Abstract
Purpose: Studies were conducted to test whether fever-range whole body thermal therapy would boost the efficacy of oxaliplatin chemotherapy without substantial toxicity.
Materials and methods: The effect of mild heat (40°C) on oxaliplatin cytotoxicity, cellular uptake, and platinum-DNA adduct formation was studied in vitro using the MTLn3 tumour cell line. In vivo oxaliplatin was administered at various doses and times before, during and after fever-range thermal therapy (6 h at 40°C) to rats bearing an MTLn3 mammary adenocarcinoma. Tumour growth, survival, and toxicity were measured to determine treatment outcome.
Results: Heating halved the oxaliplatin IC-50 dose for MTLn3 cells. Cellular uptake of platinum and platinum adducts increased by 34% and 36%, respectively, with heat. In vivo, 50% of all rats given 10 mg/kg oxaliplatin 24 h before thermal therapy were completely immunologically cured, while a further 11% regressed their primary tumour but ultimately succumbed to metastases, and 17% experienced a limited response with increased survival. The curative response occurred only in a narrow range of doses, with most cures at 10 mg/kg. Thermochemotherapy-treated, but uncured, animals had delayed incidence and slowed growth of metastases. Anti-tumour efficacy was greatest, and toxicity was least, when oxaliplatin was administered 12 or 24 h before fever-range whole body thermal therapy.
Conclusions: When properly dosed and scheduled, oxaliplatin thermochemotherapy achieved permanent eradication of all primary and metastatic tumours in 50% of animals, seemingly through an immune response. Successful clinical translation of this protocol would yield hitherto unseen cures and substantial improvement in quality of life.
Introduction
The holy grail of cancer therapy is to effect complete cures or at least durable cancer responses. While surgery, radiation, and chemotherapy, singly or in combination, can achieve complete responses in many localised primary cancers, rarely is a durable disease-free state achieved in patients with advanced metastatic cancers. This paper presents pre-clinical studies in an orthotopic rat model of metastatic breast cancer, closely resembling clinical inflammatory breast cancer, that reveal the curative potential of a multi-modality treatment combining whole body thermal therapy (hyperthermia) with chemotherapy.
Thermal therapy, or hyperthermia, for cancer treatment was originally developed on the premise that tumour cells have intrinsically higher heat sensitivity than normal cells. This is not universally true, and clinical hyperthermia may result in tumour cell kill as much because of heat effects on tumour microvasculature as due to direct thermal destruction of tumour cells Citation[1]. Hyperthermia is also a radiosensitiser Citation[2], and loco-regional hyperthermia is now an established adjunct to radiotherapy in breast Citation[3–5] as well as other cancers Citation[6], Citation[7]. Whole body thermal therapy, the deliberate heating of the entire body to achieve an elevated core temperature, can treat not only primary tumours but also metastatic disease. By increasing systemic blood flow, increasing tumour blood vessel permeability Citation[8], Citation[9], and decreasing tumour interstitial pressure Citation[10], Citation[11], whole body hyperthermia enhances the delivery of chemotherapy agents to tumours, sometimes directly augmenting the mechanism of drug action Citation[2], Citation[12]. Combined with chemotherapy against cancer, systemic temperatures of 39.5°C–40.5°C maintained for 4–6 hours, much like a fever, result in better or equal anti-tumour efficacy than maximally tolerated systemic thermal therapy, generally result in less toxicity, and reduce the requirements for intensive patient care Citation[13], Citation[14]. Studies of numerous chemotherapy drugs combined with fever-range whole body thermal therapy in animal models have defined effective combinations Citation[15], Citation[16] and have led to a number of successful clinical whole body thermochemotherapy regimens Citation[17–19]. Also, the critical influence of the timing of chemotherapy with respect to whole body hyperthermia in determining both the efficacy and toxicity of thermochemotherapy treatments has been demonstrated Citation[20–22].
The effect of fever-range temperatures on the immune system has been less well investigated. Fever-range whole body thermal therapy has been shown to enhance the host immune response, whereas extreme systemic thermal therapy (41.5–42°C) may impair immune function Citation[23], Citation[24]. Fever-range hyperthermia can induce expression of heat shock proteins Citation[25], Citation[26], enhance antigen presentation by dendritic cells Citation[24], Citation[27], promote dendritic cell maturation Citation[23], Citation[27] and activate immune effector cells (T lymphocytes and NK cells) Citation[24], Citation[33–35]. New studies suggest that fever-like temperatures also activate an innate immune response by promoting toll-like receptor 4 (TLR4) signalling Citation[31]. Furthermore, dying tumour cells resulting from some cancer chemotherapy agents can cause surface expression of chaperones that determine the uptake of tumour antigens by dendritic cells and affect dendritic cell maturation via interaction with TLR4 receptors Citation[32]. Indeed, it appears that such immune responses are required for a successful anti-cancer response Citation[33–35] and that low, non-cytotoxic doses of some chemotherapeutic drugs, including oxaliplatin, cause cell death with immunostimulatory consequences Citation[36], Citation[37]. Oxaliplatin is a third generation platinum agent with broader clinical efficacy than the heat-interactive drugs cisplatin and carboplatin Citation[15], Citation[16]. Like these older drugs, oxaliplatin's cytotoxicity has been correlated with platinum-DNA adduct formation Citation[38].
We therefore hypothesised that combining the immune stimulus of fever-range whole body thermal therapy with that of moderate doses of the immunogenic cell death-causing chemotherapy agent oxaliplatin Citation[39], Citation[40], could produce durable responses in a clinically relevant model of advanced breast cancer. We first examined the effect of heat treatment on oxaliplatin-induced tumour cell kill, cellular platinum uptake, and platinum-DNA adduct formation in vitro. In vivo, combining oxaliplatin with fever-range whole body thermal therapy (FR-WB-TT) in the MTLn3 rat model of metastatic breast cancer, we demonstrated a substantial, apparently immune-mediated, critically dose- and schedule-dependent, anti-tumour effect of the thermochemotherapy that, when optimally dosed and scheduled, reproducibly achieved complete immunologic cures.
