Abstract
Purpose: To evaluate therapeutic efficacy of ultrasound (US)-guided high-intensity focused ultrasound (HIFU) ablation for treatment of needle-track seeding of hepatocellular carcinoma (HCC).
Materials and methods: Nine patients with needle-track seeding of HCC were treated as outpatients using US-guided HIFU ablation. The mean size of the lesion was 1.8 cm (range 1.1 to 2.6 cm), two lesions were located on the abdominal wall, seven lesions were located on the chest wall. An acoustic power of 200 to 400 W was used determined by the echo changes after energy exposures, intermittent HIFU exposures of 1 to 2 s were used during treatment. Treatment was considered complete when the entire nodule and a surrounding 0.5 cm margin become hyperechoic on US. The outcome of HIFU ablation was observed by US and contrast-enhanced magnetic resonance imaging during follow up.
Results: HIFU ablation was performed smoothly in all patients, the treatment lasted for 6 to 21 min (mean 12 min). No major complications occurred. During a mean follow up of 10.3 months (range 7–15 months), persistent absence of lesion enhancement was seen in eight patients, one patient developed a recurrent lesion near the treated area, which was successfully treated by a repeat HIFU ablation.
Conclusions: US-guided HIFU ablation may be an effective treatment for needle-track seeding of HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumours around the world Citation[1]. Fine-needle aspiration and cutting-needle biopsy are commonly used to establish the pathological diagnosis Citation[2], Citation[3]. Image-guided tumour ablation using ethanol Citation[4] or thermal energies such as radiofrequency (RF) or microwave (MW) Citation[5], Citation[6] are well-established techniques for treating localised HCC. These techniques require percutaneous insertion of needle-like instruments. Viable HCC cells may be disseminated along the needle pathway during the interventional procedures and grow into implantation lesions Citation[2–8]. Although the incidence of iatrogenic needle-track seeding of HCC is low, it is generally regarded as a delayed major complication of the techniques, requiring due attention. Surgical excision is currently the most common option for treating needle-track seeding of HCC Citation[3], Citation[6], Citation[7], Citation[8]. Small subcutaneous lesions can be readily resected, whereas for large lesions, especially those involving muscles along the needle-track, wide excision with a safety margin may be technically difficult. Radical resection may sacrifice a large area of skin and muscle influencing the integrity of the abdominal or chest wall, while inadequate resection may have a high rate of tumour recurrence. Besides, as radical resection of the seeding lesion is aggressive, some patients with poor hepatic reserve may not be amenable to surgical procedure Citation[8]. Therefore, a minimally invasive yet effective therapy may be of value for treatment of needle-track seeding of HCC.
High-intensity focused ultrasound (HIFU) ablation is a conformal extracorporeal treatment which can yield thermal coagulation necrosis of target lesion without surgical exposure or insertion of applicators Citation[9–15]. HIFU energy is delivered from a therapeutic ultrasound (US) transducer that is focused at the target area. With the motion of the therapeutic transducer, well-delineated coagulation necrosis can be induced at depth in the focal area of the US beam, through the intact skin. Under the guidance of real-time imaging, large-volume or irregular lesions can be effectively ablated Citation[13], Citation[14]. In the past decade, HIFU has been applied for the treatment of various malignant tumours; effective local tumour control had been obtained Citation[9–15]. However, to our knowledge, there has been no report of HIFU ablation for treatment of needle-track seeding of HCC so far. Thus, the purpose of this study was to evaluate the therapeutic efficacy of HIFU for treatment of needle-track seeding of HCC.
Materials and methods
Patients
From May 2008 to January 2009, nine patients (eight men, one woman) with needle-track seeded HCC lesion were enrolled in this prospective study, which was approved by the institutional ethics committee. Informed consent was obtained from all patients at enrolment.
The inclusion criteria for HIFU ablation were as follows: (1) the entire lesion could be clearly seen on US; (2) the lesion was located more than 2 mm from the skin surface; (3) the lesion had not invaded the ribs. In this study one patient was excluded because the seeding lesion was located less than 2 mm from the skin surface, another patient was excluded because part of the lesion could not be shown by US due to the shadowing of ribs.
