Abstract
Purpose: Recent advances in nanotechnology have resulted in the manufacture of a plethora of nanoparticles of different sizes, shapes, core physicochemical properties and surface modifications that are being investigated for potential medical applications, particularly for the treatment of cancer. This review focuses on the therapeutic use of customised gold nanoparticles, magnetic nanoparticles and carbon nanotubes that efficiently generate heat upon electromagnetic (light and magnetic fields) stimulation after direct injection into tumours or preferential accumulation in tumours following systemic administration. This review will also focus on the evolving strategies to improve the therapeutic index of prostate cancer treatment using nanoparticle-mediated hyperthermia.
Conclusions: Nanoparticle-mediated thermal therapy is a new and minimally invasive tool in the armamentarium for the treatment of cancers. Unique challenges posed by this form of hyperthermia include the non-target biodistribution of nanoparticles in the reticuloendothelial system when administered systemically, the inability to visualise or quantify the global concentration and spatial distribution of these particles within tumours, the lack of standardised thermal modelling and dosimetry algorithms, and the concerns regarding their biocompatibility. Nevertheless, novel particle compositions, geometries, activation strategies, targeting techniques, payload delivery strategies, and radiation dose enhancement concepts are unique attributes of this form of hyperthermia that warrant further exploration. Capitalising on these opportunities and overcoming these challenges offers the possibility of seamless and logical translation of this nanoparticle-mediated hyperthermia paradigm from the bench to the bedside.
Prostate cancer
Prostate cancer accounted for 25% of estimated newly diagnosed cases of non-skin cancer in men in the USA in 2009. It was predicted that 192,280 new cases of prostate cancer would be diagnosed in the US in 2009, with one in six men developing the disease during their lifetime. Nearly 90% of men with prostate cancer have clinically localised disease. The aggressiveness of prostate cancer is largely determined by the prostate-specific antigen levels, clinical extent and volume of disease, and Gleason histologic score (a measure of glandular differentiation). By predicting the likelihood of adjacent organ invasion, nodal metastasis, distant metastasis, recurrence following treatment, and likelihood of progression without treatment, these factors help stratify tumours into low-, intermediate- and high-risk groups, and aid the choice of treatment options. For low-risk clinically localised disease, a potentially curable stage of disease, common treatments include watchful waiting, radical prostatectomy, external beam radiation therapy (RT) and interstitial RT (brachytherapy), freezing the prostate (cryotherapy), and androgen deprivation therapy (ADT). The choice of treatment is often governed by the patient's life expectancy, the likelihood of cancer progression without treatment, efficacy of treatment, convenience of treatment, treatment costs, and adverse effects (treatment-related urinary, bowel, and sexual dysfunction). For locally advanced cancers, the treatment options are based on the extent of disease but typically involve combinations of the treatments mentioned above with ADT usually being one of them. Less frequently, patients present with metastatic disease and treatment begins with ADT and palliative interventions but may proceed to involve chemotherapy when tumours become resistant to ADT. Locally recurrent prostate cancer is often treated with ADT, salvage radical prostatectomy, salvage brachytherapy, cryotherapy or thermal therapy. The focus of this article is on one form of thermal therapy – hyperthermia.
Hyperthermia for prostate cancer
Hyperthermia generally refers to temperatures between 40°C and 45°C whereas temperatures >45°C are considered thermoablative. Mild temperature hyperthermia mediates its antitumour effects via subtle influences on the tumour microenvironment Citation[1], induction of apoptosis Citation[2], Citation[3], activation of immunological processes Citation[4–6], and induction of gene and protein synthesis Citation[1], Citation[7], Citation[8]. While these effects do not independently cause tumour cell cytotoxicity, they lead to greater effectiveness of other conventional treatment modalities such as RT, chemotherapy, and immunotherapy Citation[9], Citation[10]. In its role as an adjunct to RT, hyperthermia serves as a dose-modifying agent that increases the therapeutic ratio of RT (i.e. enhanced effectiveness of a given dose of RT without additional toxicity). Hyperthermia can be achieved a number of ways including local hyperthermia by external or internal energy sources, regional hyperthermia by irrigation of body cavities or perfusion of organs or limbs, and whole body hyperthermia. Regardless of the mechanism of heating, clinical trials of hyperthermia as stand-alone therapy or in combination with RT have demonstrated promising outcomes in the treatment of many cancers including prostate cancer Citation[11–21]. Although an entirely non-invasive treatment approach is preferred, minimally invasive techniques such as intraluminal or intracavitary treatments are particularly appealing due to the flexibility of positioning endorectal applicators close to the posterior aspect of the prostate or transurethral catheters in the centre of the prostate. This strategy has been employed successfully in the treatment of locally advanced prostate cancer using modalities such as ultrasound, radiofrequency and microwaves with appropriate applicators positioned either externally, intraluminally or interstitially to generate heat. Multiple studies report gratifying outcomes with the use of endorectal microwave or ultrasound applicator mediated hyperthermia in conjunction with conventional RT (thermoradiotherapy) for the treatment of locally advanced prostate cancer Citation[4], Citation[29–32]. Similarly, radiofrequency-induced hyperthermia has been used in the treatment of prostate tumours through intracavitary applicators Citation[29–32]. The main challenge with the intracavitary or intraluminal delivery of hyperthermia is the generation of adequate heat in the prostate without excessive temperature in critical adjacent structures such as the neurovascular bundle, urethra, bladder and rectum Citation[33], Citation[34]. Alternatively, external regional hyperthermia can be used in combination with RT for the treatment of prostate cancer Citation[13]. The lack of significant temperature conformality at the interface between the prostate and the rectum remains a major challenge with this technique. Interstitial hyperthermia offers the possibility of generating uniform temperatures within the prostate without any significant temperature rise in surrounding normal structures, but is an invasive procedure Citation[19], Citation[20], Citation[44–46].
Clinical hyperthermia experience has led to the recognition that high minimum temperatures achieved in most parts of the target volume correlate better with clinical outcome than maximum temperatures attained in small parts of the target volume Citation[38]. A standardised nomenclature has been proposed and validated for the representation of variable time-temperature data as an Arrhenius isoeffect relationship where the total thermal dose is expressed as the cumulative equivalent minutes at 43°C achieved or exceeded in 90% of the prostate (CEM 43°C T90) Citation[12], Citation[39], Citation[40]. The targeted clinical thermal dose for hyperthermia when combined with RT is a CEM 43°C T90 of 5–10 min Citation[12], Citation[26], Citation[41], Citation[42]. Despite the increasingly convincing evidence for clinical hyperthermic radiosensitisation and the evolving consensus in reporting these data, it is underutilised in routine clinical practice due to: (1) the invasive means of achieving and maintaining hyperthermia, (2) the lack of good thermal dosimetry, and (3) the inability to achieve localised hyperthermic temperatures Citation[43]. Hence, a relatively non-invasive approach with externally regulatable and quantifiable prostate-specific hyperthermia could provide renewed enthusiasm for this treatment paradigm.
The above mentioned hyperthermia strategies solely rely on the ability of the cancer tissues to convert the imparted electromagnetic energy into heat. In contrast to methods relying on modifying the energy source to generate heat, there is evolving interest in methods to preferentially enhance the heat generating capacity of the cancer tissues by introducing exogenous materials in to them. Along these lines, ferromagnetic seeds have been used in conjunction with a magnetic field to induce hyperthermia in prostate cancer Citation[44–46]. Ferromagnetic seeds or thermoseeds are needle-shaped devices that are interstitially placed into the tumour, similar to brachytherapy implants, and the heating is accomplished by an externally applied magnetic field. The uniqueness of thermoseed hyperthermia are (1) the lack of requirement for external power connections and (2) the automatic regulation of temperature of the implanted thermoseeds depending on the compositional characteristics of the implants Citation[45], Citation[46]. Being an interstitial modality, thermoseed mediated hyperthermia has limitations similar to other interstitial hyperthermia techniques. Further, due to the heating equipment size and the requirement to limit electromagnetic radiation to meet federal (FCC) regulations, thermoseed implant hyperthermia treatments are performed in special electromagnetic shielded rooms located in dedicated hyperthermia suites. Alternatively, nanoscale materials, particularly metal nanoparticles that are activatable by externally applied electromagnetic fields, can be used to induce cancer-specific hyperthermia.
