Abstract
Our previous studies found small intestine epithelial tissues from several different animals (including rats, pigs and chickens) became significantly damaged following exposure to extreme heat. However, damaged tissue was rapidly repaired or regenerated in the following few days. Growth-related molecules are critical for cellular survival and promote endothelial cell proliferation and migration. The ERK1/2 signalling pathway is reported to regulate the growth and adaptation of endothelial cells to both physiological and pathological stimuli. However, little information is available concerning both growth-related molecules and ERK1/2 in response to heat stress. Herein, we employed both live rats and rat IEC-6 cells to investigate growth-related molecule expression and ERK1/2 activation in heat stress. Heat stress caused significant morphological damage to rat intestinal tissue and IEC-6 cells, reduced cell growth and proliferation, induced apoptosis, altered growth-related molecule mRNA expression and increased ERK1/2 phosphorylation. Addition of U0126 (a selective inhibitor of MEK kinase responsible for ERK phosphorylation) combined with heat stress exacerbated the morphological damage and apoptosis. With the addition of U0126, further up- or down-regulation of Egfr, Ctgf, Tgif, Vegfa, Okl38 and Gdf15 in response to heat stress was observed. In conclusion, extreme heat stress caused obvious damage to rat jejunum and IEC-6 cells. Both growth-related molecule expression and ERK1/2 phosphorylation were involved in response to heat stress. ERK1/2 inhibition exacerbated apoptosis and affected growth factor mRNA expression in heat stress.
Introduction
The intestinal epithelium is directly exposed to a large assortment of nutrients, microbes and exogenous toxins providing both a barrier and absorptive function Citation[1]. However, many stressors such as radiation, lipopolysaccharides (LPS), pharmacological drugs, endotoxins and heat can cause damage to the intestinal epithelium Citation[2]. When animals become exposed to environmental temperatures greater than their thermoneutral temperature, their peripheral blood flow is increased to dissipate internal body heat, thereby resulting in a significant reduction in blood flow to the small intestine Citation[3], Citation[4]. During this time, the epithelial tissue of the small intestine can experience ischaemic and hypoxic conditions, resulting in tissue damage, which, in turn, can lead to diarrhoea and an increased risk of morbidity and mortality Citation[5]. The intestinal epithelium has very rapid turnover rates as mature differentiated epithelial cells are shed into the lumen and are continuously replaced by the progeny of stem cells, so the damaged epithelial cells are continually and rapidly replaced by new cells, crucial for maintaining sufficient intestinal function Citation[6], Citation[7].
Growth-related molecules refer to naturally occurring agents capable of stimulating cellular growth, proliferation and cellular differentiation, which are important in regulating a variety of cellular processes Citation[8]. Growth-related molecules can promote epithelial proliferation and migration, as well as provide critical survival factors for epithelial cells Citation[9]. The extracellular-regulated kinase 1/2 (ERK1/2) signalling pathway is known as a critical regulator of cellular differentiation, proliferation, stress responsiveness, and apoptosis Citation[10]. ERK1/2 activation is understood to regulate cellular growth and adaptation in response to both physiological and pathological stimuli Citation[11]. Thus, investigating the expression of specific growth-related molecules and ERK1/2 activation may provide insight into the possible mechanisms underlying heat stress-induced damage and repair/regeneration in the small intestinal epithelium. The rat intestinal epithelial cell line (IEC-6), is a homogenous population of epithelial-like cells commonly used as a model to elucidate the mechanism of intestinal epithelial cell differentiation, growth, and wound healing Citation[12]. Using the rat IEC-6 cell line, we investigated cell morphology, viability, apoptosis, growth-related molecule mRNA expression, as well as ERK1/2 signalling in response to heat stress.
Materials and methods
In vivo studies
Ethical approval. All experimental protocols were approved by the Committee for the Care and Use of Experimental Animals, Beijing University of Agriculture.
Animal experimental groups. Forty-eight male Sprague-Dawley rats weighing 200 ± 20 g (obtained from Beijing Vital River Laboratory, Animal Technology Co., Beijing, China) were acclimated to 25°C, 60% relative humidity (RH) and maintained under a 12 h:12 h light:dark cycle. Food and water were provided ad libitum for 7 days. On day 8 the rats were randomly assorted into control or heat-stressed groups. Each group consisted of 24 rats, which were housed in plastic cages (400 mm × 300 mm × 180 mm) with a layer of soft woodchips. Feed (200 g) and water (400 mL) were provided daily.
Treatment. Rats in the control group were housed in a controlled environment (25°C, 60% relative humidity (RH)); while rats in the heat-stressed group were housed under control group conditions, but exposed to 40°C and 60% relative humidity between 11:00 am and 1:00 pm daily for ten consecutive days. On days 1, 3, 6 and 10, six rats from each group were sacrificed immediately following the 2-h heat exposure time period.