The age of the patients ranged from 45 to 68 years (mean 55.1 years). All patients had hepatitis virus-related liver cirrhosis (hepatitis B in eight patients, hepatitis C in one patient). The Child–Pugh score was A in two patients, B in six patients and C in one patient at the time of HIFU ablation. The diagnosis of needle-track seeding of HCC was made according to the following clinical standards: (1) previous history of percutaneous biopsy or percutaneous ablation of pathologically proven HCC; (2) development of an abdominal or chest wall lesion along the needle pathway after percutaneous interventional procedures; (3) the lesion showed enhancement on contrast-enhanced images consistent with needle-track seeding. Two patients had history of percutaneous 18-gauge cutting-needle biopsy of the liver tumour before transarterial chemoembolisation. Seven patients were previously treated by US-guided percutaneous thermal ablation using RF (n = 5) or MW (n = 2), of which four received percutaneous 18-gauge cutting-needle biopsy immediately before the placement of needle applicators. A palpable non-tender mass was found on the abdominal or chest wall at the insertion site of needle applicators 4–10 months after the percutaneous interventions. Three patients received a percutaneous biopsy of their lesion in other hospitals before referral to our department. To avoid possible further seeding, biopsy was not performed in the remaining six patients before HIFU ablation.
Two lesions were located on the abdominal wall, seven lesions were located on the right chest wall. The mean size of the nodule (the maximum diameter in three orthogonal directions) was 1.8 cm (range 1.1 to 2.6 cm) (). Three lesions were located in the layer of abdominal or chest muscle, six lesions were predominantly located in the abdominal or chest muscle layer with subcutaneous invasion. All lesions could be clearly seen as well-delineated hypoechoic nodules on US, which showed enhancement on contrast-enhanced magnetic resonance imaging (MRI).
Table I. Patient and tumour characteristics.
Equipment
A HIFU system (model JC; Chongqing Haifu Technology, Chongqing, China) was used to treat the seeding lesions using real-time US guidance. The system has been described in detail in previous studies Citation[9], Citation[11], Citation[12], Citation[15]. It can be operated by using one of several therapeutic transducers with different focal lengths. In this study the focused US energy is produced from a 20-cm diameter therapeutic transducer with a focal length of 120 mm operating at a frequency of 0.9 MHz. The focal region is 6.8 mm along the beam axis and 1.2 mm in the transverse direction. In the centre of the HIFU transducer, a 5–8 MHz linear array diagnostic US probe is mounted coaxially as the imaging unit of the system to provide real-time imaging for targeting. The integrated transducer is immersed in a water reservoir filled with degassed water maintained at a temperature of 12–15°C. Through computer control, the integrated transducer can be moved smoothly in six directions, including three orthogonal directions (x, y and z), rotation along the US beam axis (θ), and rotation along the long (γ) or short (φ) axis of the bed. During treatment, the HIFU beam was directed upward towards the seeding lesion.
HIFU ablation procedures
All patients were treated as outpatients in our department. No specific preparation was required before treatment. For lesions located on the abdominal wall, HIFU ablation was performed under conscious sedation by administration of fentanyl and midazolam via the peripheral vein. The patients were placed in a prone position with the abdominal skin in contact with the degassed water. According to our experience of HIFU ablation of liver cancer adjacent to ribs, the pain in the treated area is usually greater than that experienced during HIFU ablation of liver cancer behind the abdominal wall. Therefore, for better patient tolerance and immobilisation of the lesion, intravenous anaesthesia was administered by a combination of propofol and ketamine for patients with lesions located on the chest wall. The patients were carefully placed in a right decubitus position with the chest wall in contact with the degassed water. HIFU energy was applied through the intercostal space. During treatment, the patient's blood pressure, pulse, respiration rate, and peripheral oxygenation were monitored closely.