Nanoparticles
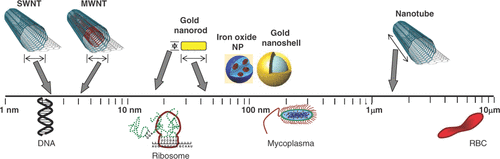
In the broadest sense, nanotechnology involves utilising the unique properties and behaviours of materials made at the nanoscale, a scale that ranges from 1 to 100 nm. At the simplest level, what drives these unique behaviours and properties is the significantly larger surface area per unit volume of nanoscale materials than the same material in the bulk scale – the greater surface area affords greater opportunities for interactions with adjacent materials. Capitalising on these observations, recent advances in nanotechnology have resulted in the manufacture of a plethora of nanoparticles with different sizes, shapes, core physicochemical properties and surface modifications that are being investigated for potential medical applications. From a biological perspective, the size of such particles tends to be similar to that of a DNA doublestrand (2 nm thick), a ribosome (20 nm), or the smallest bacteria (200 nm Mycoplasma) and considerably smaller than the typical eukaryotic cell (7 micron diameter of a small red blood cell). Therefore, systemically administered nanoparticles readily extravasate out of blood vessels and can interact with biomolecules at the cellular and molecular level. The most common nanoparticles studied for biomedical applications are liposomes and uni- or multi-lamellar vesicles (organic biolipid layers encapsulating imaging and therapeutic payloads), dendrimers (repeatedly branched polymers), quantum dots (metallic core-shell nanoparticles that are intensely fluorescent at specific wavelengths), gold nanoparticles (ranging in shape from spheres and shells to rods and cages), paramagnetic nanoparticles (iron oxide-laden particles), and carbon nanotubes. In the arena of cancer research, there has been an explosion of knowledge and research regarding oncological uses of such nanoparticles. In addition to several diagnostic applications of nanoparticles using optical, magnetic resonance, positron emission tomography, computed tomography and X-ray techniques, the therapeutic application of nanoparticles via tumour heating is emerging as a novel form of ‘nanothermal therapy’ of tumours Citation[47]. Although several potential hyperthermic particles such as silver, lanthanum and zinc nanoparticles are available Citation[48], the thermal activation properties of gold nanoparticles, magnetic nanoparticles and carbon nanotubes have been extensively characterised preclinically and they are furthest along in potential translation to clinical biomedical applications. In addition, these particles serve as platforms for development of additional novel nanoparticle ensembles comprised of alloys, dopants and hybrid particles. Hence, this review will focus on the potential application of gold nanoparticles, magnetic nanoparticles and carbon nanotubes (see ) in the thermal therapy of prostate cancer.
Gold nanoparticle-induced hyperthermia
A number of features of gold nanoparticles have rendered them particularly attractive to biomedical researchers and account for their popularity in preclinical research leading up to potential clinical translation. The most striking feature is the familiarity of the medical community with gold as a clinically useful therapeutic agent for various ailments such as melancholy, fainting, fevers, syphilis and arthritis Citation[49], Citation[50]. The most prominent use of gold has been for the treatment of rheumatic arthritis. Treatment of rheumatoid arthritis with a cumulative dose of a little less than 2 g/year for 10 years without any appreciable toxicity speaks to the good overall tolerance of gold in humans Citation[51]. Due to its physical inertness, gold is unlikely to interact chemically with biomolecules in humans. In addition to its apparent clinical safety and tolerability, gold can be used to synthesise nanoparticles with very precise sizes, shapes and surface chemistries at the nanoscale using simple techniques and relatively inexpensive reagents Citation[52]. The most unique property that lends itself to hyperthermia applications is the photothermal activation of gold nanoparticles. However, there are concerns regarding the biocompatibility of these gold nanoparticles for clinical applications. For instance, a recent study noted activation of the immune complement system by gold Citation[53]. While this raises concerns about its clinical safety, the study also reports that when coated on Bactiguard commercial surfaces the gold nanoparticles are less effective in activating the immune complement system, a feature attributed to the altered nanostructure and chemistry of gold nanoparticles and nanogalvanic effects. Furthermore, similar biocompatible coatings such as polyethylene glycol or dextran provide ‘stealth’ characteristics to these particles for evasion of capture by and accumulation within the reticuloendothelial system, further enhancing their biocompatibility.
It is known that at the nanoscale bulk metals exhibit optical resonances of their surface plasmons. In colloidal form, these metals typically absorb and scatter light strongly at a characteristic wavelength (plasmon resonance) in the visible region of the spectrum. Working with wavelengths in the near infrared (NIR) region of the spectrum is clinically meaningful because light penetrates deep within tissue (up to several cm) at these wavelengths. Indeed, certain geometries (spheres, rods and shells) of metal nanoparticles have optical plasmon resonances that can be tuned to the NIR region Citation[54]. While gold nanospheres and nanorods are made of solid gold, nanoshells consist of a dielectric core (e.g. silica) surrounded by a thin gold shell. Nanospheres exhibit resonances around 540 nm without much tuneability of this peak, whereas nanoshells and nanorods have peak resonances that can be tuned throughout the NIR spectrum Citation[55], Citation[56]. Nanoshells are tuned via their core-to-shell ratio while nanorods are tuneable through their aspect ratio (i.e. ratio of the length to diameter). For instance, gold nanoshells comprised of an aminated colloidal silica (120 nm diameter) core with a 14 nm thick shell of gold colloid adsorbed onto it as sequential nucleating sites result in an absorption peak between 780 and 800 nm. Furthermore, due to their metal structure, gold nanoparticles are extremely efficient photothermal coupling devices. Their large absorption cross sections convert light to heat, and their high thermal conductivity couples this heat to the surrounding tissue. Lastly, the handling of gold nanoparticles as devices rather than drugs could reduce time and expense incurred in translating their use from the bench to the bedside.
For clinically pertinent oncological applications, interstitial injection of these particles within tumours is a readily available option but a more exciting and possibly elegant option is to deliver these nanoparticles systemically and have them accumulate within tumours either passively or via active targeting of tumour-specific molecules. Passive yet selective sequestration of nanoparticles within tumours capitalises on a phenomenon termed enhanced permeability and retention (EPR) effect Citation[57], Citation[58] where macromolecules and nanoparticles passively extravasate from leaky tumour vasculature containing wide interendothelial junctions, incomplete or absent basement membranes, dysfunctional lymphatics, and numerous transendothelial channels Citation[59]. In contrast, active targeting is facilitated by functionalising the gold nanoparticle with biomolecules including peptides, antibodies, and oligonucleotides that are specific to the target of interest Citation[60–63].