Methods
In vitro studies
Oxaliplatin cytotoxicity. MTLn3 tumour cells (obtained from Danny R. Welch, Department of Pathology, University of Pittsburgh; characterised by L. Clifton Stephens, Division of Veterinary Medicine and Surgery, M.D. Anderson Cancer Center Citation[41]) were treated in a 96-well plate with various concentrations of oxaliplatin ranging from 0.0005 to 100 µg/mL then placed in a water-jacketed incubator with 5% CO2 for 6 h at 37°C (normothermia) or 40°C (fever-range thermal therapy). Oxaliplatin (Sigma-Aldrich, St Louis, MO; CAS 61825-94-3) was obtained as a lyophilised powder and reconstituted to 5 mg/mL in sterile water USP according to the manufacturer's recommendations. A stock solution of 2.5 mg/mL was prepared by dilution with equal parts 5% dextrose in water USP (D5W) and sterile water and further diluted as needed. The platinum content of the stock solution was checked by atomic absorption spectrophotometry (see below). Cells were incubated 72 h later, for 4 h with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. Cell survival was determined from absorbance at 570 nm. A sigmoid curve of the formwas least-squares fitted to the cell survival versus dose curves. The IC-50 dose was determined as that dose resulting in 50% cell survival on the curve fit over the range 0.0001 to 100 µg/mL oxaliplatin.
Cellular uptake of oxaliplatin. Ten million MTLn3 tumour cells were treated with 200 µM (0.0795 mg/mL) oxaliplatin for 6 h at 37°C or 40°C. The cell pellet was re-suspended in PBS to a volume of 1.6 mL of which 0.3 mL was used for cellular uptake measurement, 0.2 mL for determination of protein concentration, and 1.1 mL for DNA concentration and DNA adduct measurement. After re-suspending in 2 N NaOH, the cell pellets were incubated at 50°C for 2 h and protein concentration was determined by absorbance at 570 nm compared to protein standards. Intracellular platinum was measured in cell pellets solubilised in hyamine hydroxide (methylbenzethonium hydroxide) by graphite furnace atomic absorption spectrophotometry (see below) and normalised to the protein content to yield the cellular uptake as ng/mg protein.
Platinum-DNA adducts. Cell pellets were re-suspended overnight in TE buffer with SDS, RNase A, and proteinase K. DNA was extracted from lysed tumour cells by phenol-chloroform-isoamylalcohol extraction. DNA concentration was determined by spectrophotometric measurement of UV absorbance at 260 nm. Platinum bound to DNA (Pt-DNA adducts) was measured as ng/mg DNA by graphite furnace absorption spectrophotometry.
Graphite furnace atomic absorption spectroscopy. Cells were dissolved in hyamine hydroxide in a 55°C water bath overnight and acidified with 0.3 N HCl. Cellular platinum concentration (ng/mg protein) in cell lysates or bound to DNA (ng/mg DNA) was determined by measurement of light absorption at 265.9 nm with a spectrophotometer (Varian AA 300, Varian, Palo Alto, CA) equipped with a graphite furnace (Varian model GTA-96) for flameless sample vaporisation. Cellular and DNA platinum levels were calculated by comparison with platinum standards by a method developed previously for cisplatin Citation[42].
In vivo studies
Tumour model. The rat MTLn3 orthotopic mammary adenocarcinoma model used for the in vivo studies has been described previously Citation[43], Citation[44]. It is a syngeneic, highly aggressive, metastatic, mammary tumour with many of the characteristics of advanced human breast cancer and is generally highly resistant to chemotherapy. Spontaneous metastases in inguinal and axillary lymph nodes are palpable by 3 weeks after tumour inoculation, while macroscopic lung metastases are present at 4 weeks. 5 × 105 tumour cells were inoculated subcutaneously into the mammary fat pad of 6–7 week-old, immunologically normal female Fischer 344 rats weighing 100–120 g (Harlan Sprague-Dawley, Indianapolis, IN). Palpable tumours developed by day 10. Animals with progressive disease were subsequently euthanised according to institutional animal welfare guidelines if, in the absence of signs of treatment response, tumours reached either the smaller of 2.5 cm3 or 10% of body weight, and if there were any signs of morbidity. The animal treatment protocol was approved by the University of Texas Health Science Center Animal Welfare Committee.
Oxaliplatin. Oxaliplatin, prepared as described above for the in vitro studies, was injected at a concentration of 2.5 mg/mL into the lateral tail vein as a slow bolus (of around 0.6 mL volume for 10 mg/kg dose). To assess dose response, doses of 6, 8, 10, 12, and 14 mg/kg were administered. The maximally tolerated dose (MTD) of oxaliplatin given 24 h before fever-range whole body thermal therapy is around 14 mg/kg. For tumour response and toxicity experiments, a dose of 10 mg/kg was used.
Thermal therapy. Fever-range whole body thermal therapy was administered by partially immersing halothane or isoflurane-anaesthetised rats into a thermostatically controlled circulating water-bath maintained at 40°C ± 0.1°C as described previously Citation[20], Citation[22]. Rectal temperature was monitored continuously in each rat using small animal rectal thermistor probes (YSI model 402, Yellow Springs Instruments, Yellow Springs, OH) inserted to a depth of 6 cm in the rectum and connected to a 12-channel switch box (YSI model 4002) and digital tele-thermometer (YSI model 49A), and was recorded every 5–10 min. The probes were calibrated against a mercury thermometer (Ertco ASTM 64°C) certified by the National Bureau of Standards. Approximately 15 min was required for the rectal temperature to reach 40°C. In whole body hyperthermia, once temperature equilibrium is reached, thermal gradients throughout the body are small Citation[45], and since the rectal probe is located close to the tumour site, it is a good indicator of tumour temperature. Each rat was individually maintained at 40°C ± 0.1°C for 6 h by covering with foil and/or adjusting the depth of immersion in the water bath. The optimal duration of FR-WB-TT in these rats was previously determined to be 6 to 8 h Citation[13], Citation[41]. For logistical reasons, 6 h of hyperthermia treatment was chosen.
Anaesthesia. All animals received inhalation halothane or, more recently, isoflurane anaesthesia during the 6 h hyperthermia treatments, as well as during the corresponding 6 h sham treatments as described previously Citation[46]. Halothane anaesthesia in combination with whole body hyperthermia is safe even when administered for many hours, and affects neither tumour growth nor normal tissue toxicity Citation[46]. Isoflurane anaesthesia is similarly safe and appears not to affect tumour response. The rats were initially exposed to 3% halothane or isoflurane in pure oxygen in an induction chamber for approximately 10 min in order to induce surgical level anaesthesia prior to the start of hyperthermia or sham treatment. 1.5% halothane or 2% isoflurane mixed with pure oxygen in a standard hospital grade vaporiser was delivered through a custom-made polymethyl methacrylate mask throughout the warm-up and treatment time, or equivalent sham treatment. The rats showed no evidence of discomfort during anaesthesia and after treatment they recovered without ill effects, quickly becoming ambulatory.
