Abstract
Purpose: The cytotoxic effect of the combination treatment of TNF-α and hyperthermia on L929 and TNF-α-resistant L929 (rL929) cells was investigated.
Materials and methods: L929 cells were treated with TNF-α (5 ng/mL), heating at 43°C or the combination of TNF-α and heating. The cells were harvested at different time within the 24-hour period. The viability and the type of cell death of the harvested cells were examined.
Results: When L929 cells were treated with a combination of TNF-α and heating the cells died quickly and apoptosis increased to an overwhelming extent, especially in the group pre-treated with TNF-α for 1 h prior to heating. Although rL929 cells were resistant to TNF-α alone, the cells became sensitive to TNF-α treatment when combined with heating. Similar to the L929 cell, the cells also died rapidly and exhibited apoptosis to a higher extent. Using an Annexin-V-FITC kit and flow cytometer, we found that both necrosis and apoptosis occurred. Agarose gel electrophoresis of DNA extracted from treated cells showed that the DNA fragments were multiples of approximately 200 bp. Furthermore, by studying the kinetics of cell death and apoptosis, we found that the loss of cell membrane integrity preceded the DNA fragmentation in both L929 and rL929 cells.
Conclusion: The results suggested that hyperthermia may enhance the necrotic and apoptotic effects of TNF-α on some tumour cells and overcome the resistance of some tumour cells to TNF-α.
Introduction
Tumour necrosis factor-α (TNF-α), a macrophage-derived multifunctional cytokine, was originally described as a polypeptide able to produce haemorrhagic necrosis of some tumours in experimental animals Citation[1], Citation[2]. TNF-α is cytostatic or cytotoxic in vitro against a variety of tumour cell lines Citation[1]. Because of its cytotoxic effect on tumour cells, TNF-α is a candidate for anti-tumour agent. However, its cytotoxic spectrum is limited. Relatively few human tumours respond to TNF-α clinically when it is used alone as an antitumour agent Citation[3]. Moreover, TNF-α is also a mediator in immune responses and this activity leads to toxic side-effects to the hosts when TNF-α is administered to cancer patients Citation[4]. Many attempts have been made to enhance TNF-α cytotoxicity for clinical applications, such as combining with various anticancer agents or hyperthermia, which may provide more potent therapeutic effects.
A number of reports which studied the cytotoxic interactions between TNF-α and hyperthermia have found that TNF-α treatments at >39°C resulted in much more cell death in many tumour cell lines in vitro and murine tumour models in vivo Citation[5–8]. It is very interesting that some TNF-α-resistant cells are sensitised to the combination of TNF-α and heat treatment Citation[5], Citation[6]. Some clinical trials using the treatment of TNF-α followed by hyperthermia showed impressive results Citation[9], Citation[10]. Indeed, high-dose TNF-α can be administered safely to treat patients via isolated limb perfusion (ILP), a surgical technique in which a cancer-bearing limb is perfused in isolation from the systemic circulation with TNF-α and melphalan under hyperthermic conditions Citation[11]. Hyperthermic ILP with TNF-α and melphalan is an effective treatment for advanced in-transit melanoma Citation[12–14], as well as unresectable extremity sarcoma Citation[14], Citation[15]. It is important to understand the mechanism of combination treatment so as to design a better treatment regimen. In this study we have explored the apoptotic and necrotic effects of TNF-α, hyperthermia and their combination treatment on L929, a TNF-α sensitive fibrosarcoma cell line, and rL929, a TNF-α-resistant fibrosarcoma cell line.
Materials and methods
Materials
RNase A, proteinase K, propidium iodine and trypan blue were purchased from Sigma (St. Louis, MO). RPMI-1640 medium, foetal calf serum (FCS), penicillin and streptomycin were obtained from Gibco BRL (Grand Island, NY). Recombinant human TNF-α was purchased from R&D Systems Europe (Abingdon, Oxfordshire, UK). L929 was from ATCC (American Type Culture Collection, Rockville, MD). rL929 was kindly provided by Dr. H.K. Cheng (Department of Biochemistry, CUHK). 100bp DNA ladder was obtained from Pharmacia Biotech (Hong Kong). Annexin V-FITC kit was purchased from Trevigen (Gaithersburg, MD).
Cell culture
Murine fibrosarcoma cell line L929 and rL929 cells were cultured in RPMI-1640 medium supplemented with 5% FCS, penicillin (50 U/mL) and streptomycin (50 ug/mL).
Heat treatment
Cells were heated in culture flasks with tightly screwed caps or in culture plates sealed in bags containing 5% CO2, 95% air. The flasks or plates were put into a 43°C incubator. It took 15 min for the culture medium to reach 43°C. They were kept at that temperature for 1 h. After that the flasks or plates were transferred to a 37°C incubator for further incubation.