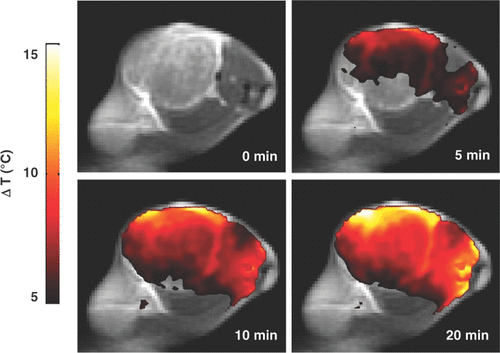
The seminal report on the use of systemically administered non-targeted NIR-activatable gold nanoshells for thermal ablation of tumours in an animal model Citation[64] is built on the initial characterisation of laser dosimetry studies and pharmacokinetic and biodistribution analyses. A subsequent efficacy study in BALB/c mice inoculated subcutaneously with CT26/wt murine colorectal cancer cells demonstrated that 100 µL of 2.4 × 1011 nanoshells/mL administered intravenously resulted in tumour accumulation at 6 h – NIR laser treatment at 4 W/cm2 for 3 min resulted in 90% survival of nanoshell-treated and irradiated mice and 0% survival of nanoshell-treated and unirradiated controls and 0% survival of saline-treated and irradiated controls Citation[65]. Subsequently, several studies have been reported on the use of gold nanoparticles such as gold nanorods and nanocages for the thermal therapy of cancer Citation[66–73]. More recently, the feasibility of using gold nanoshells and optical fibre-based NIR illumination for interstitial thermoablation of intracranial tumours was demonstrated Citation[74]. While these reports involve passive targeting of gold nanoparticles to achieve the photothermal ablation, several other studies document the feasibility and efficacy of tumour-specific active targeting of gold nanoparticles for photothermal therapy of tumours Citation[75–82]. Gold nanoshells have also been used to induce mild temperature hyperthermia in murine tumour models to enhance the therapeutic efficacy of RT. Initial experiments defined the laser parameters for mild temperature hyperthermia and demonstrated that non-invasive magnetic resonance thermal imaging accurately predicted temperature measurements obtained using thermocouples inserted into the tumours (see ). When mild temperature hyperthermia (41°C for 20 minutes) was followed immediately by a single 10 Gy dose of radiation (125 kVp X-rays), there was an approximately 2-fold increase in tumour growth delay (time taken for tumours to double in volume) when compared to the animals treated with radiation alone Citation[83]. As expected, hyperthermia led to an immediate increase in perfusion of the centre of tumours (documented on dynamic contrast enhanced magnetic resonance imaging). Unexpectedly, however, there were large areas of necrosis in the combined treatment group at a later time point. This necrotic pattern of tumour cytotoxicity was attributed to a decrease in microvessel density, possibly due to focal vascular disruption mediated by perivascularly concentrated gold nanoshells that generate intense focal temperature elevations. Presumably, gold nanoshells that are too large to diffuse freely into tumour interstitium but large enough to leak out of tumour vasculature remain sequestered in the perivascular region. Conceivably, as an extension of this concept gold nanorods that are smaller than nanoshells will penetrate deeper into tumours and provide more global temperature elevations within tumours, particularly if they are conjugated to tumour-specific biomarkers.
Figure 2. Spatial temperature map of magnetic resonance thermal imaging indicating temperature rise (°C) above the baseline following near infrared illumination of gold nanoshell-laden tumours (Reproduced with permission from Diagaradjane P, et al. Modulation of in Vivo Tumor Radiation Response via Gold Nanoshell-Mediated Vascular-Focused Hyperthermia: Characterizing an Integrated Antihypoxic and Localized Vascular Disrupting Targeting Strastegy. Nano Letters, 8(5). Copyright 2008 American Chemical Society.)
In spite of these extensive reports on the use of gold nanoparticle-mediated ablation and hyperthermic radiosensitisation, very few reports document its utility in the treatment of prostate cancer. In vitro studies of gold nanoshell-mediated photothermal ablation of PC-3 and C4-2 prostate cancer cells demonstrated a complete loss of cell viability while maintaining intact cellular morphology Citation[84]. This observation correlates well with the intact cellular morphology of clinical biopsies obtained after radiofrequency ablation Citation[85]. A subsequent in vivo study on a murine subcutaneous prostate cancer model compared the therapeutic efficacy of two doses of gold nanoshells (7 µL/g and 8.5 µL/g of body weight) and demonstrated enhanced therapeutic efficacy (93% tumour necrosis and regression with an average temperature rise to 65.4°C) with the high concentration of gold nanoshells Citation[86]. Targeted thermal therapy of PC-3 prostate cancer cell lines using prostate-specific EphrinA1-conjugated gold nanoshells demonstrated localised thermal damage to cells that were bound to the conjugates Citation[87]. Prostate cancer cell specific uptake and toxicity studies of different nanoparticles (gold nanoshells and gold nanorods) have also demonstrated size-dependent uptake and negligible toxicity Citation[88]. It is reasonable to assume that findings from other tumours can be reproduced in prostate cancers to provide justification for advancing gold nanoparticle-mediated hyperthermia in clinical scenarios.
Magnetic nanoparticle induced hyperthermia
Thermotherapy using magnetic nanoparticles involves the coupling of an external magnetic field to tumour-laden magnetic particles to generate high-energy photons through a magnetic field induced locally in the vicinity of the nanoparticle. These high-energy photons result in the observed magnetic hyperthermia effect by the Neel's relaxation process Citation[10]. Although the concept of magnetic hyperthermia was introduced 50 years ago, its clinical potential has recently been recognised based on the achievable selective and relatively homogenous temperature distribution in deep-seated tumours when compared to conventional hyperthermia modalities Citation[89]. Initial studies on the physical evaluation of ferromagnetic particles and magnetic fluids suggested that intratumoural power absorption from a moderate concentration of 5 mg ferrite per g tumour (i.e. 0.5% w/w) and clinically acceptable magnetic fields is comparable to radiofrequency heating with local applicators and superior to regional radiofrequency heating (by comparison with clinical specific absorption rate measurements from regional and local hyperthermia treatments), which is attributed to the much larger number and surface area of magnetic particles Citation[90]. Subsequent in vivo studies were performed in murine models of mammary carcinoma with intratumoural injection of 1.5 × 10−2 mg ferrite/mm3 followed 20–30 min later by intratumoural hyperthermia (steady-state temperatures of 47°C for 30 min) generated by whole-body alternating magnetic fields of 6–12.5 kA/m at 520 kHz. In spite of the inhomogeneity in the intratumoural distribution of magnetic nanoparticles and local tumour regrowth, widespread tumour necrosis was observed after hyperthermia treatment and tumour growth was slightly delayed in comparison with untreated controls Citation[91]. The first in vivo preliminary evaluation in a rat model of prostate cancer demonstrated successful intraprostatic nanoparticle infiltration, excellent tolerability and stable steady-state thermoablative intratumoural temperatures (50°C using a field strength of 15 kA/m) induced by an alternating magnetic field Citation[92]. Further investigation of an orthotopic Dunning R3327 rat prostate cancer model revealed an intra-prostatic temperature of 70°C with a maximum field strength of 18 kA/m resulting in significant growth inhibition of 44–51% over control animals Citation[93]. Subsequent studies of magnetic nanoparticle-mediated hyperthermia in combination with RT (20 Gy) in a rat prostate cancer model demonstrated a therapeutic efficacy equivalent to a single radiation of 60 Gy Citation[94]. Similar results were observed with complete tumour regression 30 days after hyperthermic treatment of DMBA-induced rat mammary cancers using magnetic nanoparticles Citation[95]. In an animal model of metastatic prostate cancer to bone, application of an alternating magnetic field to magnetic nanoparticles conjugated to cationic liposomes demonstrated potent suppression of tumour proliferation in the bone microenvironment Citation[96]. The promising preclinical activity of magnetic nanoparticle-mediated hyperthermia has now been advanced to early clinical experiences discussed later.