Sampling. Rat body temperature was recorded daily before and after heat exposure using a thermistor probe connected to a digital thermometer. The probe was inserted 40 mm into the rectum of each rat and the animal held stationary for 30 s to record the true rectal temperature. Body surface temperature was also recorded daily before and after heat exposure using both an infrared and contact thermometer (Fluke 561, Evered, Washington, USA). Blood samples collected were stored at 37°C for 60 min, prior to being centrifuged at 3000 g for 10 min; serum was collected and stored at −80°C until required. Following removal of the intestine, it was immediately irrigated with physiological saline to remove all intestinal contents before being sectioned into the duodenum (15 mm from the pylorus), the distal jejunum–ileum (half of the remaining small intestine up to the caecum) and the ileum (20 mm proximal to the caecum). Each intestinal section was divided into three pieces: one fixed in 10% buffered formalin phosphate for histological analysis; one for cDNA microarray analysis; and one stored at −80°C. Total serum cortisol concentration was determined using an iodine (I125) cortisol radioimmunoassay kit, performed according to the manufacturer's instructions (Beijing Chemclin Biotech).
Fixing intestinal sections and staining. Small intestine tissue samples were promptly rinsed with physiological saline and immediately fixed in 10% buffered formalin phosphate following removal from the animal. The formalin-fixed samples were embedded in paraffin and transversely sectioned (5 µm thick). After deparaffinisation and dehydration, the sections of duodenum, jejunum and ileum (HyClone, Logan, Utah, USA) were stained with hematoxylin and eosin (Sigma, St. Louis, MO, USA). Microstructures of the small intestine were observed using a BH2 Olympus microscope (DP71, Olympus, Tokyo, Japan) and analysed using an Olympus Image Analysis System (Olympus 6.0).
In vitro studies
IEC-6 cell culture. IEC-6 cells (CRL21592, obtained from Peking Union Medical College) were cultured in DMEM containing 5% (v/v) fetal bovine serum (HyClone, Logun, Utah, USA), 2 mg/L insulin, 50 IU/mL penicillin and 50 µg/mL streptomycin at 37°C and 5% (v/v) CO2. The medium was changed 24 h following initial cell plating.
Treatment and morphology observation. The control cells were strictly regulated at 37°C and 5% CO2, while heat-stressed cells were exposed to 42°C for 3h at 5% CO2. Changes in cell morphology following heat stress were observed using a phase-contrast inverted biological microscope (IX71/IX2, Olympus).
MTT and FACS assay. To measure cell proliferation, equivalent numbers of IEC-6 cells were plated on 96-well multiplates and cultured in DMEM containing 5% fetal bovine serum at a density of 1.2 × 105 cells/mL. After the cells were attached to the multiplate, control cells were maintained at 37°C, while heat-stressed cells were submitted to 42°C for 2, 3, 4 or 5 h. Following the heat stress period, 10 µL of MTT (10 mg/mL) was added to each well then incubated at 37°C for 4 h. Well media was aspirated and the formazan product dissolved using dimethyl sulphoxide. The remaining formazan product was analysed using a microplate reader (BIO-RAD, Foster, California, USA) at a fixed absorption wavelength of 570 nm. Using the MTT assay it was determined that subjecting the intestinal cells to 42°C for 3 h caused the greatest level of stress.
To analyse apoptosis, IEC-6 cells were divided into control (37°C), heat-stressed (42°C for 3 h), and ERK1/2 inhibitor (42°C for 3 h + 20 µmol/L U0126 Tocris Bioscience, St. Louis, Missouri, USA) groups. Following each specific treatment, all IEC-6 cells were stained with annexin V/propidium iodide (PI) (Invitrogen, Carlsbad, California, USA), before FACS analysis was performed according to manufacturer's instructions (Beckman, Fullerton, California, USA).
Total RNA expression and reverse transcription. Total RNA was isolated from the small intestine and IEC-6 cells using a phenol and guanidine isothiocyanate-based TRIzol® reagent (Invitrogen). RNA concentration and purity were assessed using a spectrophotometer (SmartSpec plus, BIO-RAD) utilising the OD260/OD280 ratio. Total RNA was reverse transcribed as follows: 2 µg of RNA isolated from each sample was added to 25 µL of reaction solution comprising 2 µL oligo-dT18, 5 µL dNTP, 1 µL RNase inhibitor, 1 µL M-MLV transcriptase, 5 µL M-MLV reverse transcription (RT) reaction buffer (Promega, Madison, Wisconsin, USA) and 11 µL RNase-free water. The parameters for the reverse-transcription procedure, based on the manufacturer's instructions (Promega), were: 70°C for 5 min followed by 42°C for 2 h. The RT products (cDNA) were stored at −20°C for subsequent PCR.
DNA microarray
RNA extraction and target labelling. Total RNA was isolated from small intestine tissue using a phenol and guanidine isothiocyanate-based TRIzol reagent in accordance with manufacturer's instructions. RNA quality of each sample was determined and recorded using an RNA 6000 LabChip Kit, and the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA consistently had a 28S/18S ratio of ∼1.4. RNA was purified using a QIAGEN RNeasy ® Mini Kit (74106, QIAGEN, Valencia, California, USA) and amplified using a low RNA input linear amplification kit (5184-3523, Agilent). Each RNA sample was annealed with a primer containing a poly-dT and a T7 polymerase promoter. Reverse transcriptase produced primary and secondary cDNA strands. T7 RNA polymerase was then used to create cRNA from the double stranded cDNA by incorporating cyanine-3-labelled cytidine 5-triphosphate. The quality of the labelled cRNA was again verified and the absolute concentration was measured using a spectrophotometer (Nanodrop ND1000, Wilmington, Delaware, USA).