Treatment groups. On day 10 after tumour inoculation, rats with primary tumours around 100 mm3 in volume were randomised to treatment and control groups. At this time there was no overt evidence of metastases, however, microscopic inguinal and/or axillary lymph node metastases, but no microscopically visible metastatic lung deposits, would have been established Citation[44]. For dose-response, toxicity, and curative response studies the groups were control, and oxaliplatin administered 24 h before fever-range whole body thermal therapy. Control, oxaliplatin alone, oxaliplatin 24 h before, 12 h before, 3 h before, simultaneously with, and 24 h after FR-WB-TT were investigated for the schedule-dependency study. Control animals received sham treatment comprising injection of the same solution used to dilute the oxaliplatin (D5W in water) on the same days after tumour inoculation, and the same anaesthesia protocol, as treated animals. They were maintained at a normal body temperature by placement on a microprocessor-controlled, warmed water blanket (Blanketrol II Hyper-Hypothermia System and PlastiPad®, Cincinnati Sub-Zero Products, Cincinnati, OH].
Tumour assessment. Tumour size (primary and inguinal and axillary metastases) was measured every 2 days using a vernier caliper to determine three orthogonal dimensions (d), and the tumour volume (V) was approximated according to the formula V = (d1 × d2 × d3) ÷ 2. The incidence and size of any axillary and inguinal lymph node metastases were recorded.
Treatment response criteria. Animal response to treatment was graded into one of four categories by taking into account primary tumour and metastatic tumour volume, body weight change, and survival:
Cure complete regression of all primary and metastatic tumours, normal life span, and/or immune to MTLn3 tumour re-challenge;
CR complete regression of primary tumour, weight gain, but eventual late death from new metastatic disease;
LR limited response comprising temporary regression or growth arrest of primary tumour and/or metastases, weight gain or stabilisation, some increase in survival;
NR no reduction of primary tumour or metastases, weight loss, no increase in survival.
Note that these definitions are not the same as standard clinical oncologic response designations.
Toxicity assessment. Rat body weight was recorded every other day as a general indication of treatment-induced toxicity. Haematocrit, an indicator of haematological toxicity, was measured in centrifuged blood samples withdrawn on days 8, 15, and 22 after treatment.
Photographic image capture and processing. Colour images of primary and metastatic tumours were recorded with a pocket digital camera (Canon Powershot SD10, 2272 × 1704 pixels). For publication purposes, each original jpeg image was processed with Microsoft Office Picture Manager software, 1) to standardise the white balance by making the centre of the upper ruler white, and then 2) to convert the white-balanced image to grey-scale. The grey-scale images were imported into Microsoft Publisher, resized, and arranged to fit the page in a 3 × 3 grid. Grid lines were superimposed on the images and cell titles added in text boxes. Arrows and corresponding labels in text boxes were superimposed on the images. The composite image was saved in Tagged Image File Format (TIFF).
Statistical analysis. The significance of differences in oxaliplatin IC-50 dose between 37°C and 40°C, and in primary tumour and metastatic lymph node tumour size, body weight, haematocrit, and survival between the experimental groups was calculated by an appropriate Student's t test. A P-value of <0.05 was considered statistically significant.
Results
In vitro
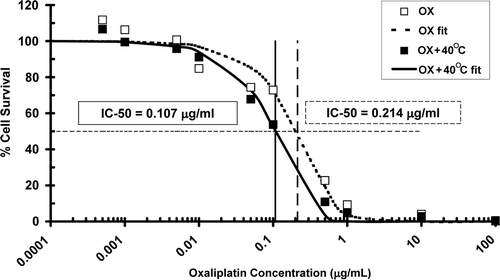
Six hours of heating to 40°C following oxaliplatin administration increased the intracellular concentration of platinum by 34% from 307 to 412 ng/mg protein (p = 0.008) as shown in . The number of platinum-DNA adducts also increased 36% from 60 to 82 ng/mg DNA (p = 0.039) in cells heated for 6 h at 40°C. The ratio of cellular platinum uptake to DNA adducts was, however, unchanged by the higher temperature (5.09 at 37°C and 5.02 at 40°C). MTLn3 cells treated with oxaliplatin at doses from 0.0005 to 100 µg/mL were more effectively killed when incubated for 6 h at 40°C compared to 37°C. The oxaliplatin dose at which half the cells were killed (IC-50 dose) was halved from 0.214 µg/mL at 37°C to 0.107 µg/mL at 40°C ().
Figure 1. Comparison of in vitro cellular platinum uptake (solid black bars) and platinum-DNA adducts (open bars) between cells incubated with 200 µM oxaliplatin (OX) for 6 h at 37°C and 40°C. Cellular uptake of platinum (ng/mg protein) was significantly increased in heated cells (*p < 0.01) and platinum-DNA adducts (ng/mg DNA) were also increased (**p < 0.04) compared to oxaliplatin alone. Error bars indicate standard deviation.
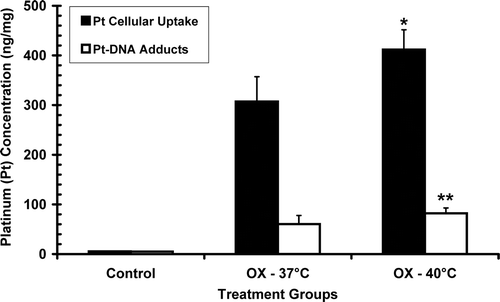
Figure 2. Percentage of surviving MTLn3 tumour cells in vitro, as measured by MTT assay, after administration of oxaliplatin in the dose range 0.0005 to 100 µg/mL, with (▪) and without (□) 6 h of 40°C heating, along with sigmoid curves least-squares fitted to the measured data (––OX, ___ OX + 40°C). Heat treatment increased the efficacy of cell killing by oxaliplatin as evidenced by a shift to the left of the survival curve. The IC-50 dose was halved from 0.214 µg/mL to 0.107 µg/mL.