Carbon nanotube-induced hyperthermia
Carbon nanotubes (CNTs) are another class of nanomaterials that holds great potential for various biomedical applications including extrinsically activated hyperthermia. CNTs are nested, cylindrical grapheme structures with diameters ranging from a few to hundreds of nanometres and lengths up to a few micrometers Citation[97]. The extraordinary photon-to-thermal energy conversion efficiency of CNTs with high absorption cross-section in the NIR region of the electromagnetic spectrum stimulated several investigations to exploit their potential for anticancer therapy Citation[98], Citation[99]. Several in vitro studies have demonstrated the use of targeted and non-targeted CNTs (single walled) for photothermal ablation of cancer cells Citation[98], Citation[109–112]. While these studies have used NIR irradiation to generate hyperthermia, radiofrequency fields have also been shown to induce thermal toxicity in malignant cells. In this study, internalised single walled CNTs (SWCNTs) in human cancer cells were exposed to a 13.56 mHz radiofrequency field to induce non-invasive, selective, concentration-dependent thermal destruction. Direct intratumoural injection of SWCNTs followed by radiofrequency treatment at 48 h demonstrated complete necrosis of tumours when compared to the controls Citation[105]. In vivo obliteration of solid human epidermoid tumour xenografts in mice without harmful side effects or tumour recurrence for 6 months was demonstrated by combining intratumoural injections of SWCNTs (∼120 mg/ml, 100 µL) with NIR irradiation (808 nm, 76 W/cm3) for 3 min. SWCNTs were completely excreted (in 2 months) via the biliary or urinary pathways Citation[106]. Recently, multi walled CNTs (MWCNTs) have been explored as a mediator for photothermal therapy of cancer because of their enhanced absorption cross-section when compared to SWCNTs Citation[98]. A long-term survival study following a single treatment of kidney tumours with MWCNTs (100 µg/mouse) and NIR radiation (1064 nm; 3 W/cm2) for 30 s demonstrated tumour ablation with minimal local or systemic toxicity. The tumour ablation achieved with a relatively low laser power and very minimal exposure time suggests that the photothermal efficacy of MWCNTs is considerably greater than SWCNTs Citation[107]. More recently, treatment of prostate cancer xenografts in nude mice with MWCNTs demonstrated that DNA-encasement enhanced the heat emission from MWCNTs following NIR irradiation with a 3-fold lower concentration of MWCNTs than that required to impart a 10°C temperature increase in bulk solution. A single intratumoural injection of MWCNTs (100 µL of a 500 µg/mL solution) followed by laser irradiation at 1064 nm, 2.5 W/cm2 resulted in complete eradication of PC3 xenograft tumours Citation[108]. Despite these promising results, toxicity concerns about carbon nanotubes have been attributed to factors such as surface chemistry, degree of aggregation, and chemical functionalisation Citation[109–112]. In addition, the route of administration also contributes the likelihood of toxicity. For instance, exposing the mesothelial lining of the body cavity of mice to long MWCNTs resulted in granuloma formation similar to that noted with asbestos exposure Citation[113]. Similarly, in a manner reminiscent of asbestos-associated pleural fibrosis and mesothelioma formation, MWCNTs reach the subpleura in mice after a single inhalation exposure Citation[114]. Nonetheless, at low doses, ranging from 20–850 µg/kg body weight, carbon nanotubes are non-toxic in mice Citation[115–118]. Even at high oral doses of 1000 mg/kg body weight, a more recent study has demonstrated that carbon nanotubes are non-toxic Citation[119]. Clearly, the toxicity of carbon nanotubes needs to be carefully evaluated in parallel with continued exploration of the use of carbon nanotubes for biomedical applications.
Clinical experience
The first indication of clinical feasibility of nanoparticle-based hyperthermia treatment was provided by a German pilot study in 10 patients with biopsy-proven locally recurrent prostate cancer following prior RT. In the absence of an extant standard treatment for recurrent prostate cancer, patients were eligible as long as they were either not suitable for or refused salvage radical prostatectomy. In an approach similar to prostate brachytherapy, patients under general anaesthesia underwent transrectal ultrasound/fluoroscopy-guided external template-assisted transperineal intraprostatic injection of a nanoparticle dispersion to achieve a three-dimensional distribution paralleling a preplan Citation[120]. The magnetic nanoparticles had an average core size of 15 nm with an aminosilane-type shell and remained stable in the injected location for all six weeks of treatment. Computed tomography images allowed visualisation of the spatial distribution of nearly 90% of all magnetic nanoparticles in suspension (112 mg/mL of ferrites in aqueous solution) whereas ultrasound and MRI were incapable of providing similar anatomic definition. A customised magnetic field applicator MFH 300 F (MagForce Nanotechnologies, AG, Berlin, Germany) Citation[121] provided the 100 kHz alternating magnetic field at a variable field strength beginning at 2.5 kA/m and escalated to 18 kA/m as tolerated by the unanaesthetised patient. A median temperature of 40.1°C was attained in 90% of prostates and a median CEM 43°C T90 of 7.8 min was achieved over six 60 min weekly treatments. A 4–5 kA/m constant magnetic field strength was tolerated for the entire hour by all patients. With a median follow-up of 17.5 months, no systemic toxicity was noted Citation[122]. Acute urinary retention occurred in four patients with pre-existing urethral strictures. Quality of life assessments detected acute (midway through and upon completion of 6-week course) decline in social and sexual functioning, and increased fatigue, pain, and urinary symptoms. Later (3–6 months from treatment), only deterioration in social functioning was recorded.
Challenges with nanoparticle-mediated hyperthermia
Biodistribution
Even a single injection of nanoparticles directly into the prostate, in an idealised hypothetical scenario, leads to a non-uniform spatial distribution of particles that is defined by the injection volume, the injection rate, the concentration of the particles and the resilience of the tissue Citation[123]. The heterogeneity in intraprostatic biodistribution of nanoparticles is compounded by the need for multiple injections to encompass the entire prostate. It is well recognised that the spatial distribution of nanoparticles dominates the resulting spatial distribution of temperature within the prostate Citation[124]. In the case of systemically delivered nanoparticles not only is there heterogeneous accumulation within tumours, there is also considerable variability in organ/tissue biodistribution and pharmacokinetics. For instance, the kinetics of accumulation within tumours is influenced by the hydrodynamic diameter Citation[125], shape Citation[126], surface charge Citation[127], and the extent Citation[127], length Citation[128] and branching Citation[129] of the polyethylene glycol surface coating often used to confer ‘stealth’ properties for immune evasion from the reticuloendothelial system. Experimental evidence suggests that spherical nanoparticles with a hydrodynamic diameter of approximately 5.5 nm and a zwitterionic surface charge are cleared by the kidneys and not entrapped within the reticuloendothelial system Citation[125], whereas larger nanoparticles (20 nm) are captured by the reticuloendothelial macrophages Citation[130]. At the tissue-level, leaky vascular fenestrations and chaotic immature vascular architecture largely determine the geographic distribution of nanoparticles within tumours – untargeted nanoparticles, irrespective of their size, leak out of tumour vasculature and remain sequestered and spatially confined to the perivascular zone Citation[130] Citation[83]. The heterogeneity in vascular architecture and the consequent heterogeneity in intratumoural distribution of nanoparticles results in non-uniform temperature profiles within tumours when these nanoparticles are activated. Some reports would suggest that this heterogeneity is advantageous when combined with radiation since focal vascular disruption may ensue following the juxtaposition of this form of hyperthermia with RT Citation[83]. Therefore, in contrast to the vascular compartment of tumours serving as a heat sink with traditional forms of hyperthermia, in this instance the preferential heating of nanoparticles entrapped within perivascular spaces focuses thermal energy on endothelial cells, a prime target for radiosensitisation strategies. Despite this potential advantage, the heterogeneous distribution of nanoparticles, the inhomogeneity of NIR density (greater intensity closer to the illumination source), and difficulty with modelling and dosimetry make nanoparticle-mediated hyperthermia challenging. To some extent, the heterogeneity in the nanoparticle distribution can be overcome by specifically targeting tumour interstitium-penetrating small nanoparticles to tumour-specific biomarkers for a relatively uniform and homogeneous distribution Citation[130].