Treatment of L929 and rL929 cells with TNF-α (5 ng/mL), hyperthermia (43°C, 1 h) and their combination
The cells were subcultured into culture flasks or culture plates. After 12 h, the cells were treated with either TNF-α (5 ng/mL), hyperthermia (43°C, 1 h) or their combination. For the combination treatment the cells were treated with either of the 2 sequences: the addition of TNF-α to the medium 1 h before heating, or the reversed sequence, i.e. TNF-α was added after heating for 1 h. Once TNF-α was added, it remained in the medium until the cells were harvested.
Measurement of viability
Cells were harvested at various time points after treatment and re-suspended in 0.4% trypan blue-containing medium. The total number of cells that were not stained was immediately counted in a series of microscopic fields.
Characterise the cell death with Annexin V-FITC kit
Cells were trypsinised and washed once in cold PBS buffer, then gently re-suspended in the Annexin V incubation reagent with propidium iodine (PI). This was followed by incubation in the dark for 10 min at room temperature. Before analysing the result with flow cytometry, 400 uL 1X binding buffer was added into each sample.
DNA preparation and agarose gel electrophoresis
Cells treated with or without TNF-α, heat and the combination of both treatments were harvested, washed and re-suspended in 0.4 mL cell lysis buffer (100 mM Tris/HCl, 50 mM Ethylenediaminetetraacetic acid [EDTA], 1% SDS) with proteinase K (final concentration 0.5 mg/mL). After incubation at 50°C for 12 hours, the lysate was extracted twice with phenol followed by two extractions with chloroform. The supernatant DNA was precipitated at −20°C with a mixture of two volumes of absolute ethanol and 5M NaCl. The precipitate was pelleted by centrifugation at 13,000 g for 10 min. The resulting pellet was air-dried and re-suspended in 20 µL TE buffer (10 mM Tris-HCl, 0.5 mM EDTA, pH 7.4) with RNase A at a concentration of 0.2 mg/mL. After incubation at 37°C for 2 h, the concentration of DNA was quantified by absorbance at 260 nm, and 12 µg DNA was loaded with loading buffer on a 1.5% agarose gel (containing 0.5 ug/mL ethidium bromide) and run in TBE buffer (90 mM Tris-HCl, 90 mM boric acid, 2 mM EDTA, pH 8.0) for 3 hours at 30 V Citation[16]. After electrophoresis, the gel was photographed under UV illumination. 100bp DNA ladder was used as marker.
Flow cytometry analysis
Single cell suspensions were obtained from monolayers of cells as follows. Cells were washed with PBS and detached with Trypsin-EDTA. Then the cells were harvested by low-speed centrifugation. The cells were washed and re-suspended in 1 mL PBS. Aliquots of cells were counted using a haemocytometer. For the flow cytometry analysis Citation[16], cells were fixed with ice-cool 70% ethanol, incubated at 4°C for at least 30 min. After centrifugation, cells were rehydrated in PBS for 30 min with ribonuclease A at 0.1 mg/mL. Cells were then incubated with propidium iodine (PI) (final concentration 50 µg/mL) for 30 min. Subsequently, flow cytometric analysis was carried out in a fluorescence-activated cell sorter (FACSort, Becton Dickinson). Laser excitation of 488 nm was provided and the fluorescence signal was collected using a 570 nm-long pass emission filter.
Statistical analysis
All experiments were repeated at least three times and similar results were obtained. Unpaired t-test was employed for statistical analysis. A value of p < 0.05 was considered significant.
Results
Cytotoxic effect of TNF-α, hyperthermia and their combination treatments
As shown in , the combination treatment of TNF-α and hyperthermia resulted in more tumour cell death than TNF-α or hyperthermia alone. The sequence of the treatment was noted to be important in this combination treatment. When L929 cells were incubated with TNF-α at the concentration of 5 ng/mL, it took at least 6 h before cell death. The cell viability reduced to 72% after 24 h incubation (). Heating at 43°C for 1 h alone led cells to die gradually. The cell viability decreased to 43% 24 h after heating. After being treated with a combination of TNF/hyperthermia, the L929 cells lost their viability at higher rate, especially in the group of cells that were pretreated with TNF-α for 1 h prior to heating at 43°C for 1 h: immediately after this treatment, the cell viability reduced to 68%. The viability further dropped to 23% and 4% after 3.5 h and 9 h, respectively. In the reversed sequence, the viability was 74%, 53%, 27% at 0 h, 3.5 h and 9 h after treatment, respectively.