In vivo
compares the average MTLn3 tumour growth in controls with rats in a representative experiment in which 6 rats were treated with 10 mg/kg oxaliplatin alone, and 6 rats were given 10 mg/kg oxaliplatin followed 24 h later by 6 h of fever-range whole body thermal therapy at 40°C. The tumour grew rapidly in control animals to almost 100 times the initial tumour volume and the rats died between day 20 and day 28 (median survival 27 days). Animals treated with oxaliplatin alone showed initial slowing of tumour growth for few days but the tumours soon began to grow rapidly again and survival was barely longer than in controls (median survival 28 days). In animals treated with oxaliplatin and thermal therapy, tumour growth was initially much the same as in those animals given oxaliplatin alone, but between day 10 and 16 there was a further slowing of tumour growth and 5 out of 6 animals (83%) began to regress their primary tumours. Three of these rats (50%) went on to be cured of all primary and metastatic tumours and live a normal life span.
Figure 3. (A) Average relative primary tumour volume (RTV) in a representative experiment in controls, animals treated with 10 mg oxaliplatin alone (○), and after treatment with 10 mg oxaliplatin followed 24 h later by of fever-range whole body thermal therapy at 40°C (▪, n = 6 per group). Day 0 is the day of oxaliplatin treatment. Error bars indicate standard error. (B) Primary tumour growth averaged over six experiments comprising 36 treated animals that either did not regress their tumours (○, 22%), were limited responders that temporarily regressed their tumours (□, 17%), or completely regressed their primary tumours (▪, 61%), compared to controls (no symbol). Error bars indicate standard error. Of the 22 complete responders, 18 (50% of treated rats) were completely cured of all primary and metastatic tumours and went on to live a normal life span. (C) Photographic comparison of tumours in an untreated control animal (left-hand column), a treated animal with limited response (centre column), and an animal that was ultimately cured (right-hand column) on day 8 (top row), day 16 (middle row), and day 23 (bottom row) after oxaliplatin treatment followed 24 h later by fever-range whole body thermal therapy. Arrows point to the primary tumour (P) and an inguinal lymph node metastasis (IM); *indicates remodelling and ulceration of the progressing primary tumour on day 16 in the control rat, while ** illustrates how the primary tumour and inguinal metastasis in the control rat have merged by day 23, the day of death; # in the limited responder's day 23 image denotes regrowth of the initially responding inguinal metastasis at the same time as regression of the primary tumour. In the bottom right image, N indicates the nipple, now visible thanks to substantial regression of the primary tumour and inguinal metastasis by day 23 (complete disappearance of tumours in cured animals typically occurred around day 30).
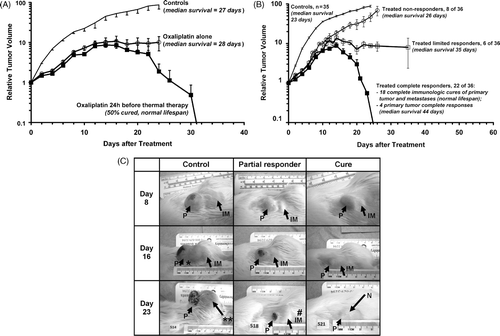
Panel B in plots the average tumour response in a series of six experiments conducted over a period of three years in which animals were treated with 10 mg/kg oxaliplatin followed 24 h later by 6 h of fever-range whole body thermal therapy at 40°C. In all treated animals there was an initial growth inhibition compared to controls but in 8 of the 36 treated animals (22%) rapid tumour growth resumed after day 4 and the animals went on to die of their tumours between day 22 and 30 after oxaliplatin treatment (NR). Another 6 animals (17%) displayed a limited response (LR) in which tumour growth slowed again after day 10 and there was tumour regression or control for some time, leading to increased survival compared to control animals (median survival = 35 days, see ). However, in 22 of the 36 treated animals (61%) a late slowing of tumour growth around day 10 was followed by complete regression of the primary tumours, although 4 animals (11%) later succumbed to metastases after an average survival of 50 days post-treatment. The other 18 animals (50%; from 33–67% in any single experiment with 6 animals) were cured of all primary and metastatic tumours and lived out an essentially normal life span. These animals were immune to multiple (up to 4) re-challenges with MTLn3 cells yet grew a control tumour (glioblastoma), suggesting a tumour-specific immunologic cure. illustrates representative tumour appearance in situ on days 8, 15, and 22 after oxaliplatin administration in a control animal, a treated animal that didn’t respond beyond early growth suppression, and an animal that went on to be cured.
Table I. Treatment outcome measures of 36 rats treated with oxaliplatin 24 h before fever-range whole body thermal therapy; maximum relative tumour volume, median, and mean survival in controls, non-responders (NR), limited responders (LR), complete responders (CR) and cured rats.
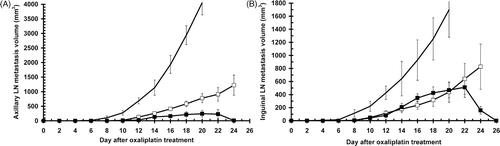
As seen in , oxaliplatin thermochemotherapy was not only effective against primary tumours, but also against metastases that were not present at the time of treatment but appeared later. Axillary and inguinal metastases were completely eliminated in animals cured by oxaliplatin 24 h before FR-WB-TT. In animals that were not cured, both axillary and inguinal metastases appeared later, and grew more slowly, than in rats treated with oxaliplatin alone. The median day on which inguinal metastases first could be measured was day 6, 8, and 10, respectively in control, oxaliplatin, and oxaliplatin 24 h before FR-WB-TT groups, while axillary metastases were first seen on median day 7, 11, and 13, respectively. Even in the uncured animals, metastases began to regress late after treatment, around day 20–22, much like the primary tumours. In contrast, in animals treated with oxaliplatin alone, both axillary and inguinal metastases grew steadily until the animal's death, albeit at a slower rate than in controls.
Figure 4. Metastatic tumour growth in uncured animals in a representative experiment: controls (no symbol, n = 6), 10 mg/kg oxaliplatin (□, n = 5), 10 mg/kg oxaliplatin 24 h before FR-WB-TT (▪, n = 3). (A) Axillary metastases, (B) inguinal metastases. Error bars represent standard error.