Quantification and visualisation
One of the challenges of using nanoparticles for cancer therapy is the inability to readily quantify and/or visualise these particles after they have accumulated within tumours. As noted above, magnetic nanoparticles are not visualised on MRI due to a signal void in the areas containing high concentrations of interstitially injected iron oxide nanoparticles Citation[131]. Similarly, they are not readily visualised on transrectal ultrasound. CT imaging was able to detect large deposits of injected magnetic nanoparticles but this sensitivity would be much lower if the concentration of nanoparticles is considerably lower, as in the case of systemically administered nanoparticles. For such instances, dedicated techniques are needed to quantify the amount of nanoparticles globally present within tumours as well as to visualise geographic locations of these nanoparticles within tumours. In the case of gold nanoparticles, one technique that non-invasively estimates the quantity of gold (and therefore, the number of gold nanoparticles) within tumours in real time is diffuse optical spectroscopy (DOS). In DOS, light is delivered to and collected from tissue via an optical fibre probe, and the specific reflectance spectra of gold nanoparticles are used to indirectly measure gold concentrations after accounting for the contribution of oxy-haemoglobin and deoxy-haemoglobin. In one such study, DOS measurements accurately quantified the concentration of gold nanoshells in tissue phantoms within 10% of the known concentration as well as in vivo where gold content measurements were validated by neutron activation analysis, the standard method of measuring gold nanoshell concentrations in tissues that are excised, dehydrated and irradiated within a nuclear reactor Citation[132]. While DOS provides a global estimate of gold nanoparticle concentration within tumours, it does not provide spatial information on the distribution of these nanoparticles within tumours. One technique that offers this option is narrowband imaging which capitalises on the strong NIR absorption of gold nanoshells to distinguish between blood and nanoshells in the tumour by imaging in narrow wavelength bands in the visible and NIR, respectively Citation[133]. By clearly discriminating between blood and gold nanoshells, this technique allows imaging of the heterogeneous spatial distribution of nanoshells within tumours. This geographic distribution imaging can be verified ex vivo by two-photon luminescence imaging. Alternative strategies include radiolabelling the nanoparticle for visualisation by positron emission tomography or single photon emission computed tomography, fluorescent dye labelling for optical tomography of enhanced fluorescence Citation[134], X-ray fluorescence computed tomography Citation[135], optical coherence tomography Citation[136], or NIR diffuse optical tomography Citation[137].
Modelling and dosimetry
Visualising and quantifying gold nanoparticles accurately in tumours is a prelude to modelling and mapping the temperature elevations within tumours and generating dosimetry outputs similar to RT. For instance, continued development and clinical translation of gold nanoshell-mediated thermal therapy requires computational tools for estimating heat distribution within the tumour and surrounding tissues. One model for estimating heat from NIR laser activation of gold nanoshells uses a modified bioheat equation with a finite element model based on alterations in the optical properties of the medium when laden with nanoshells to predict temperature rise and magnetic resonance thermal imaging to validate it Citation[138]. However, this model requires prior knowledge of the altered optical properties of the medium with nanoshells, which might not be readily obtained during routine in-vivo experiments or in future clinical applications. An alternative technique is to use the light transport theory with a diffusion approximation to model the temperature rise within nanoshell-free media due to NIR laser power dissipation and combine this with modelling of plasmonic heat generated by NIR irradiated individual gold nanoshells due to photothermal effect to estimate the global elevation of temperature within nanoshell-laden media Citation[139]. This model accounts for the response of tissue to laser illumination as well as the optical and thermal effects due to embedded individual gold nanoshells on the temperature rise in tissues. Similarly, in the clinical study described earlier, real-time thermal measurements in four catheters (two in each lobe of the prostate) were fitted to thermal dosimetry calculations using the bio-heat transfer equation solved on a finite element basis using iron-mass (derived from CT density), specific absorption rate (SAR) of magnetic nanoparticles, magnetic field strength, and an estimated perfusion Citation[131]. In the earliest incarnation of individualised non-invasive three-dimensional thermal modelling based on spatial distributions of intraprostatic magnetic nanoparticles, the model overestimated temperatures near the urethra and bladder base, and underestimated temperatures at the prostatic apex and along skin folds. On the flip side, the heterogeneity of nanoparticle distribution (a function of injection flow rate, concentration and firmness of a previously radiated prostate) Citation[123] and consequent non-uniform intraprostatic temperatures also results in an overestimation of actual temperature by intraluminal measurements. Nonetheless, the reasonable agreement between non-invasive temperature calculations and invasive thermometry within the prostate suggests that thermal modelling could provide a global assessment of temperature across a target volume that complements focal thermal monitoring. Admittedly, unlike radiation dosimetry that is based on physical parameters (radiation quality, radiation quantity, tissue density and tissue geometry), thermal dosimetry also needs to account for tissue physiology (heat dissipation and transfer being modulated by vascularity, degree of necrosis/fibrosis, tissue conductivity/perfusion, and the influence of heat itself on these parameters) Citation[140]. Continued refinement of thermal dosimetry algorithms incorporating normal tissue avoidance constraints could guide the selection of injection coordinates for highly conformal thermotherapy Citation[124]. If predictive models do not turn out to be sufficiently accurate, magnetic resonance thermometry currently provides accurate and real-time spatiotemporal resolution of thermal dose without requiring accurate heat transfer models and precise knowledge of local particle concentrations. Magnetic resonance thermometry capitalises on the correlation between a shift in proton resonance frequency and elevation of temperature within tissue to facilitate mapping of temperature distributions Citation[141]. Typically, after obtaining a reference (baseline) scan and a measurement scan, phase subtraction allows computation of a proton resonance frequency shift. Since the proton resonance frequency of adipose tissue does not shift as a function of temperature, nulling these regions (fat correction) can be utilised for calibration and accounting for baseline drift. This novel non-invasive thermal imaging remains one of the most significant technical advances in clinical applications of thermal therapy that allows real-time temperature monitoring, confirmation of adequate thermal dose coverage and adaptive course-correction during treatment.
Biocompatibility
A legitimate concern with all classes of nanoparticles is their toxicity, which is in turn determined by the dose, core composition, surface chemistry, and location and duration of confinement to the body. Whereas the use of gold and iron oxide in medicine for many decades offers some degree of familiarity with the safety profile of these bulk metals, the unique properties and biodistribution of nanoscale formulations of these metals calls for systematic evaluation and characterisation of their biocompatibility. The National Institutes of Health also recognises the need for toxicity testing of nanoparticles to parallel preclinical efficacy assessment - the Nanotechnology Characterisation Laboratory, working in concert with the National Institute of Standards and Technology (NIST) and the US Food and Drug Administration (FDA), facilitates such testing and regulatory review. Gold nanoshells have been extensively tested preclinically and are now in clinical trials as investigational new devices.
Unique opportunities with nanoparticle- mediated hyperthermia
Active targeting
As noted above, a unique feature of systemic administration of nanoparticles for hyperthermia is the ability to potentially target the nanoparticle preferentially to tumour cells, thereby increasing tumour specificity and reducing collateral damage to surrounding critical structures. This has been demonstrated in vitro using ephrinA 1 Citation[87]. Similarly, circulating nanoparticles Citation[142] could be guided onto prostate-specific membrane antigen, an integral transmembrane glycoprotein expressed on the surface of prostate carcinoma at all stages of the cancer Citation[143] but highly restricted in extraprostatic tissues and normal endothelial cells Citation[144]. Alternatively, rather than focusing delivery on prostate cancer cells, the nanoparticles can be delivered to prostate cancer neovasculature by targeting molecules such as integrin αvβ3, a cell adhesion molecule that is significantly up-regulated on endothelia during angiogenesis and on fast-growing solid tumour cells, but not on quiescent endothelium and normal tissues Citation[145]. Nevertheless, although targeted therapy is highly appealing as a means of treating prostate cancer without excessive collateral damage to surrounding normal tissue, a major limitation of such treatments for prostate cancer, which is typically a multifocal disease, is the lack of contemporary diagnostic imaging modalities to visualise localised foci of involvement.
