y, Sugiura S, Nakajima M, Satake M, Noguchi M, Ohkohchi N. Consistent liver metastases in a rat model by portal injection of microencapsulated cancer cells. Cancer Res 2006; 66: 11131–11139
- Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT-2) producing carcinoembryonic antigen and carbohydrate antigen 19-9. Jpn J Cancer Res 1987; 78: 54–62
- Hashimoto S, Oda T, Yamada K, Takagi M, Enomoto T, Ohkohchi N, Takagi T, Kanamori T, Ikeda H, Yanagihara H, et al. The measurement of small magnetic signals from magnetic nanoparticles attached to the cell surface and surrounding living cells using a general-purpose SQUID magnetometer. Phys Med Biol 2009; 54: 2571–2583
- Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: Optimization of the heat absorption pattern. Int J Hyperthermia 2009; 25: 309–321
- Ivkov R, DeNardo SJ, Daum W, Foreman AR, Goldstein RC, Nemkov VS, DeNardo GL. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res 2005; 11: S7093–7103
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 2009; 25: 3–20
- Horng TL, Lin WL, Liauh CT, Shih TC. Effects of pulsatile blood flow in large vessels on thermal dose distribution during thermal therapy. Med Phys 2007; 34: 1312–1320
- Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense?. Int J Hyperthermia 2002; 18: 194–202
- Kalambur VS, Longmire EK, Bischof JC. Cellular level loading and heating of superparamagnetic iron oxide nanoparticles. Langmuir 2007; 23: 12329–12336
- Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. Limits of localized heating by electromagnetically excited nanoparticles. J Appl Phys 2006; 100: 054305
- Levy M, Wilhelm C, Siaugue J, Horner O, Bacri J, Gazeau F. Magnetically induced hyperthermia: Size-dependent heating power of γ-Fe2O3 nanoparticles. J Phys Condens Matter 2008; 20: 204133
- International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys 1998; 74: 494–522
- Wang X, Gu H, Yang Z. The heating effect of magnetic fluids in an alternating magnetic field. J Magn Magn Materials 2005; 293: 334–340
- Kita E, Yanagihara H, Hashimoto S, Yamada K, Oda T, Kishimoto M, Tasaki A. Hysteresis power-loss heating of ferromagnetic nanoparticles designed for magnetic thermoablation. IEEE Tran Magn 2008; 44: 4452–4455
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release 2000; 65: 271–284
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res 1986; 46: 6387–6392
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 2006; 66: 6732–6740
- Chang IA, Nguyen UD. Thermal modeling of lesion growth with radiofrequency ablation devices. Biomed Eng Online 2004; 3: 27