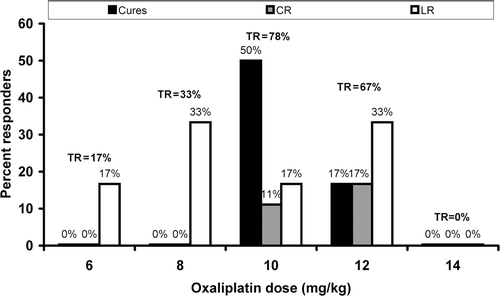
As shown in , the number of curative and complete responses was highly dose-dependent. Cures occurred only at 10, and 12 mg/kg of oxaliplatin administered 24 h before thermal therapy, with the highest cure rate resulting from 10 mg/kg. Similarly, complete responses were observed at an oxaliplatin dose of 10 and 12 mg/kg only. At 6 and 8 mg/kg there were only a few partial responses, while at 14 mg/kg, close to the MTD dose, there were no responses at all.
Figure 5. Dependence of tumour response on dose of oxaliplatin given 24 h before fever-range whole body thermal therapy. Percent limited responses (LR, white bars), complete responses (CR, grey bars) and cures (black bars) are shown for each of 5 doses: 6, 8, 10, 12, and 14 mg/kg (n = 6, 8, 36, 8, 6 respectively). Total response, TR = cures + CR + LR.
The efficacy of oxaliplatin combined with fever-range whole body thermal therapy was also dependent on the schedule of administration of the drug with respect to the thermal therapy. compares the survival and cure rate of rats treated with various relative timings of oxaliplatin with respect to thermal therapy to survival with oxaliplatin alone and in untreated control animals. Oxaliplatin (10 mg/kg) given 12 h or 24 h before thermal therapy resulted in a significant increase in median survival over controls, and compared to oxaliplatin alone, with 50% of rats being cured and living out a normal life span. There was one cure in the 10 mg/kg oxaliplatin alone group, but the other five rats had an average survival only 15% greater than controls (not significant), much shorter than rats receiving the drug 12 or 24 h before thermal therapy.
Table II. Survival and toxicity measures from two experiments for different schedules of oxaliplatin thermochemotherapy: average median survival, percent cures, maximum body weight loss, and decrease in haematocrit to nadir for rats treated with fever-range whole body thermal therapy before, simultaneously with, and after oxaliplatin. The dose of oxaliplatin was either 10 mg/kg or 12 mg/kg.
Not only was anti-tumour efficacy and survival schedule-dependent, but so also was toxicity. gives two measures of toxicity after various schedules of oxaliplatin thermochemotherapy: maximum body weight loss and nadir haematocrit decrease, and change in white blood cell number (as indicated by the buffy coat thickness) between days 8 and 15 after treatment. Oxaliplatin given 24 h before thermal therapy resulted in the least body weight loss, the least decrease in haematocrit, and the greatest increase in white blood cell count of all the tested schedules.
Discussion
Oxaliplatin in vitro
Oxaliplatin forms intra-strand platinum-DNA adducts that induce a number of signal transduction pathways leading to apoptosis Citation[47]. Enhanced oxaliplatin efficacy against ovarian carcinoma cells following 1 h of concurrent, 42°C hyperthermia, along with increased G2/M arrest has been reported Citation[48]. Our in vitro data indicate that 40°C heat increases the sensitivity of MTLn3 tumour cells to oxaliplatin by increasing intracellular uptake of platinum and formation of platinum adducts. Since the ratio of platinum adducts to intracellular platinum remained unchanged after heating () it would appear that heat did not increase the reaction rate between platinum and DNA, rather that the increased adduct formation was due to a thermally induced increase in cellular drug uptake, presumably because of increased cell permeability. Heat-induced, and exposure-related, increases in cell membrane permeability have been reported at temperatures as low as 40°C Citation[49]. Increased nanoparticle extravasation from tumour vasculature in response to loco-regional and fever-range hyperthermia have also been reported Citation[3], Citation[4]. Even greater cellular uptake of oxaliplatin, and consequent tumour cytotoxicity, is to be expected from thermal therapy in vivo. Hyperthermia may also inhibit repair of DNA adducts.
In-vivo tumour response to thermochemotherapy
The remarkable tumour responses observed in vivo as illustrated in cannot, however, be explained by increased drug uptake and heat augmentation of drug action alone. The late tumour regression observed in so many of the rats treated with oxaliplatin 24 h before FR-WB-TT, together with the inability of cured rats to grow tumours even when re-challenged multiple times with the same tumour type, suggests an immune response that led to formation of memory T cells. The cured animals that were re-challenged with MTLn3 tumour cells displayed marked skin reactivity on days 3–5 after inoculation, also indicative of an immune response. There was also a significant difference in peripheral white blood cell count between responders and non-responders (unpublished data). Furthermore, our preliminary data from ongoing flow cytometry and immunoblot studies suggest that 1) while in vitro stimulation of peripheral blood mononuclear cells with irradiated MTLn3 tumour cells results in a marked increase in the number of CD8+ cells in both cured rats and limited responders compared to controls with progressing tumours, the number of CD25-expressing CD8+, i.e. functionally activated T cells increases only in cured rats; and 2) lymphocytes extracted from peripheral blood of cured animals and stimulated with irradiated MTLn3 cells show interferon-γ activity. The precise mechanism of immune activation has yet to be elucidated, but we hypothesise that oxaliplatin-induced cell kill, largely by apoptosis, causes tumour antigen cross-presentation to dendritic cells. Thermal stimulation of both dendritic and NK cells Citation[27], Citation[28], and possible down-regulation of regulatory T cells Citation[50], may additionally lead to an increased anti-tumour response. Additional immunotherapy might result in an even higher cure rate.
Tumours in all the treated animals followed the same initial growth pattern with some growth inhibition on days 2–4, followed by tumour re-growth (). Regression of responding tumours did not begin until around day 10 and tumour cure or complete response could only be distinguished on the basis of tumour volume after about day 16. Indeed, the first sign of long-term response was generally a gain in the animal's weight beginning around day 12. Similarly late responses with oxaliplatin in both a fibrosarcoma and a pancreas cancer model (unpublished data), and with gemcitabine in the MTLn3 model Citation[22], have been seen. Despite the initial tumour growth inhibition, there was no significant survival advantage of animals treated with oxaliplatin alone, or those thermochemotherapy-treated non-responders. While limited responders had only slightly increased survival, the sustained tumour growth inhibition throughout their remaining life, and their reduced maximum tumour volume (), likely translated into an improved quality of life. Complete responders had durable regression of their primary tumours, but like the majority of advanced cancer patients, ultimately succumbed to metastases. Notably, 78% of the rats were cured, complete responders, or limited responders, a substantially higher response rate than observed with other heat-interactive chemotherapy agents, perhaps due to the particular immunogenicity of oxaliplatin cell death Citation[39]. Since the studies reported here, we have been using the clinical formulation of oxaliplatin (Eloxatin, Sanofi Aventis) and continue to see a consistently high cure rate, in one experiment as high as 83%.