Targeted payload delivery
Another unique feature of nanoparticle-mediated hyperthermia is the possibility of delivering an entirely different payload to the tumour when the nanoparticle concentrates within it. This is in contrast to using hyperthermia to trigger release of payloads contained with separate thermosensitive nanoparticles – this form of hyperthermia-triggered payload release would potentially be applicable to any form of hyperthermia. Instead, this feature refers to hybrid nanoparticles that serve as activatable thermal sources as well as therapeutic payload carriers. As a first step in achieving this goal it has been shown that pretreatment with intravenously administered TNF-α-coated gold nanoparticles enhances thermally induced tumour growth delay in a mouse model of breast cancer Citation[146]. Future applications could entail externally triggered, thermally mediated release of payloads within the confines of the tumour alone – the payloads could include targeted therapeutic peptides/proteins, toxins, oligonucleotides and small interfering RNA.
Radiation dose enhancement
One additional benefit of combining RT with metal nanoparticle-mediated hyperthermia is the possibility of radiation dose enhancement due greater photoelectric interactions in the presence of higher atomic number (Z) gold preferentially within tumours. Irradiation of tumour-bearing mice after injecting gold nanoparticles was shown to induce remarkable tumour regression and long-term survival without any significant toxicity when compared to mice irradiated without gold nanoparticles Citation[147]. This dramatic outcome was attributed to significant radiation dose enhancement within gold nanoparticle-laden tumours and their vasculature during X-ray irradiation Citation[148]. A subsequent Monte Carlo computational study confirmed that the macroscopic (or average) tumour dose enhancement in the original animal study was dependent on gold concentration within the tumour and the photon beam quality, ranging from several hundred per cent for diagnostic X-rays to a few per cent for typical megavoltage photon beams Citation[149]. Since low-energy photons interact with gold nanoparticles within the tumour predominantly via photoelectric effect, additional computational studies suggested that macroscopic dose enhancement might be more pronounced with low energy gamma ray sources such as I-125 and Pd-103 typically used in prostate brachytherapy Citation[150], Citation[151].
Conclusions
Nanoparticle-mediated hyperthermia promises to be a new minimally invasive tool in the armamentarium for the treatment of prostate cancer. A greater understanding and increasing research related to novel particle compositions, geometries, activation strategies, targeting techniques, payload delivery strategies and radiation dose enhancement concepts are likely to energise this field in the coming years. Accurate modelling and dosimetry are likely to facilitate seamless and logical transition from the bench to the bedside.
Acknowledgements
We wish to thank David Aten from the medical graphics and photography department at the M.D. Anderson Cancer Center for assistance with preparing figures.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Horsman MR, Overgaard J. Can mild hyperthermia improve tumour oxygenation?. Int J Hyperthermia 1997; 13: 141–147
- Harmon BV, Takano YS, Winterford CM, Gobe GC. The role of apoptosis in the response of cells and tumours to mild hyperthermia. Int J Radiat Biol 1991; 59: 489–501
- Fuller KJ, Issels RD, Slosman DO, Guillet JG, Soussi T, Polla BS. Cancer and the heat shock response. Eur J Cancer 1994; 30A: 1884–1891
- Servadio C, Leib Z. Local hyperthermia for prostate cancer. Urology 1991; 38: 307–309
- Stawarz B, Zielinski H, Szmigielski S, Rappaport E, Debicki P, Petrovich Z. Transrectal hyperthermia as palliative treatment for advanced adenocarcinoma of prostate and studies of cell‐mediated immunity. Urology 1993; 41: 548–553
- Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: A temperature's story. Cancer Lett 2008; 271: 191–204
- Roti Roti JL. Introduction: Radiosensitization by hyperthermia. Int J Hyperthermia 2004; 20: 109–114
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int J Radiat Biol 2001; 77: 399–408
- vander Zee J. Heating the patient: A promising approach?. Ann Oncol 2002; 13: 1173–1184
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345: 540–543
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Anscher MS, Samulski TV, Dodge R, Prosnitz LR, Dewhirst MW. Combined external beam irradiation and external regional hyperthermia for locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 1997; 37: 1059–1065
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, vander Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- vander Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- Satoh T, Seilhan TM, Stauffer PR, Sneed PK, Fike JR. Interstitial helical coil microwave antenna for experimental brain hyperthermia. Neurosurgery 1988; 23: 564–569
- Shimm DS, Hynynen KH, Anhalt DP, Roemer RB, Cassady JR. Scanned focussed ultrasound hyperthermia: Initial clinical results. Int J Radiat Oncol Biol Phys 1988; 15: 1203–1208
- Sherar MD, Trachtenberg J, Davidson SR, Gertner MR. Interstitial microwave thermal therapy and its application to the treatment of recurrent prostate cancer. Int J Hyperthermia 2004; 20: 757–768
- Sherar MD, Trachtenberg J, Davidson SR, McCann C, Yue CK, Haider MA, Gertner MR. Interstitial microwave thermal therapy for prostate cancer. J Endourol 2003; 17: 617–625
- Baronzio G, Gramaglia A, Fiorentini G. Review. Current role and future perspectives of hyperthermia for prostate cancer treatment. In Vivo 2009; 23: 143–146
- Mendecki J, Friedenthal E, Botstein C, Paglione R, Sterzer F. Microwave applicators for localized hyperthermia treatment of cancer of the prostate. Int J Radiat Oncol Biol Phys 1980; 6: 1583–1588
- Yerushalmi A, Servadio C, Leib Z, Fishelovitz Y, Rokowsky E, Stein JA. Local hyperthermia for treatment of carcinoma of the prostate: A preliminary report. Prostate 1982; 3: 623–630
- Algan O, Fosmire H, Hynynen K, Dalkin B, Cui H, Drach G, Stea B, Cassady JR. External beam radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate carcinoma. Cancer 2000; 89: 399–403
- Fosmire H, Hynynen K, Drach GW, Stea B, Swift P, Cassady JR. Feasibility and toxicity of transrectal ultrasound hyperthermia in the treatment of locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 1993; 26: 253–259
- Hurwitz MD, Kaplan ID, Hansen JL, Prokopios‐Davos S, Topulos GP, Wishnow K, Manola J, Bornstein BA, Hynynen K. Hyperthermia combined with radiation in treatment of locally advanced prostate cancer is associated with a favourable toxicity profile. Int J Hyperthermia 2005; 21: 649–656
- Hurwitz MD, Kaplan ID, Svensson GK, Hynynen K, Hansen MS. Feasibility and patient tolerance of a novel transrectal ultrasound hyperthermia system for treatment of prostate cancer. Int J Hyperthermia 2001; 17: 31–37
- Hurwitz MD, Kaplan ID, Hansen JL, Prokopios-Davos S, Topulos GP, Wishnow K, Manola J, Bornstein BA, Hynynen K. Association of rectal toxicity with thermal dose parameters in treatment of locally advanced prostate cancer with radiation and hyperthermia. Int J Radiat Oncol Biol Phys 2002; 53: 913–918
- Zargar Shoshtari MA, Mirzazadeh M, Banai M, Jamshidi M, Mehravaran K. Radiofrequency-induced thermotherapy in benign prostatic hyperplasia. Urol J 2006; 3: 44–48
- Bhowmick S, Swanlund DJ, Coad JE, Lulloff L, Hoey MF, Bischof JC. Evaluation of thermal therapy in a prostate cancer model using a wet electrode radiofrequency probe. J Endourol 2001; 15: 629–640
- Dawkins GP, Harrison NW, Ansell W. Radiofrequency heat-treatment to the prostate for bladder outlet obstruction associated with benign prostatic hyperplasia: A 4-year outcome study. Br J Urol 1997; 79: 910–914