Hybridisation, scanning and feature extraction. The cRNA was hybridised in equal amounts to six arrays using a gene expression hybridisation kit (5188-5242, Agilent). Hybridisation was performed at 60°C for 17 h with Agilent whole rat genome arrays (G4131F, Agilent). The arrays were washed using a gene expression wash buffer kit (5188-5327, Agilent), Stabilisation and dehydration was performed (5185-5979, Agilent). The arrays were scanned on a Microarray (G2565BA, Agilent) and the subsequent data compiled with Agilent feature extraction software. All steps from RNA amplification to the final scanner output were conducted by a private contractor (Shanghai Biochip, China).
Microarray data analysis. Array normalisations and error detection were carried out using Silicon Genetics’ GeneSpring GX Version 10.0 (Agilent), via the enhanced Agilent feature extraction import preprocessor. First, values of poor quality intensities and low dependability were removed using a ‘filter on flags’ feature, where standardised software algorithms determined which spots were ‘present’, ‘marginal’, or ‘absent’. Spots were considered ‘present’ only when the output was uniform, non-saturated and significantly greater than the background. Spots that satisfied these requirements but were deemed outliers relative to the typical values for the other genes were considered ‘marginal’. Filters were set to retain only the present and marginal values for further analysis. Data were normalised using algorithms supplied with the feature extraction software. After data normalisation, a final quality-control filter was applied, where genes expressing excessive biological variability were discarded. Data were further analysed using GeneSpring to reveal significant genes.
Validation of growth factor mRNA expression using real time PCR
To corroborate the relative changes in gene expression in response to heat stress both in vivo and in vitro identified by the oligonucleotide microarrays, we employed real time quantitative reverse transcriptase-PCR (RT-PCR). Quantitative PCR analysis was carried out using the DNA Engine Mx3000P® fluorescence detection system (Stratagene, La Jolla, California, USA) according to optimised PCR protocols, and using Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, California, USA), containing a double-stranded DNA-specific fluorescent dye. β-actin was always amplified in parallel with the target gene. The cDNA of each sample was subjected to real-time RT-PCR using the primer pairs listed in . The PCR reaction system (20 µL in total) comprised of 10 µL SYBR Green qPCR mix, 0.3 µL reference dye, 1 µL of each primer (both 10 µmol/L), and 1 µL of cDNA template. Cycling conditions were: 94°C for 10 min, followed by 40 cycles of 94°C for 30 s, 56°C for 30 s and 72°C for 40 s. Dissociation was initiated by 95°C for 1 min, and the melting curve (from 55°–95°C at 0.2°C/s) was measured continuously by fluorescence. Expression levels were determined using the relative threshold cycle (CT) method as described by the manufacturer (Stratagene). Each gene was calculated by evaluating the expression 2ΔΔCT, where ΔΔCT is the result of subtracting heat-stressed [CTgene–CTβ-actin] from control [CTgene–CTβ-actin].
Table I. Primers used for PCR.
SDS-polyacrylamide gel electrophoresis and western blotting
Protein from rat jejunum and IEC-6 cells was extracted using a total protein extraction kit (K3011010, Biochain, Hayward, California, USA) and quantified using a BCA protein assay kit (23225, Pierce, Rockford, USA). 30 µg of total protein was loaded and electrophoresced for 40 min at 200 V, before being transferred onto nitrocellulose membranes (88585, Pierce, Rockford, USA). Blots were blocked overnight at 4°C in SuperBlock T20 (TBS) blocking buffer (37536, Pierce, Rockford, USA). Phospho-ERK1/2, ERK1/2 (4376, 4695, CST, MA, USA) and Actin (SC-1615, Santa, USA) antibodies were added to the block buffer at 1:1000 dilutions and incubated for 2 h under agitation. Blots were washed in PBST20 for 5 min with shaking. Goat anti-rabbit IgG-HRP (SC-2004, Santa) and donkey anti-goat IgG-HRP (SC-2033, Santa) secondary antibodies were diluted to 1:500 and incubated for 2 h under agitation. Blots then were washed in PBST20 for 5 min with shaking and visualised using a SuperSignal® West Pico Chem luminescent substrate (34080, Pierce, Rockford, USA). Blots then were exposed to X-ray film (Kodak, USA).
Statistical analysis
All results are presented as mean ± SD. Statistical analysis was performed by independent-sample T-tests using SPSS 12.0 software. A p-value of less than 0.05 was considered to indicate a significant difference. Microarray analysis was conducted using GeneSpring GX_10.0.
Results
In vivo studies
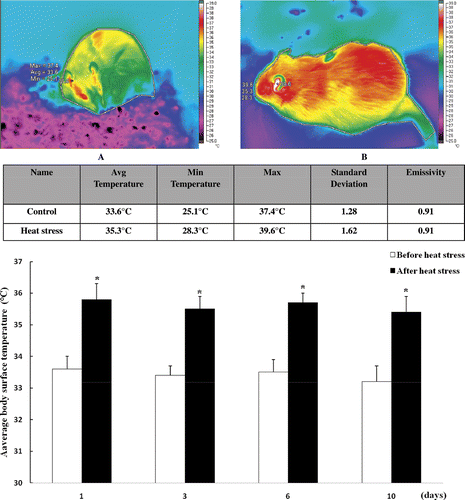
Assessment of heat treatment. Changes in body temperature and serum glucocorticoid levels were used as primary criteria for indicating heat stress in mammals. Therefore, rectal temperature, surface temperature and cortisol concentration were recorded to compare the changes in response to heat treatment. Rat rectal and body surface temperatures were found to be significantly increased after 2 h of heat treatment ( and ). Serum cortisol levels were significantly increased in heat-stressed rats compared with control rats on days 1, 3 and 6; however, no significant difference was found on the 10th day of heat stress (). The significantly elevated body temperature and serum glucocorticoid levels after heat exposure indicate the rats underwent conditions of significant heat stress. There was no significant difference in serum cortisol levels on day 10 after heat treatment revealing the animals had adapted to heat stress.