Metastases. Also important is the effect of thermochemotherapy on the subsequent appearance and course of metastases. Metastasis appearance was delayed after oxaliplatin 24 h before FR-WB-TT compared to oxaliplatin alone. Cured animals completely regressed their metastases as well as their primary tumours. Notably, both inguinal and axillary metastasis volume was lower in uncured animals treated with oxaliplatin 24 h before FR-WB-TT than in those animals treated with oxaliplatin alone (). Delayed incidence of metastases has also been reported for irinotecan combined with fever-range whole body thermal therapy Citation[44]. Control of metastatic disease is extremely important for increased survival, and also determines quality of life.
Dose response. The dose of oxaliplatin administered prior to thermal therapy is critically important to outcome. Cures and complete responses occurred only within a narrow window of doses, between 70% and 85% of the maximally tolerated dose (MTD). At lower doses, around 40–60% of MTD) little acute tumour growth delay, no long-term responses, and no cures occurred (). When the dose approached the MTD, despite significant short-term tumour growth arrest, no animals had long-term tumour responses. We hypothesise that sufficient tumour cell kill by oxaliplatin is required for effective antigen cross-presentation, but that too high a drug dose, while resulting in initial tumour killing or growth arrest, also kills many antigen-presenting and immune effector cells and thus disables a curative immune response, leading to rapid, fatal, tumour re-growth. Low doses of chemotherapy, as used in metronomic therapy, can initiate an immune response Citation[51].
Schedule and immunostimulation. The relative timing of thermal therapy with respect to oxaliplatin is very important for anti-tumour efficacy (), as has been observed with other chemotherapy agents Citation[20–22]. When 10 mg/kg oxaliplatin was administered simultaneously with, or after, thermal therapy the tumour response and survival was no different than with oxaliplatin alone. Similarly, oxaliplatin given 3 h or 48 h before thermal therapy was no more effective than oxaliplatin alone. However, when oxaliplatin was given 12 or 24 hours before thermal therapy there was significantly increased survival and 50% of the animals were completely cured. The need for a substantial delay between oxaliplatin treatment and subsequent thermal therapy is consistent with the hypothesis that oxaliplatin-induced cell cycle arrest and tumour cell death initiates an immune stimulus that is then augmented by thermal therapy to cause substantial tumour responses. While platinum-DNA adducts form immediately after oxaliplatin administration, induction of apoptosis is a later event following S phase delay and G2/M phase arrest Citation[52], Citation[53]. Cisplatin-induced apoptosis of MTLn3 cells has been detected 16 h after treatment Citation[54], but as oxaliplatin-DNA adducts are more efficient at producing irreparable cellular damage than cisplatin, oxaliplatin-induced apoptosis may occur sooner. Actually, full progression to apoptosis may not be necessary to initiate immune activation since damage response-associated expression of NKG2D receptor ligand may decrease regulatory T cells enough to expand the effector cell population Citation[55], and/or increased expression of cyclins during G2/M arrest may act as tumour antigens Citation[56].
T cell activation follows pro-inflammatory maturation signals (here presumably heat-induced), and efficient T cell cross-priming requires fully mature dendritic cells Citation[27], Citation[28]; dendritic cell maturation in vivo can take 7–10 days Citation[27], Citation[57]. Activation of T cells after satisfactory contact with primed dendritic cells Citation[58] is then a rapid process of a few hours Citation[59]. The tumour responses reported here, typically occurring around day 14–16 after oxaliplatin treatment, but occasionally much later, are consistent with T cell activation following a prolonged period of dendritic cell maturation after drug-induced tumour cell death. Interestingly, the kinetics of our curative tumour response are virtually identical to those of Kelly et al. following NK cell-mediated tumour rejection Citation[60] which may argue towards a significant contribution of NK cells. Stimulation of NK cell activity after fever-range thermal therapy has been reported Citation[28], Citation[30], Citation[61].
Modulation of drug toxicity by systemic hyperthermia. Not only did oxaliplatin thermochemotherapy result in significant anti-tumour responses, but toxicity was also reduced in comparison to oxaliplatin alone. Treatment with oxaliplatin resulted in an acute, dose-dependent drop in body weight, indicative of drug toxicity. Additionally, a decline in body weight was observed over several weeks after treatment in all groups including controls, presumably due to increasing tumour burden and cachexia. In hyperthermia-treated animals, however, body weight reached a nadir, usually between days 7 and 14, increasing again sooner rather than later with the more efficacious treatments. The changes in body weight shown in appear to correlate inversely with treatment efficacy. Indeed, increasing body weight was generally the first sign when treatment led to a complete response or cure. The least weight loss of all the schedules was observed for oxaliplatin followed 24 h later by thermal therapy. Haematocrit, which also decreases to a nadir after chemotherapy, fell significantly less when oxaliplatin preceded hyperthermia than after oxaliplatin alone and in controls.
Clinical potential. Given the notable tumour responses obtained with oxaliplatin thermochemotherapy, along with the acceptable toxicity, and the fact that pre-clinical results in this clinically relevant tumour model have formed the basis for several ongoing clinical trials Citation[17–19], oxaliplatin combined with fever-range whole body thermal therapy could form the basis of a superior clinical treatment for advanced metastatic cancers. Harnessing the innate immune system through lower-than-MTD chemotherapy combined with immune-enhancing therapy, such as thermal therapy, is a promising approach for treatment of patients with cancer. Perhaps the goal of complete cures, or at least significantly extended survival along with improved quality of life following treatment, may no longer be so elusive.
Conclusion
Mild heating in vitro of MTLn3 tumour cells exposed to oxaliplatin increased tumour cell kill through increased uptake of oxaliplatin by tumour cells and subsequent formation of oxaliplatin-DNA adducts. In vivo, in an animal model that closely parallels rapidly progressing inflammatory human breast cancer, fever-range whole body thermal therapy combined with properly dosed and scheduled oxaliplatin chemotherapy consistently achieved a hitherto unseen level of tumour responses, delayed appearance and slowed growth of metastases, reduced toxicity, and increased survival. Complete, durable, immunologic cures were achieved in 50% of animals. A further 11% of animals completely regressed their primary tumours although they ultimately succumbed to metastases, and 17% showed limited tumour responses. Fever-range thermal therapy, when optimally timed with respect to correctly dosed oxaliplatin chemotherapy, appears to augment the immune response to drug-induced cell death sufficiently to result in tumour cures. Thermochemotherapy with sub-MTD oxaliplatin and fever-range whole body thermal therapy therefore holds great potential as a safe and effective clinical treatment against advanced, metastatic cancer.