- Sofras F, Sakkas G, Kontothanassis D, Lyssiotis F, Tamvakis N. Transurethral thermotherapy in the management of benign prostatic hyperplasia. Int Urol Nephrol 1996; 28: 673–679
- Gillett MD, Gettman MT, Zincke H, Blute ML. Tissue ablation technologies for localized prostate cancer. Mayo Clin Proc 2004; 79: 1547–1555
- Marberger M. Energy-based ablative therapy of prostate cancer: High-intensity focused ultrasound and cryoablation. Curr Opin Urol 2007; 17: 194–199
- Prionas SD, Kapp DS, Goffinet DR, Ben-Yosef R, Fessenden P, Bagshaw MA. Thermometry of interstitial hyperthermia given as an adjuvant to brachytherapy for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys 1994; 28: 151–162
- Emami B, Scott C, Perez CA, Asbell S, Swift P, Grigsby P, Montesano A, Rubin P, Curran W, Delrowe J, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the Radiation Therapy Group. Int J Radiat Oncol Biol Phys 1996; 34: 1097–1104
- Lancaster C, Toi A, Trachtenberg J. Interstitial microwave thermoablation for localized prostate cancer. Urology 1999; 53: 828–831
- Dewhirst MW, Sim DA, Sapareto S, Connor WG. Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res 1984; 44: 43–50
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L, Case B, Rosner G, Azuma C, Poulson J, Pruitt AF, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res 2005; 11: 5206–5014
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, George SL. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993; 25: 289–297
- Tilly W, Gellermann J, Graf R, Hildebrandt B, Weissbach L, Budach V, Felix R, Wust P. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol 2005; 181: 35–41
- Moros EG, Corry PM, Orton CG. Thermoradiotherapy is underutilized for the treatment of cancer. Med Phys 2007; 34: 1–4
- Brezovich IA, Meredith RF. Practical aspects of ferromagnetic thermoseed hyperthermia. Radiol Clin North Am 1989; 27: 589–602
- Meredith RF, Brezovich IA, Weppelmann B, Henderson RA, Brawner WR, Jr, Kwapien RP, Bartolucci AA, Salter MM. Ferromagnetic thermoseeds: Suitable for an afterloading interstitial implant. Int J Radiat Oncol Biol Phys 1989; 17: 1341–1346
- Partington BP, Steeves RA, Su SL, Paliwal BR, Dubielzig RR, Wilson JW, Brezovich IA. Temperature distributions, microangiographic and histopathologic correlations in normal tissue heated by ferromagnetic needles. Int J Hyperthermia 1989; 5: 319–327
- Sharma R, Chen CJ. Newer nanoparticles in hyperthermia treatment and thermometry. Journal of Nanoparticle Research 2009; 11: 671–689
- Naruse S, Higuchi T, Horikawa Y, Tanaka C, Nakamura K, Hirakawa K. Radiofrequency hyperthermia with successive monitoring of its effects on tumors using NMR spectroscopy. Proc Natl Acad Sci U S A 1986; 83: 8343–8347
- Higby GJ. Gold in medicine: Review of its use in the west before 1900. Gold Bull 1982; 15: 130–140
- Parish RV. Biologically-active gold(III) complexes. Met Based Drugs 1999; 6: 271–276
- Abrams MJ, Murrer BA. Metal compounds in therapy and diagnosis. Science 1993; 261: 725–730
- Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004; 104: 293–346
- Hulander M, Hong J, Andersson M, Gerven F, Ohrlander M, Tengvall P, Elwing H. Blood interactions with noble metals: Coagulation and immune complement activation. ACS Appl Materials Interfaces 2009; 1: 1053–1062
- Oldenburg SJ, Jackson JB, Westcott SL, Halas NJ. Infrared extinction properties of gold nanoshells. Appl Phys Lett 1999; 75: 2897–2899
- Jain PK, Lee KS, El‐Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J Phys Chem B 2006; 110: 7238–7248
- Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chem Phys Lett 1998; 288: 243–247
- McDonald DM, Choyke PL. Imaging of angiogenesis: From microscope to clinic. Nat Med 2003; 9: 713–725
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 2003; 163: 1801–1815
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng 1999; 1: 241–263
- Han S, Lin J, Zhou F, Vellanoweth RL. Oligonucleotide-capped gold nanoparticles for improved atomic force microscopic imaging and enhanced selectivity in polynucleotide detection. Biochem Biophys Res Commun 2000; 279: 265–269
- Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine 2006; 1: 149–154
- Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett 2005; 5: 709–711
- Khan MK, Minc LD, Nigavekar SS, Kariapper MS, Nair BM, Schipper M, Cook AC, Lesniak WG, Balogh LP. Fabrication of {198Au0} radioactive composite nanodevices and their use for nanobrachytherapy. Nanomedicine 2008; 4: 57–69
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell‐mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA 2003; 100: 13549–13554
- O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 2004; 209: 171–176
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 2006; 128: 2115–2120
- Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X, Marquez M, Xia Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem Soc Rev 2006; 35: 1084–1094
- Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomed 2007; 2: 125–132
- von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res 2009; 69: 3892–3900
- Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett 2008; 269: 57–66
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 2008; 23: 217–228
- Skrabalak SE, Au L, Lu X, Li X, Xia Y. Gold nanocages for cancer detection and treatment. Nanomed 2007; 2: 657–668
- Wu X, Ming T, Wang X, Wang P, Wang J, Chen J. High-photoluminescence-yield gold nanocubes: For cell imaging and photothermal therapy. ACS Nano 2010;4:113–120.
- Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Feasibility study of particle‐assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res 2009; 69: 1659–1667
- Cheng FY, Chen CT, Yeh CS. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica/Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology 2009; 20: 425104
- Kawano T, Niidome Y, Mori T, Katayama Y, Niidome T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjug Chem 2009; 20: 209–212
- Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir 2008; 24: 11860–11865
- Liu SY, Liang ZS, Gao F, Luo SF, Lu GQ. In vitro photothermal study of gold nanoshells functionalized with small targeting peptides to liver cancer cells. J Mater Sci Mater Med 2010;21:665–674.
- Bernardi RJ, Lowery AR, Thompson PA, Blaney SM, West JL. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J Neurooncol 2008; 86: 165–172
- Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson TA, Tam JO, Ingram DR, Paramita V, Villard JW, et al. Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. ACS Nano 2009; 3: 2686–2696
- Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res 2003; 63: 1999–2004
- Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat Protoc 2008; 3: 314–320
- Diagaradjane P, Shetty A, Wang JC, Elliott AM, Schwartz J, Shentu S, Park HC, Deorukhkar A, Stafford RJ, Cho SH, et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: Characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett 2008; 8: 1492–1500
- Stern JM, Stanfield J, Lotan Y, Park S, Hsieh JT, Cadeddu JA. Efficacy of laser-activated gold nanoshells in ablating prostate cancer cells in vitro. J Endourol 2007; 21: 939–943
- Margulis V, Matsumoto ED, Lindberg G, Tunc L, Taylor G, Sagalowsky AI, Cadeddu JA. Acute histologic effects of temperature-based radiofrequency ablation on renal tumor pathologic interpretation. Urology 2004; 64: 660–663
- Stern JM, Stanfield J, Kabbani W, Hsieh JT, Cadeddu JA. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol 2008; 179: 748–753
- Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine 2008; 3: 351–358
- Malugin A, Ghandehari H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J Appl Toxicol 2009;30:212–217.