Figure 1. (A) Rat rectal temperature before and after heat treatment. Rat rectal temperatures were significantly elevated following 2 h heat exposure at 40°C. (B) Cortisol concentrations from the heat-stressed group were significantly higher than those of the control group on day 1, 3 and 6 after heat treatment. Cortisol concentration was greatest on day 3 before returning to levels equal to the control group by day 10. Values represent the mean ± SE, n = 6 rats for each group. *P < 0.05 for the heat-stressed group versus the control group.
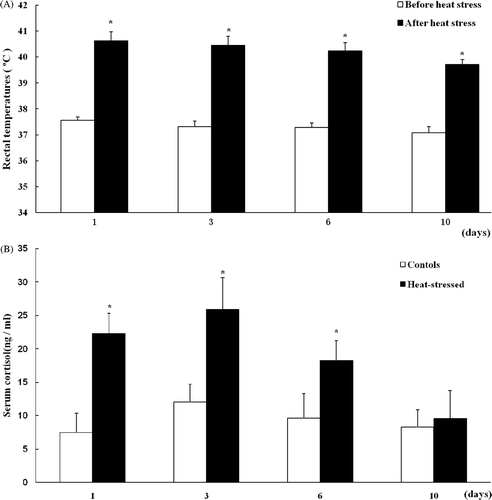
Figure 2. Body surface temperature of rats before (A) and after (B) heat stress. Rat body surface temperature was significantly increased after 2 h heat exposure at 40°C. Values represent the mean ± SE, n = 6 rats for each group. *P < 0.05 for the temperature after heat stress versus before heat stress.
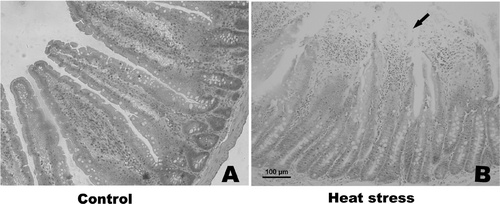
Histological changes in the small intestine following heat stress. To investigate histological changes in the small intestine due to heat treatment, sections of duodenum, jejunum and ileum were stained with hematoxylin and eosin, and the histological structure was observed using microscopy. Bleeding in the intestinal villi and desquamation at the tips of the intestinal villi were observed in the small intestine after heat treatment. The desquamation of mucosal epithelial cells exposed the lamina propria, with such damage found to be most severe in the jejunum on day 3 of the experiment (). Thus, the samples of jejunum taken on day 3 were used for microarray analysis.
Figure 3. Photomicrographs of hematoxylin and eosin-stained sections of rat jejunum on day 3 (200× magnification). (A) control; (B) heat treatment (on day 3). Severe damage to rat intestinal villi is apparent, with desquamation at the tips of the intestinal villi and the lamina propria is exposed. Abnormal microstructures are indicated with arrowheads. Scale bar represents 100 µm.
Microarray data analysis. To gain insight into the effects of heat stress on gene expression in rat jejunum we performed six rat genome microarrays. Genes known to have a significant role in the defence response and regulate cell growth are listed in . The expression levels of genes related to defence response including Hsps, Abcb11, Herpud1, Bcl2l1, Abcc2, Stip1, Ddit3, Prkab1 and Cryab were all significantly increased after heat exposure. Expression levels of genes related to cell proliferation, differentiation and growth including Gdf15, Gdf9, Pdgfa, Vegfa, Okl38 and Xrn2_ were all up-regulated, while Alb, Bmp2, Casp3, Ccl2, Ccne1, Egfr, Fgfr2, Mmp9, Igfbp3 were all down-regulated. Hierarchical clustering was conducted using GeneSpring GX_10 (Distance metric: Pearson centred; Linkage rule: average linkage) and are listed in . To examine gene ontology, including biological processes, cellular components, molecular functions, and genetic networks, the data obtained were analysed using Ingenuity Pathways Analysis tools. We predicted that the interaction of growth-related molecules detected in the current study will provide insight into the possible mechanisms of heat stress-induced cellular damage and regeneration ().
Figure 4. Hierarchical clustering of dysregulated genes conducted using GeneSpring GX. Control groups comprise: Control A, Control B and Control C. Heat-stressed groups comprise: SJ G, SJ H, and SJ I. Red and green colours in the heat map represent up-regulation and down-regulation relative to control, respectively.