Figure 1. The effects of TNF-α, hyperthermia and their combination treatments on cell viability detected by trypan blue exclusion method. The treated cells were harvested at different times after the treatments and the number of viable cells was counted. TNF-α concentration was 5 ng/mL, and it remained in the medium until the cells were harvested; H: cells were incubated at 43°C for 1 h; T + H: TNF-α was added 1h before heating; H + T: TNF was added after heating 43°C for 1 h. The data presented are the mean and standard deviation of three independent determinations. (A) L929 cells, (B) rL929 cells.
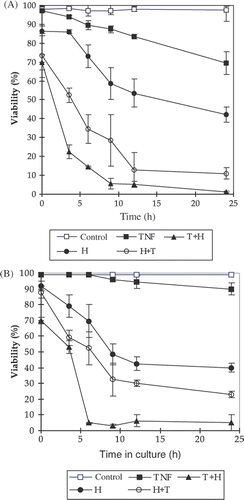
rL929 is a TNF-α-resistant L929 cell line. Few cells died after 24-h incubation with TNF-α (5 ng/mL) in the medium (). But after the combination treatment of TNF-α and hyperthermia, the cell viability quickly reduced. Similar to what happened to L929, the effect is sequence dependent, i.e. adding TNF-α before heating resulted in more cell death.
Type of cell death induced by the treatments
In order to determine whether necrosis or apoptosis was the main type of cell death after the TNF-α and hyperthermia treatments, we stained the cells with Annexin V-FITC kit. The binding of Annexin V-FITC to phosphatidylserine (PS) on the surface of apoptotic cells may be used as an indicator of early apoptosis, preceding DNA fragmentation and membrane disruption Citation[17]. In conjunction with the addition of a membrane impermeable DNA stain, propidium iodide (PI), the kit allowed us to distinguish early apoptosis from late apoptosis plus necrosis Citation[17]. Early apoptotic cells bind Annexin V-FITC but exclude PI. Late apoptotic and necrotic cells bind Annexin V-FITC and are stained by PI, while living cells do not bind Annexin V-FITC and are not stained by PI. Both apoptosis and necrosis were detected in L929 cells after TNF-α, hyperthermia and their combination treatments. At earlier time points, such as 3.5 h and 6 h, the percentage of early apoptosis was similar to that of necrosis plus late apoptosis. At later time points, the percentage of early apoptosis appeared to be prominent. In rL929 cells, hyperthermia and TNF-α/heating combination treatments also resulted in apoptosis and necrosis. In both cell lines, the apoptosis occurred continuously after different treatments rather than at a specific time point, and combination treatments induced apoptosis more quickly than either single treatment.
The effects of different treatments on the apoptotic rate
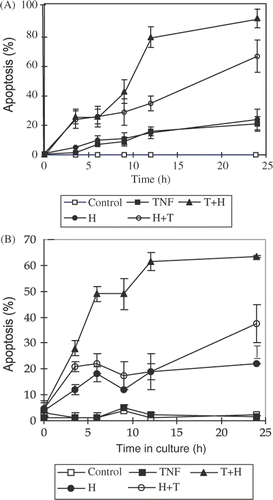
Flow cytometry was applied to detect the DNA content of single cells. Apoptotic cells were recognised by flow cytometry by their lower DNA content due to the leakage of low molecular weight DNA after being fixed by ethanol. The quantitative data of this experiment is presented in . As is evident, either TNF-α or hyperthermia alone resulted in 12-15% of apoptotic cells in L929 after 12 h and 14%−21% after 24 h respectively (). In both sequences of the combination treatments, the percentage of apoptotic cells increased more rapidly than either single treatment. After treating with TNF-α followed by heating, the percentage of apoptotic cells was 43%, 79%, and 91% at 9 h, 12 h and 24 h respectively. In rL929 cells, except that TNF-α treatment alone failed to result in apoptosis, the effects of hyperthermia and TNF-α/heat combination treatments were similar to those in L929 cells (). The cell cycle distribution of the non-apoptotic cell subpopulation revealed that the percentage of G0/G1 phase cells was lowered compared to that of control cells (data not shown).
Figure 2. The kinetics of apoptosis induced by TNF-α, hyperthermia and their combination treatments on L929 and rL929 cells. The percentage of apoptosis induced by different treatments was detected by flow cytometer after staining DNA in single cell with propidium iodine (PI). The procedures of different treatments including TNF-α, H, T + H and H + T are the same as in . The data presented are the mean and standard deviation of three independent determinations. (A) L929 cells, (B) rL929 cells.