Declaration of interest: The authors gratefully acknowledge the support of research grants from the National Institutes of Health (NCI R01 CA 43090 and NCI R01 CA127263), the Susan G. Komen Breast Cancer Foundation, Sanofi-Aventis, and the University of Texas Hyperthermia Research Laboratory Miscellaneous Donors Fund.
References
- Nishimura Y, Shibamoto Y, Jo S, Akuta K, Hiraoka M, Takahashi M, Abe M. Relationship between heat-induced vascular damage and thermosensitivity in four mouse tumors. Cancer Res 1988; 48: 7226–7230
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Rowe RW, Strebel FR, Sumiyoshi K, Kirpotin DB, Bull JMC. Time-course of liposome uptake in MTLn3 tumors and normal tissue with and without whole body hyperthermia. Proc Am Assoc Cancer Res 2002; 43: 417, (abstract)
- Leunig M, Goetz AE, Dellian M, Zetterer G, Gamarra F, Jain RK, Mesmer K. Interstitial fluid pressure in solid tumors following hyperthermia: Possible correlation with therapeutic response. Cancer Res 1992; 52: 487–490
- Sem A, Capitano M, Hylander B, Spernyak J, Schueckler J, Singh A, Repasky E. Fever-range systemic hyperthermia increases tumor vascular perfusion, decreases interstitial fluid pressure and hypoxia and sensitizes tumors to subsequent radiation therapy. Paper presented at the Society for Thermal Medicine Annual Meeting, Tucson, Arizona, (2009) (Symposium IV: Thermal Therapy, Hypoxia and the Tumor Microenvironment). Abstract available at http://stm.conference-services.net/resources/467/1527/pdf/STM(2009)_0118.pdf. Accessed March 25, 2010.
- Van der Zee J, Vernon CC. Thermoradiotherapy for advanced and recurrent breast tumors. Thermoradiotherapy and Thermochemotherapy, HM Seegenschmiedt, P Fessenden, CC Vernon. Clinical Applications Berlin, Springer. 1996; 2: 35–48
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, González González D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Jones EL, Marks LB, Prosnitz LR. Point: Hyperthermia with radiation for chest wall recurrences. J Natl Compr Canc Netw 2007; 5: 339–344
- Hehr T, Wust P, Bamberg M, Budach W. Current and potential role of thermoradiotherapy for solid tumours. Onkologie 2003; 26: 295–302
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18
- Rowe RW. Hyperthermia, systemic. Encyclopedia of Medical Devices and Instrumentationsecond (Table 6)., JG Webster. Wiley, New York 2006; 57
- Matsuda H, Strebel FR, Kaneko T, Danhavser LL, Jenkins GN, Toyota N, Bull JM. Long duration mild whole body hyperthermia of up to 12 hours in rats: Feasibility, and efficacy on primary tumour and axillary lymph node metastases of a mammary adenocarcinoma: Implications for adjuvant therapy. Int J Hyperthermia 1997; 13: 89–98
- Yamada Y, Itoh Y, Aoki S, Nakamura K, Taki T, Naruse K, Tobiume M, Zennami K, Katsuda R, Kato Y, et al. Preliminary results of M-VAC chemotherapy combined with mild hyperthermia, a new therapeutic strategy for advanced or metastatic transitional cell carcinoma of the urothelium. Cancer Chemother Pharmacol 2009; 64: 1079–1083
- Sakaguchi Y, Makino M, Kaneko T, Stephens LC, Strebel FR, Danhauser LL, Jenkins GN, Bull JM. Therapeutic efficacy of long duration-low temperature whole body hyperthermia when combined with tumor necrosis factor and carboplatin in rats. Cancer Res 1994; 54: 2223–2227
- Toyota N, Strebel FR, Stephens LC, Matsuda H, Bull JM. Long-duration, mild whole body hyperthermia with cisplatin: Tumour response and kinetics of apoptosis and necrosis in a metastatic rat mammary adenocarcinoma. Int J Hyperthermia 1997; 13: 497–506
- Nagle V, Berry J, Bull JM. Whole body hyperthermia with carboplatin (CBDCA) for treatment of advanced or metastatic GI adenocarcinomas. Proc Am Assoc Ca Res 1999; 40: 345
- Bull JM, Scott GL, Nagle VL, Strebel FR, Koch SM. Phase I study of long-duration, low-temperature whole-body hyperthermia (LL-WBH) with liposomal doxorubicin (Doxil), 5-fluorouracil (5-FU), and interferon-a (IFN-a). Proc Am Soc Clin Oncol 2002; 21, abstract No. 2126. Accessed March 25, 2010 from http://www.asco.org/ASCOv2/meetings/abstracts?&vmview=abst_detail_view&confID=16&abstractID=2126
- Bull JM, Scott GL, Strebel FR, Nagle VL, Oliver D, Redwine M, Rowe RW, Ahn CW, Koch SM. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: A description of a phase I-II protocol. Int J Hyperthermia 2008; 24: 649–662
- Baba H, Siddik ZH, Strebel FR, Jenkins GN, Bull JM. Increased therapeutic gain of combined cis-diamminedichloroplatinum (II) and whole body hyperthermia therapy by optimal heat/drug scheduling. Cancer Res 1989; 49: 7041–7044
- Strebel FR, Sumiyoshi K, Jenkins GN, Rowe RW, Bull JMC. Drug sequence effect on therapeutic outcome (tumor growth, toxicity, and survival) of irinotecan combined with epirubicin in the in vivo MTLn3 rat mammary adenocarcinoma. Proc Am Assoc Cancer Res 2002; 43: 587
- Bull JMC, Strebel FR, Jenkins GN, Deng W, Rowe RW. The importance of schedule in whole body thermochemotherapy. Int J Hyperthermia 2008; 24: 171–181
- Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs 2009; 10: 550–558
- Zhang H-G, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: A temperature's story. Cancer Lett 2008; 271: 191–204
- Tulapurkar ME, Asiegbu BE, Singh IS, Hasday JD. Hyperthermia in the febrile range induces HSP72 expression proportional to exposure temperature but not to HSF-1 DNA-binding activity in human lung epithelial A549 cells. Cell Stress Chaperones 2009; 14: 499–508