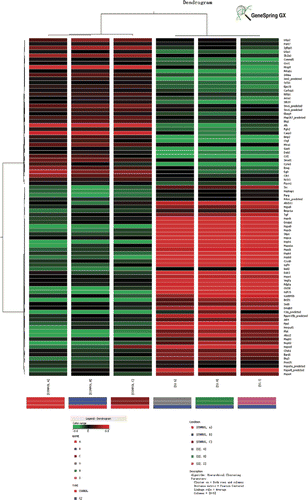
Figure 5. Gene network map of growth-related molecules including Alb, Egfr, Fgfr2, Tgif, Pdgfa, Vegfa, Gdf-9, Okl18, Ctgf and Gdf-15 by GeneSpring GX_10.0 based on the database.
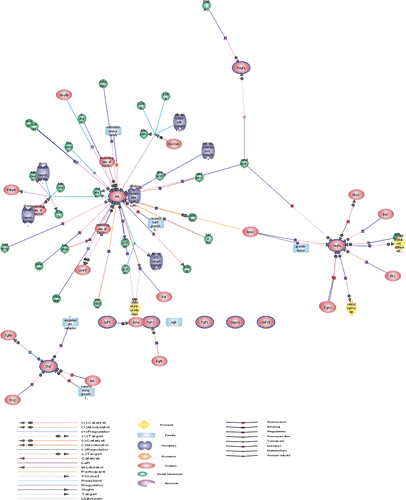
Table II. Microarray analysis of the effects of heat stress on cell growth-related gene expression profiles in rat jejunum analysed on day 3 following heat exposure.
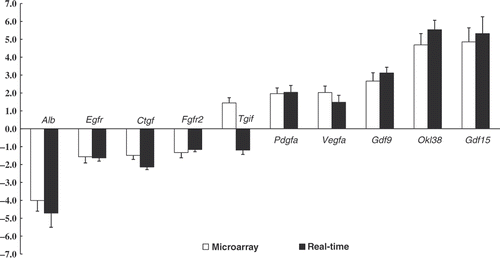
Validation of growth-related molecules mRNA expression. To validate the changes in expression of growth-related genes which had significant variation in cDNA microarray analysis, ten selected genes were confirmed by real-time quantitative RT-PCR and displayed in . The one exception to the trend was Tgif RNA expression, which may have been due to the poor specificity of the Tgif probe in the microarray. The change in gene expression levels of most genes was found to be closely correlated with the corresponding microarray data, although the exact fold change differed between the two assays.
In vitro experiment
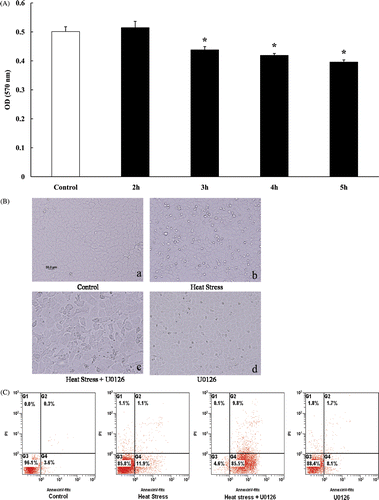
Cell viability, morphology and apoptosis due to heat treatment. IEC-6 cells were subjected to 42°C, based on our previous study. The growth-inhibitory effect of heat treatment was measured using the MTT assay. Cellular proliferation and viability were significantly reduced after exposure to 42°C for 3 h, 4 h, and 5 h but not 2 h (), therefore, 3 h of exposure to 42°C was chosen as the treatment for IEC-6 cells in the following experiments. Following heat exposure, IEC-6 cells were examined under the phase-contrast inverted microscope. The morphology was markedly altered, differing in cell shape, and a greater dead cell mass was clearly observed in the supernatant (). To detect and measure apoptosis, IEC-6 cells were stained with annexin V/propidium iodide (PI) and submitted to FACS analysis. Apoptosis significantly increased after heat stress, which was exacerbated with the presence of U0126 in rat IEC-6 cells (). The results indicated that heat treatment reduced cell viability, caused cell damage and induced apoptosis, ERK1/2 inhibition exacerbated cell damage and apoptosis in the heat stress group.
Figure 7. (A) Cell proliferation and viability were measured by MTT analysis after IEC-6 cells were submitted to 42°C for 2, 3, 4 and 5 h respectively. Compared with the control group, cell proliferation and viability were significantly reduced following heat stress for 3, 4, and 5 h, but no significant difference for 2 h. (B) Photomicrographs of IEC-6 cells demonstrate gross cell morphology (200× magnification). a: control, b: heat stress (42°C for 3 h), c: U0126 (20 µmol/L) combined with heat stress (42°C for 3 h) d: U0126 (20 µmol/L) without heat stress. (C) Cell apoptosis analysis displaying control, and heat stressed cells (42°C for 3 h) with or without U0126 (20 µmol/L), after treatment all attached cells were collected and stained with annexin V/propidium iodide for apoptosis analysis. Cell apoptosis was significantly increased after heat stress and could be augmented by the addition of U0126.