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and future directions. Int J Hyperthermia 2002; 18: 267–284
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993; 9: 51–68
- Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia 1997; 13: 587–605
- Johannsen M, Jordan A, Scholz R, Koch M, Lein M, Deger S, Roigas J, Jung K, Loening S. Evaluation of magnetic fluid hyperthermia in a standard rat model of prostate cancer. J Endourol 2004; 18: 495–500
- Johannsen M, Thiesen B, Jordan A, Taymoorian K, Gneveckow U, Waldofner N, Scholz R, Koch M, Lein M, Jung K, et al. Magnetic fluid hyperthermia (MFH) reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate 2005; 64: 283–292
- Johannsen M, Thiesen B, Gneveckow U, Taymoorian K, Waldofner N, Scholz R, Deger S, Jung K, Loening SA, Jordan A. Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic rat model of prostate cancer. Prostate 2006; 66: 97–104
- Motoyama J, Yamashita N, Morino T, Tanaka M, Kobayashi T, Honda H. Hyperthermic treatment of DMBA-induced rat mammary cancer using magnetic nanoparticles. Biomagn Res Technol 2008; 6: 2
- Kawai N, Futakuchi M, Yoshida T, Ito A, Sato S, Naiki T, Honda H, Shirai T, Kohri K. Effect of heat therapy using magnetic nanoparticles conjugated with cationic liposomes on prostate tumor in bone. Prostate 2008; 68: 784–792
- Zheng LX, O'Connell MJ, Doorn SK, Liao XZ, Zhao YH, Akhadov EA, Hoffbauer MA, Roop BJ, Jia QX, Dye RC, et al. Ultralong single-wall carbon nanotubes. Nat Mater 2004; 3: 673–676
- Kam NW, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 2005; 102: 11600–11605
- O'Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002; 297: 593–596
- Chakravarty P, Marches R, Zimmerman NS, Swafford AD, Bajaj P, Musselman IH, Pantano P, Draper RK, Vitetta ES. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc Natl Acad Sci USA 2008; 105: 8697–8702
- Wang CH, Huang YJ, Chang CW, Hsu WM, Peng CA. In vitro photothermal destruction of neuroblastoma cells using carbon nanotubes conjugated with GD2 monoclonal antibody. Nanotechnology 2009; 20: 315101
- Torti SV, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti FM, Akman S, Liu J, Ajayan PM, et al. Thermal ablation therapeutics based on CN(x) multi-walled nanotubes. Int J Nanomedicine 2007; 2: 707–714
- Biris AS, Boldor D, Palmer J, Monroe WT, Mahmood M, Dervishi E, Xu Y, Li Z, Galanzha EI, Zharov VP. Nanophotothermolysis of multiple scattered cancer cells with carbon nanotubes guided by time-resolved infrared thermal imaging. J Biomed Opt 2009; 14: 021007
- Mahmood M, Karmakar A, Fejleh A, Mocan T, Iancu C, Mocan L, Iancu DT, Xu Y, Dervishi E, Li Z, et al. Synergistic enhancement of cancer therapy using a combination of carbon nanotubes and anti-tumor drug. Nanomed 2009; 4: 883–893
- Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007; 110: 2654–2665
- Moon HK, Lee SH, Choi HC. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 2009; 3: 3707–3713
- Burke A, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA 2009; 106: 12897–12902
- Ghosh S, Dutta S, Gomes E, Carroll D, D'Agostino R, Jr, Olson J, Guthold M, Gmeiner WH. Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano 2009; 3: 2667–2573
- Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Doyle CD, West JL, Billups WE, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 2006; 161: 135–142
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forro L. Cellular toxicity of carbon-based nanomaterials. Nano Lett 2006; 6: 1121–1125
- Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett 2007; 168: 121–131
- Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett 2006; 6: 1522–1528
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 2008; 3: 423–428
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol 2009; 4: 747–751
- Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol 2007; 2: 47–52
- Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci USA 2008; 105: 1410–1415
- Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol 2008; 3: 216–221
- Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA 2006; 103: 3357–3362
- Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to swiss mice. ACS Nano 2010;23:1481–1492.
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperthermia 2005; 21: 637–647
- Gneveckow U, Jordan A, Scholz R, Bruss V, Waldofner N, Ricke J, Feussner A, Hildebrandt B, Rau B, Wust P. Description and characterization of the novel hyperthermia- and thermoablation-system MFH 300 F for clinical magnetic fluid hyperthermia. Med Phys 2004; 31: 1444–1451
- Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, Jung K, Jordan A, Wust P, Loening SA. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int J Hyperthermia 2007; 23: 315–323
- Salloum M, Ma RH, Weeks D, Zhu L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int J Hyperthermia 2008; 24: 337–345
- Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: Optimization of the heat absorption pattern. Int J Hyperthermia 2009; 25: 309–321
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol 2007; 25: 1165–1170
- Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 2009; 26: 244–249
- Akiyama Y, Mori T, Katayama Y, Niidome T. The effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing mice. J Control Release 2009; 139: 81–84
- Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett 2009; 9: 2354–2359
- Veronese FM. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001; 22: 405–417
- Diagaradjane P, Orenstein-Cardona JM, Colon-Casasnovas NE, Deorukhkar A, Shentu S, Kuno N, Schwartz DL, Gelovani JG, Krishnan S. Imaging epidermal growth factor receptor expression in vivo: Pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobe. Clin Cancer Res 2008; 14: 731–741
- Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, Scholz R, Jordan A, Loening SA, Wust P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 2007; 52: 1653–1661
- Zaman RT, Diagaradjane P, Wang J, Swartz J, Gill-Sharp K, Rajaram N, Payne DJ, Krishnan S, Tunnell JW. In vivo detection of gold nanoshells in tumors using diffuse optical spectroscopy. IEEE J Sel Top Quant Elec 2007; 13: 1715–1720
- Puvanakrishnan P, Park J, Diagaradjane P, Schwartz JA, Coleman CL, Gill-Sharp KL, Sang KL, Payne JD, Krishnan S, Tunnell JW. Near-infrared narrow-band imaging of gold/silica nanoshells in tumors. J Biomed Opt 2009; 14: 024044
- Bardhan R, Grady NK, Cole JR, Joshi A, Halas NJ. Fluorescence enhancement by Au nanostructures: Nanoshells and nanorods. ACS Nano 2009; 3: 744–752
- Cheong SK, Jones BL, Siddiqi AK, Liu F, Manohar N, Cho SH. X-ray fluorescence computed tomography (XFCT) imaging of gold nanoparticle-loaded objects using 110 kVp x-rays. Phys Med Biol 2009; 55: 647–662
- Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett 2007; 7: 1929–1934
- Wu CF, Liang XP, Jiang HB. Metal nanoshells as a contrast agent in near-infrared diffuse optical tomography. Opt Commun 2005; 253: 214–221
- Elliott A, Schwartz J, Wang J, Shetty A, Hazle J, Stafford JR. Analytical solution to heat equation with magnetic resonance experimental verification for nanoshell enhanced thermal therapy. Lasers Surg Med 2008; 40: 660–665
- Cheong SK, Krishnan S, Cho SH. Modeling of plasmonic heating from individual gold nanoshells for near-infrared laser-induced thermal therapy. Med Phys 2009; 36: 4664–4671
- Hurwitz M. Editorial: Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 2007; 52: 1661–1662
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia 2006; 22: 255–262
- Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 2004; 22: 969–976
- Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein?. Am J Physiol Cell Physiol 2005; 288: C975–981
- Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81–85
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer 2002; 2: 91–100
- Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischof JC. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther 2006; 5: 1014–1020
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004; 49: N309–315
- Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol 2008; 60: 977–985
- Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Phys Med Biol 2005; 50: N163–173
- Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/X-ray sources. Phys Med Biol 2009; 54: 4889–4905
- Roeske JC, Nunez L, Hoggarth M, Labay E, Weichselbaum RR. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol Cancer Res Treat 2007; 6: 395–401