- Murapa P, Gandhapudi S, Skaggs HS, Sarge KD, Woodward JG. Physiological fever temperature induces a protective stress response in T lymphocytes mediated by heat shock factor-1 (HSF1). J Immunol 2007; 179: 8305–8312
- Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother 2006; 55: 292–298
- Milani V, Noessner E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells. Cancer Immunol Immunother 2006; 55: 312–319
- Chen Q, Fisher DT, Kucinska SA, Wang WC, Evans SS. Dynamic control of lymphocyte trafficking by fever-range thermal stress. Cancer Immunol Immunother 2006; 55: 299–311
- Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol 1998; 177: 137–147
- Zhao W, An H, Zhou J, Xu H, Yu Y, Cao X. Hyperthermia differentially regulates TLR4 and TLR2-mediated innate immune response. Immunol Let 2007; 108: 137–142
- Van der Most RG, Currie AJ, Robinson BWS, Lake RA. Decoding dangerous cell death: How cytotoxic chemotherapy invokes inflammation, immunity, or nothing at all. Cell Death Differ 2008; 15: 13–20
- Tesniere A, Panaretakis T, Kepp O, apeton L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008; 15: 3–12
- Ullrich E, Bonmort M, Mignot G, Kroemer G, Zitvogel L. Tumor stress, cell death, and the ensuing immune response. Cell Death Differ 2008; 15: 21–28
- Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Current Opin Oncol 2009; 21: 71–76
- Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol 2009; 183: 137–144
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29: 482–91
- Raymond E, Faivre S, Chaney S, Woynarowsky J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther 2002; 1: 227–235
- Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008; 68: 4026–4030
- Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, et al. High mobility group box I (HMGB1) release from tumor cells after treatment: Implications for development of targeted chemoimmunotherapy. J Immunother 2007; 30: 596–606
- Matsuda H, Strebel FR, Kaneko T, Stephens LC, Danhauser LL, Jenkins GN, Toyota N, Bull JM. Apoptosis and necrosis occurring during different stages of primary and metastatic tumor growth of a rat mammary adenocarcinoma. Anticancer Res 1996; 16: 1117–1122
- Siddik ZH, Boxall FE, Harrap KR. Flameless atomic absorption spectrophotometric determination of platinum in tissues solubilized in hyaminehydroxide. Anal Biochem 1987; 163: 21–26
- Neri A, Welch D, Kawaguchi T, Nicolson GL. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst 1982; 68: 507–517
- Sumiyoshi K, Strebel FR, Rowe RW, Bull JM. The effect of whole-body hyperthermia combined with ‘metronomic’ chemotherapy on rat mammary adenocarcinoma metastases. Int J Hyperthermia 2003; 19: 103–118
- Thrall DE, Page RL, Dewhirst MW, Meyer RE P, Hoopes J, Kornegay JN. Temperature measurements in normal and tumor tissue of dogs undergoing whole body hyperthermia. Cancer Res 1986; 46: 6229–6235
- Wondergem J, Siddik ZH, Strebel FR, Bull JM. Effect of whole body hyperthermia on cis-diamminedichloroplatinum (II)-induced antitumour activity and tissue Pt-distribution: Do anaesthetics influence the therapeutic ratio?. Eur J Cancer 1993; 29A: 549–554
- Nehmé A, Baskaran R, Nebel S, Jink D, Howell SB, Wang JY, Christen RD. Induction of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells. Br J Cancer 1999; 79: 1104–1110
- Atallah D, Marsaud V, Radanyi C, Komprobst M, Rouzier R, Zlias D, Renoir JM. Thermal enhancement of oxaliplatin-induced inhibition of cell cycle progression in human carcinoma cell lines. Int J Hyperthermia 2004; 20: 405–419
- Bischof JC, Padanilam WH, Holmes RM, et al. Dynamics of cell membrane permeability changes at supraphysiological temperatures. Biophys J 1995; 68: 2608–2614
- Terunuma H, Wada A, Deng X, Yasuma Y, Onishi T, Toki A, Abe H. Mild hyperthermia modulates the relative frequency of lymphocyte cell subpopulations: an increase in a cytolytic NK cell subset and a decrease in a regulatory T cell subset. Thermal Med (Jap J Hyperthermic Oncol) 2007; 23: 41–47
- Ehrke MJ. Immunomodulation in cancer therapeutics. Int Immunopharmacol 2003; 3: 1105–1119
- William-Faltaos S, Rouillard D, Lechat P, Bastian G. Cell cycle arrest by oxaliplatin on cancer cells. Fundam Clin Pharmacol 2007; 21: 165–172
- Toscano F, Parmentier B, El Fajoui Z, Estornes Y, Chayvialle JA, Saurin JC, Abello J. p53 dependent and independent sensitivity to oxaliplatin of colon cancer cells. Biochem Pharmacol 2007; 74: 392–406
- van Nimwegen MJ, Huigsloot M, Camier A, Tijdens IB, van de Water B. Focal adhesion kinase and protein kinase B cooperate to suppress doxorubicin-induced apoptosis of breast tumor cells. Mol Pharmacol 2006; 70: 1330–1339
- Gasser S, Raule DH. The DNA damage response arouses the immune system. Cancer Res 2006; 66: 3959–3962
- Egloff AM, Vella LA, Oliver JF. Cyclin B1 and other cyclins as tumor antigens in immunosurveillance and immunotherapy of cancer. Cancer Res 2006; 66: 6–9
- Camporeale A, Boni A, Iezzi G, Degl'Innocenti E, Grioni M, Mondino A, Bellone M. Critical impact of the kinetics of dendritic cells activation on the in vivo induction of tumor-specific T lymphocytes. Cancer Res 2003; 63: 3688–3694
- Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol 2003; 4: 579–585
- Roosnek E, Demotz S, Corradin G, Lanzavecchia A. Kinetics of MHC-antigen complex formation on antigen-presenting cells. J Immunol 1988; 140: 4079–4082
- Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol 2002; 3: 83–90
- Dayanc BE, Beachy SH, Ostberg JR, Repasky EA. Dissection the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia 2008; 24: 41–56