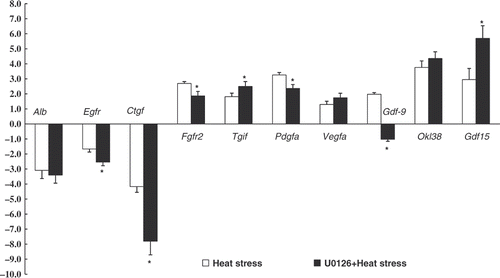
Growth-related molecules mRNA expression. mRNA expression levels of growth-related molecules in control and heat stressed rat IEC-6 cells were evaluated using real time PCR. The results indicated that Fgfr2, Tgif, Pdgfa, Vegfa, Gdf15, Okl38 and Gdf9 were all significantly up-regulated, while Alb, Egfr and Ctgf were significantly down-regulated after heat exposure. To investigate whether ERK1/2 inhibition influence on growth-related molecule mRNA expression during heat stress, IEC-6 cells were subjected to heat combined with U0126. There was a further up- or down-regulation of Egfr, Ctgf, Tgif, Vegfa, Okl38 and Gdf15 in response to heat stress with the addition of U0126 (), indicating ERK1/2 signalling can modulate growth-related molecule mRNA expression during heat stress.
Figure 8. Relative mRNA expression of growth factors in rat IEC-6 cells analysed by real-time PCR. Group protocols included: control, (cultivated in 37°C) heat stress (exposure to 42°C for 3 h), U0126 (20 µmol/L)+heat treatment. Values represent the mean ± SE, n = 6 for each group. *P < 0.05 for the heat-stressed group versus the control group.
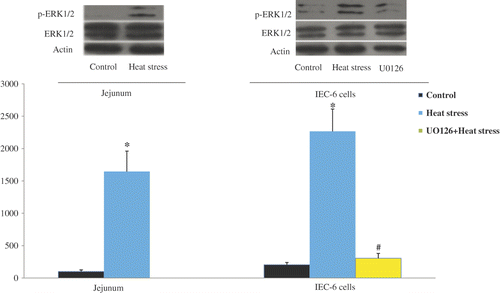
Activation of the ERK1/2 signaling pathway in rat jejunum and IEC-6 cells due to heat treatment. To investigate whether ERK1/2 was activated after heat treatment both in vivo and in vitro, ERK1/2 phosphorylation in rat jejunum and IEC-6 cells was measured by western blot analysis. Heat treatment was found to significantly increase phosphorylation of ERK1/2, reversible by the addition of U0126 ().
Figure 9. ERK1/2 phosphorylation in rat jejunum and IEC-6 cells was determined using western blotting. Cells underwent the following protocols: control, cultivated in 37°C; heat stress, temperature elevated to 42°C for 3 h; inhibition group, application of U0126 combined with heat stress (42°C for 3 h). Heat stress significantly induced ERK1/2 phosphorylation, which was abolished when U0126 was applied. Values represent the mean ± SE, n = 6 for each group. *P < 0.05 for the heat-stressed group versus the control group, *P < 0.05 for the U0126 group versus the heat-stressed group.
Discussion
Heat stress caused damage to rat intestine and IEC-6 cells
The small intestine plays a critical role both in providing a barrier to pathogens and an absorptive function Citation[1]. However, many stressors such as radiation, lipopolysaccharides (LPS), pharmacological drugs, endotoxins and heat can cause damage to the intestinal epithelium Citation[2]. Focusing on heat stress, when mammals are exposed to environmental temperatures greater than their thermoneutral temperature internal body heat is dissipated by increased peripheral blood flow which, in turn, dramatically reduces blood flow to the GI tract Citation[4], Citation[13]. The ischaemia and hypoxia experienced by the small intestine can result in necrosis and shedding of epithelial cells of the intestinal villi. Intestinal epithelial cell damage may disrupt the intestinal barrier and reduce absorptive function. In the present study, we found obvious damage in rat small intestine, and such damage was most severe in the jejunum within three days of initiation of treatment (40°C, RH 60% for 2 h), consistent with our previous studies Citation[6], Citation[7]. Furthermore, we employed a rat intestinal epithelial cell line (IEC-6) to analyse cell morphology, viability, apoptosis, growth-related molecule mRNA expression, as well as ERK1/2 signalling in response to heat stress in vitro. The effect of heat stress on IEC-6 cells was previously found to be dependent on temperature and duration of heat treatment. Based on previous experiments subjecting IEC-6 cells to between 40°C and 43°C for 2 to 5 h, a heat treatment protocol of 42°C for 3 h was chosen for the current in vitro study. Clear morphological changes were present in IEC-6 cells after heat treatment, supporting previous reports of cell damage following several different stressors Citation[14].
The MTT assay and FACS analysis determined that heat treatment caused a significant reduction in cell viability and induced apoptosis in cultured IEC-6 cells. Cells possess the capability to self-induce programmed cell death (apoptosis) in response to environment and physical stress Citation[15–17]. Oxidative stress, hyperthermia, and irradiation-induced apoptosis have been observed in many different types of cells Citation[18–20]. The ratio of apoptotic to healthy cells was found to be significantly increased during the early period of heat stress compared with control (11.9% versus 3.6%, respectively). More interesting, addition of a specific ERK1/2 inhibitor (U0126) augmented heat stress-induced apoptosis to 85% of total cells, indicating ERK1/2 activation may be vital for cellular survival during heat stress.