Real-time US was used to target the lesion by moving the integrated probe, the lesion and a surrounding 0.5-cm margin was divided horizontally into sections with 5 mm separation. An acoustic power of 200 to 400 W was used during HIFU ablation, the HIFU energy was intermittently applied, each individual shot lasted for one or two seconds to ablate one target spot. During HIFU ablation, the real-time US images obtained before and after each exposure were immediately compared to determine whether the echo changes, indicative of coagulation necrosis Citation[13–15], had covered the treated area. Generally, a power output of 200 W was used initially, if the treated area did not become hyperechoic on the US image after the energy exposure, the power output was increased in increments of 40 W until the treated spot become hyperechoic on the US image. After ablation of the spot, the transducer was moved, and a nearby spot was treated similarly. This process was repeated section by section, in successive sweeps from the deep to shallow regions until the entire nodule and a surrounding margin of at least 0.5 cm became hyperechoic on US. The HIFU treatment was performed by one doctor (W.W.).
Post-treatment observation and follow-up
After HIFU ablation, patients were carefully observed for possible complications such as skin burn and side effects such as pain, fever and pleural effusion. During the follow-up period, the treated area was examined by colour Doppler US and contrast-enhanced MRI at 1–3 months after HIFU ablation and then at a 1–3 months interval at the time when imaging examination was required to assess the therapeutic response or tumour progression in the liver. Areas of low signal intensity at MRI which did not enhance after contrast material administration were considered to represent necrotic tissue. Enhancing areas on the abdominal or chest wall were assumed to represent viable tumour. If residual or recurrent tumour on the abdominal or chest wall was detected, a further HIFU ablation session would be planned if the lesion still met the inclusion criteria.
Results
HIFU ablation was successfully performed in all patients. For patients with abdominal wall seeding lesions, piercing or burning sensation was felt in the treated area during each energy exposure; however, no patients complained of unbearable pain necessitating discontinuation of HIFU ablation. HIFU ablation was also performed smoothly for patients with chest wall seeding lesions. The treatment time ranged from 6 to 21 min (mean 12 min).
Because no skin burns were found in the treated area and the vital signs were stable, all patients were discharged after brief observation of 1–2 h. The treated area became slightly swollen, eight patients complained of mild pain in the treated area which subsided in 1–2 days requiring no analgesic medication. Post-ablation fever was encountered in no patient. No major complications were encountered after HIFU ablation.
The patients were closely followed up until 1 October 2009. During a mean follow up of 10.3 months (range 7–15 months) two patients died of HCC progression, however the treated lesion did not show enhancement on follow-up contrast-enhanced MRI. In six surviving patients, persistent absence of contrast enhancement was observed in the treated area, the treated lesion gradually shrank over time (). One surviving patient developed a recurrent lesion near the treated area four months after HIFU ablation. The new lesion was treated by a repeat session of HIFU ablation ().
Figure 1. A 56-year-old man with needle-track seeding of HCC on the abdominal wall, the lesion was located on the needle pathway of previous percutaneous RF ablation. (A) Before HIFU ablation, the lesion (arrow) showed enhancement on T1-weighted contrast-enhanced MRI. No enhancement was observed in the treated area (arrow) on T1-weighted contrast-enhanced MRI at one month (B), three months (C), and six months (D) after HIFU ablation.
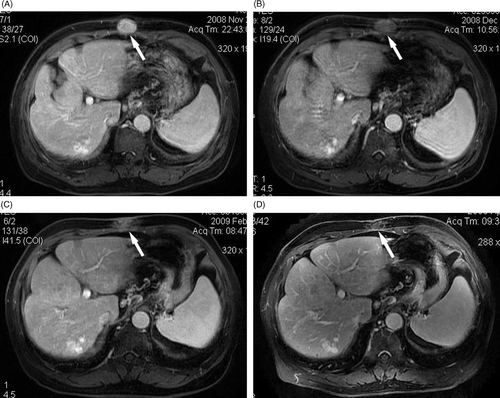
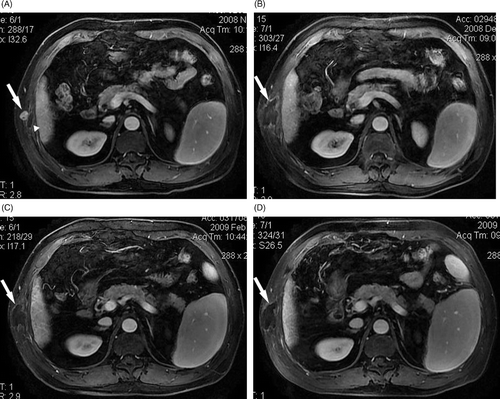
Figure 2. A 60-year-old man with needle-track seeding of HCC on the chest wall. Four months after the first HIFU ablation a recurrent lesion was detected adjacent to the treated area. (A) Before the repeat HIFU ablation a lesion (arrow) adjacent to the treated area of first HIFU ablation (arrowhead) was detected, which showed enhancement on T1-weighted contrast-enhanced MRI. No enhancement was observed in the treated area (arrow) at one month (B), three months (C), and seven months (D) after the repeat HIFU ablation.
Discussion
The incidence of needle-track seeding of HCC varies significantly amongst different studies. It ranges from 0% to an alarmingly high rate of 12.5% Citation[7], Citation[16]. The time interval between interventional procedure and the detection of seeding lesion ranges from several months to as long as 4 years Citation[17]. Possible risk factors for needle-track seeding include tumour location, tumour differentiation, multiple applicator placements and type of applicators Citation[7], Citation[18], Citation[19]. By taking prophylactic precautions, such as limiting the number of biopsies and cauterising the needle-track during removal of applicators, the incidence of needle-track seeding can be kept as low as less than 1% Citation[5], Citation[6], Citation[8]. However, if left untreated, it may change a potentially curable HCC into an untreatable situation via tumour dissemination to other organs or the peritoneum Citation[20].
Reported management of needle-track seeding of HCC included surgical resection Citation[3], Citation[6], Citation[7], Citation[8], transarterial embolisation Citation[21], percutaneous ethanol injection Citation[22], and percutaneous thermal ablation using RF Citation[23] or MW Citation[6]. Surgical resection is currently the first choice for treating the seeding tumour, though it carries the risk of haemorrhage, possible post-operative incision hernia, and tumour dissemination or recurrence if the resection margin is inadequate. Surgical resection is also the most invasive method. Some patients may not be surgical candidates due to poor medical condition Citation[8]. Transarterial embolisation of the feeding vessel of the seeding tumour may be technically challenging and it is difficult to yield a safety margin to eradicate possible microscopic tumour foci. Thus, it was rarely used for treatment of needle-track seeding of HCC. Percutaneous ablation using ethanol or thermal energy provides a less invasive alternative to surgical resection. These techniques could result in chemical or coagulative necrosis of the tumour; however, they require percutaneous placement of an applicator which may cause possible new tumour seeding along the needle pathway.
HIFU is a promising technique which offers effective ablation of tissue without the need for skin incision. HIFU ablation focuses an extracorporeal source of US to a specific target tissue. The US energy can pass harmlessly through overlying tissues en route to a tightly focused target area. The rate of energy deposition at the target tissue far exceeds the rate of heat dissipation, resulting in rapid temperature rise and subsequent coagulation necrosis Citation[13], Citation[14], Citation[15]. The ability to focus and accurately target a lesion with HIFU by using real-time imaging guidance allows precise ablation of lesions without damage to surrounding structures.
In this study HIFU was used for the treatment of needle-track seeding of HCC. Because the lesions were superficially located, we chose a therapeutic transducer with coaxially mounted high-frequency diagnostic probe for clear lesion detection and observation of the changes in lesion echogenicity during treatment. This ensured precise energy deposition and reliable real-time estimation of treatment response. Absence of lesion enhancement was obtained after scheduled HIFU ablations and no major complication occurred. These results suggest that, using accurate image guidance, HIFU may be safe and effective for treating the seeding lesion, it may present as a competitive alternative to surgical resection and percutaneous ablation. Compared with surgery, it leaves no incision on the abdominal or chest wall and has no haemorrhage. Therefore it could be more acceptable by the patients. Unlike other ablation techniques, it requires no percutaneous placement of applicators, so the risk of new treatment-related needle-track seeding could be avoided, By careful targeting of the lesion and manipulation of the focal area, superficially located lesions could also be ablated without irreversible skin burn. As long as the acoustic pathway is preserved, the procedure can be repeated as required. Residual or recurrent nodules can be readily treated. Because HIFU ablation is minimally invasive, repeatable and can be performed as an outpatient procedure, it may become an attractive option for treatment of needle-track seeding.