Heat stress affected growth factor mRNA expression and induced ERK1/2 activation
Heat stress results in a complex cellular response including alterations in gene expression and biochemical adaptations. It is now widely accepted that changes in gene expression are an integral part of the cellular response to heat stress Citation[21–23]. In the present study, mRNA expression of the genes associated with the stress and defence response, as well as cellular growth and proliferation, were significantly altered after heat stress both in vivo (in the rat jejunum) and in vitro (in rat IEC-6 cells). Growth-related molecules (including Alb, Egfr, Ctgf, Fgfr2, Pdgfa, Tgif, Vegfa, Gdf15 and Gdf9) are a group of biologically active polypeptides which function as hormone-like regulatory signalling molecules, controlling cell survival, growth, differentiation, adhesion, migration, tumour angiogenesis, wound healing and tissue repair Citation[24]. Growth-related molecules are also reported to regulate and activate cellular mitogenic and stress-associated signalling transduction pathways, leading to changes in gene expression controlling various cellular functions Citation[9], Citation[25]. For example, The epidermal growth factor receptor (EGFR) is strongly associated with healing of damaged gastric mucosa, repairing and regenerating the protective tissue Citation[26], activated by a variety of non-specific stimuli such as ionising radiation, UV-radiation, hypoxia and oxidative stress Citation[27], Citation[28]. In the present study, Egfr mRNA expression was down-regulated, both in vivo and in vitro, supporting our previous findings in heat-stressed pig jejunum Citation[6]. Both GDF-9 and GDF-15 are novel members of the TGF-β superfamily associated with cell proliferation, differentiation, extracellular matrix formation, and immune defence Citation[29], Citation[30]. Recently, GDF-15 has been reported to provide cardioprotection following ischaemia/reperfusion injury Citation[31]. In the current study, both GDF-9 and GDF-15 were found to be up-regulated after heat treatment, indicating they may play an important role in heat stress. Moreover, comparing the growth-related gene expression between in vivo (jejunum) and in vitro (IEC-6 cells) experiments after heat treatment; the expression patterns of Alb, Egfr, Ctgf, Pdgfa, Tgif, Vegfa, Gdf9 and Gdf15 correlated significantly, but the expression patterns of Fgfr2 and Tgif did not. The jejunum contains many distinct types of cells and, although the IEC-6 cell line is commonly used as a model to elucidate the mechanisms underlying intestinal epithelial cell differentiation, growth, and wound healing, some differences were apparent in gene expression in response to heat stress between the jejunum and IEC-6 cells.
The extracellular-regulated kinases MAPK p42 and p44 (ERK1/2) are from the family of classical mitogen-activated protein kinases (MAPK) which respond to extracellular stimuli and regulate various intracellular processes including gene expression, mitosis, cell differentiation, cell proliferation, cell survival and apoptosis. ERK1/2 can be activated by various stimuli including cytokines, irradiation, heat stress, and osmotic stress. Activated ERK1/2 then, in turn, phosphorylate a diverse array of their substrate proteins Citation[10], Citation[19]. In the present study, phosphorylation of EK1/2 was significantly increased following heat exposure both in vivo and in vitro, indicating the ERK1/2 signalling pathway to be activated in response to heat stress.
Inhibition of ERK1/2 phosphorylation augmented heat stress-induced cell apoptosis and altered growth factor mRNA expression
Activation of ERK1/2 can result in both protective and detrimental effects to the cell Citation[32]. For example, ERK1/2 activation protects cells from a variety of cytotoxic insults, including death receptor activation, growth factor withdrawal, DNA damage, oxidative stress and hypoxia. However, several recent studies have suggested that ERK1/2 activation is implicated in apoptotic cell death induced by glutamate, reactive oxygen, and reactive nitrogen species in several types of cells Citation[33–36]. Therefore, ERK1/2 activation may stimulate different biological outcomes dependent on cell type and the specific stimulus. To further investigate whether ERK1/2 activation was involved in cell damage and apoptosis during heat stress, we observed the effect of administering U0126 (a selective MEK kinase inhibitor) during heat stress. Western blot analysis revealed that heat-stress induced phosphorylation of ERK1/2 was abolished by U0126. In turn, inhibition of ERK1/2 phosphorylation exacerbated cell damage and dramatically elevated cell apoptosis (85%, determined by FACS assay) in heat stress, indicating ERK1/2 activation may play a role in cell survival in rat IEC-6 cells during heat stress, but further studies are required. Inhibiting ERK1/2 also altered several growth-related molecules’ mRNA expression in IEC-6 cells after heat exposure; we speculated that ERK1/2 signalling may provide a critical regenerative role where heat stress-induced damage has occurred through activating regulated cellular growth, proliferation and cellular differentiation.
In conclusion, we revealed heat stress caused obvious damage to rat intestines and IEC-6 cells, reduced cell growth and proliferation viability, induced cell apoptosis, altered growth-related molecule mRNA expression and elevated ERK1/2 phosphorylation. ERK1/2 inhibition exacerbated apoptosis and affected growth factor mRNA expression in heat stress. Further work investigating these genes at the protein level is essential in order to determine whether the altered gene expression is related to changes in the level of proteins, and in their activity or signal pathways.
Acknowledgements
We are thankful for the help from the members of CAU-BUA TCVM teaching and research team.
Declaration of interest: This work was supported by grants from the National Natural Science Foundation of China (No.30771566), Learning Innovative Group Programs of Beijing Education Committee, Beijing Natural Science Foundation (No.6082007) and the National Eleventh Five-Year Scientific and Technological Support Plan (No. 2008BADB4B01, 2008BADB4B07).