This study has some limitations. First, because of concerns for further needle-track seeding, most lesions were diagnosed by clinical means rather than pathology. Second, it was not a randomised controlled trial with surgical resection or percutaneous ablation, the patient number was small and the follow up was short. Third, HIFU ablation is fundamentally influenced by the acoustic pathway, the entire tumour had to be clearly visible on US. If part of the tumour was undetectable due to the acoustic shadowing of the ribs, it was unsuitable for HIFU ablation.
Conclusion
In conclusion, our preliminary results showed US-guided HIFU ablation may be effective for treatment of needle-track seeding of HCC. Further studies are warranted to observe its long-term efficacy and compare the results with those of other treatment options.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Colombo M. Hepatocellular carcinoma. J Hepatol 1992; 15: 225–236
- Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: A systematic review and meta-analysis. Gut 2008; 57: 1592–1596
- Chang S, Kim SH, Lim HK, Lee WJ, Choi D, Lim JH. Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: Evaluation of doubling time, frequency, and features on CT. Am J Roentgenol 2005; 185: 400–405
- Ishii H, Okada S, Okusaka T, Yoshimori M, Nakasuka H, Shimada K, Yamasaki S, Nakanishi Y, Sakamoto M. Needle track implantation of hepatocellular carcinoma after percutaneous ethanol injection. Cancer 1998; 82: 1638–1642
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003; 226: 441–451
- Liang P, Wang Y, Yu XL, Dong BW. Malignant liver tumors: Treatment with percutaneous microwave ablation–Complications among cohort of 1136 patients. Radiology 2009; 251: 933–940
- Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 2001; 33: 1124–1129
- Chang S, Kim SH, Lim HK, Kim SH, Lee WJ, Choi D, Kim YS, Rhim H. Needle tract implantation after percutaneous interventional procedures in hepatocellular carcinomas: Lessons learned from a 10-year experience. Korean J Radiol 2008; 9: 268–274
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Xie FL, Jin CB, Su HB, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med Biol 2004; 30: 245–260
- Gianfelice D, Khiat A, Boulanger Y, Amara M, Belblidia A. Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J Vasc Interv Radiol 2003; 14: 1275–1282
- Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR. High-intensity focused ultrasound for the treatment of liver tumors. Ultrasonics 2004; 42: 931–935
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advance pancreatic cancer. Radiology 2005; 236: 1034–1040
- Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: Current potential and oncologic applications. Am J Roentgenol 2008; 190: 191–199
- Haar GT, Coussios C. High intensity focused ultrasound: Past, present and future. Int J Hyperthermia 2007; 23: 85–87
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1099–1106
- Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, Francis IR. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. Am J Roentgenol 2006; 187: 1184–1187
- Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: Is needle biopsy of the liver always necessary?. Liver Transpl 2000; 6: 67–72
- Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, Sherman M, Grant DR, Greig PD, Gallinger S. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol 2005; 16: 485–491
- Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2005; 92: 856–858
- Liu YW, Chen CL, Chen YS, Wang CC, Wang SH, Lin CC. Needle tract implantation of hepatocellular carcinoma after fine needle biopsy. Dig Dis Sci 2007; 52: 228–231
- Shibata T, Shibata T, Maetani Y, Kubo T, Nishida N, Itoh K. Transcatheter arterial embolization for tumor seeding in the chest wall after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2006; 29: 479–481
- Tarantino L, Francica G, Esposito F, Pisaniello D, Parmeggiani D, Marzullo G, Sordelli IM, Sperlongano P. Seeding from hepatocellular carcinoma after percutaneous ablation: Color Doppler ultrasound findings. Abdom Imaging 2006; 31: 69–77
- Espinoza S, Briggs P, Duret JS, Lapeyre M, de Baère T. Radiofrequency ablation of needle tract seeding in hepatocellular carcinoma. J Vasc Interv Radiol 2005; 16: 743–746