References
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9: 799–809
- Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis 2009; 27: 443–449
- Yoshitake S, Noguchi T, Hoashi S, Honda N. Changes in intramucosal pH and gut blood flow during whole body heating in a porcine model. Int J Hyperthermia 1998; 14: 285–291
- Kregel KC, Wall PT, Gisolfi CV. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol 1988; 64: 2582–2588
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 2001; 280: H509–521
- Liu F, Yin J, Du M, Yan P, Xu J, Zhu X, Yu J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling. J Anim Sci 2009; 87: 1941–1949
- Yu J, Yin P, Liu F, Cheng G, Guo K, Lu A, Zhu X, Luan W, Xu J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp Biochem Physiol A: Mol Integr Physiol 2010; 156(1)119–128
- Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol 2009; 77: 508–520
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000; 97: 11307–11312
- Cheung EC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE 2004; 2004: E45
- Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA 2007; 104: 14074–14079
- Wood SR, Zhao Q, Smith LH, Daniels CK. Altered morphology in cultured rat intestinal epithelial IEC-6 cells is associated with alkaline phosphatase expression. Tissue Cell 2003; 35: 47–58
- Deja M, Ahlers O, Macguill M, Wust P, Hildebrandt B, Riess H, Kerner T. Changes in hepatic blood flow during whole body hyperthermia. Int J Hyperthermia 2010; 26: 95–100
- Roti RJ, Pandita RK, Mueller JD, Novak P, Moros EG, Laszlo A. Severe, short-duration (0-3 min) heat shocks (50–52°C) inhibit the repair of DNA damage. Int J Hyperthermia 2010; 26: 67–78
- Concordet JP, Ferry A. Physiological programmed cell death in thymocytes is induced by physical stress (exercise). Am J Physiol 1993; 265: C626–629
- Roti RJ. Cellular responses to hyperthermia (40–46°C): Cell killing and molecular events. Int J Hyperthermia 2008; 24: 3–15
- Wen D. Intracellular hyperthermia: Nanobubbles and their biomedical applications. Int J Hyperthermia 2009; 25: 533–541
- Borkamo ED, Dahl O, Bruland O, Fluge O. Global gene expression analyses reveal changes in biological processes after hyperthermia in a rat glioma model. Int J Hyperthermia 2008; 24: 425–441
- Sharif-Khatibi L, Kariminia A, Khoei S, Goliaei B. Hyperthermia induces differentiation without apoptosis in permissive temperatures in human erythroleukaemia cells. Int J Hyperthermia 2007; 23: 645–655
- Yu DY, Zhao QL, Wei ZL, Shehata M, Kondo T. Enhancement of hyperthermia-induced apoptosis by sanazole in human lymphoma U937 cells. Int J Hyperthermia. 2009; 25: 364–373
- Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 2005; 67: 225–57
- Tabuchi Y, Takasaki I, Wada S, Zhao QL, Hori T, Nomura T, Ohtsuka K, Kondo T. Genes and genetic networks responsive to mild hyperthermia in human lymphoma U937 cells. Int J Hyperthermia 2008; 24: 613–622
- Kokura S, Adachi S, Mizushima K, Okayama T, Hattori T, Okuda T, Nakabe N, Mahabe E, Ishikawa T, Handa O, et al. Gene expression profiles of diabetic mice treated with whole body hyperthermia: A high-density DNA microarray analysis. Int J Hyperthermia 2010; 26: 101–107
- Drucker DJ. Glucagon-like peptides: Regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003; 17: 161–171
- Foncea R, Carvajal C, Almarza C, Leighton F. Endothelial cell oxidative stress and signal transduction. Biol Res 2000; 33: 89–96
- Alimandi M, Wang LM, Bottaro D, Lee CC, Kuo A, Frankel M, Fedi P, Tang C, Lippman M, Pierce JH. Epidermal growth factor and betacellulin mediate signal transduction through co-expressed ErbB2 and ErbB3 receptors. Embo J 1997; 16(18)5608–5617
- Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology 1996; 110: 489–497
- Yang ZB, Yan J, Zou XP, Yi SX, Chang XR, Lin YP, Li XP. Enhanced expression of epidermal growth factor receptor gene in gastric mucosal cells by the serum derived from rats treated with electroacupuncture at stomach meridian acupoints. World J Gastroenterol 2006; 12: 5557–5561
- Bottner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene 1999; 237(1)105–111
- Wu X, Matzuk MM. GDF-9 and BMP-15: Oocyte organizers. Rev Endocr Metab Disord 2002; 3: 27–32
- Kelly JA, Lucia MS, Lambert JR. p53 controls prostate-derived factor/macrophage inhibitory cytokine/NSAID-activated gene expression in response to cell density, DNA damage and hypoxia through diverse mechanisms. Cancer Lett 2009; 277: 38–47
- Hindley A, Kolch W. Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. J Cell Sci 2002; 115: 1575–1581
- Kim GS, Choi YK, Song SS, Kim WK, Han BH. MKP-1 contributes to oxidative stress-induced apoptosis via inactivation of ERK1/2 in SH-SY5Y cells. Biochem Biophys Res Commun 2005; 338: 1732–1738
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001; 81: 807–869
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. Iubmb Life 2006; 58: 621–631