Combination treatment induced DNA fragmentation more rapidly
Apoptosis is characterised by degradation of DNA into internucleosomal fragments which can be shown as typical DNA laddering on electrophoresis; whereas necrosis cuts DNA into a continuous spectrum of sizes. Since the hypodiploid areas defined as apoptosis by PI flow cytometry could contain both types of DNA fragmentations, we analysed DNA fragmentation by electrophoresis. The results are shown in . TNF-α induced an apoptosis ladder after 24 h treatment only in L929 but not in rL929 (data not shown). Heating induced both L929 and rL929 to show an apoptosis ladder at 24 h. The combination treatment resulted in an apoptosis ladder earlier after treatment in both cell lines, which is consistent with flow cytometry results.
Figure 3. Examples of DNA fragmentation shown on agarose gel electrophoresis after (A) heat treatment and (B) TNF-α treatment in L929 cells. Lane 1: 100 bp marker, lanes 2-8 represent control, 0 h,3.5 h, 6 h, 9 h, 12 h, 24 h after the treatment, respectively. The data are representatives of three independent experiments.
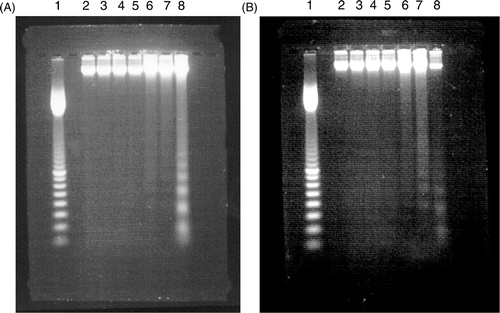
Figure 4. Examples of DNA fragmentation shown on agarose gel electrophoresis after (A) H + T treatment and (B) T + H treatment in L929 cells. Lane 1: 100 bp marker, lanes 2-9 represent control, 0 h, 3.5 h, 6 h, 9 h, 12 h, 24 h after the treatment, respectively. The data are representatives of three independent experiments.
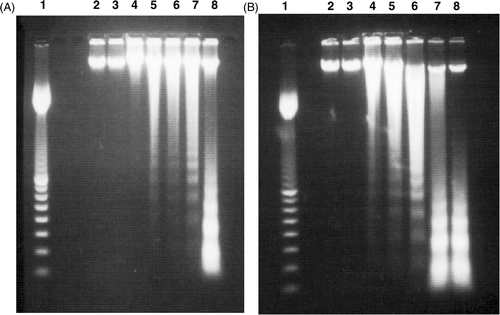
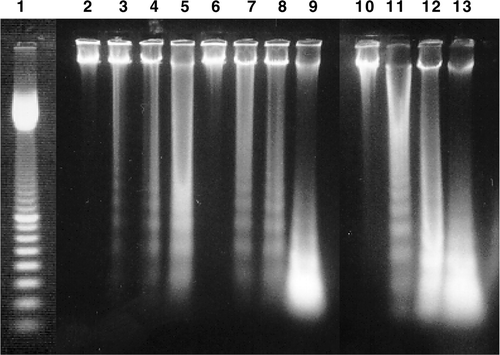
Figure 5. Example of DNA fragmentation shown on agarose gel electrophoresis after different treatments in rL929 cells. Lane 1: 100 bp marker, lanes 2–5 represent 6 h, 9 h, 12 h, 24 h after the heating treatment, respectively. Lanes 6–9 represent 6 h, 9 h, 12 h, 24 h after the H + T treatment, respectively. Lanes 10–13 represent 6 h, 9 h, 12 h, 24 h after the T + H treatment, respectively. The data are representatives of three independent experiments.
Discussion
The potentiation of TNF-α effect by hyperthermia has been reported in both in vitro and in vivo studies Citation[5–10]. The most significant aspect in this study is the determination of the best combination sequence of administering TNF-α and hyperthermia to the tumour cells, and the type of cell death induced by those treatments. Conforming to another report Citation[8], there were supra-additive effects of TNF-α and hyperthermia on L929 and synergistic effects of TNF-α and hyperthermia on rL929. Both combination sequences caused more cell death than either TNF-α or heating treatment alone, but the pretreatment with TNF-α 1 h prior to heating resulted in the most cell death. Another advantage of the combination treatment was that heating overcame the resistance of rL929 cells to TNF-α. Our previous studies found that the combination of TNF-α and hyperthermia could induce more cell death in MCF-7 and MDA-MB-231 cells in vitro compared with the single treatment (unpublished data, data not shown). Both apoptosis and necrosis were detected after the combination treatment. Hyperthermia significantly enhanced the sensitivity of cells to TNF-α cytotoxicity by speeding up the cell killing and cleavage of DNA into nucleosome fragments. The induction of rapid cell death and DNA cleavage is a great advantage for treating tumours, which reduces the chance of tumour recurrence and removed the tumours ultimately. Together with the other in vivo studies which showed enhanced antitumour effect of the combination treatment Citation[7], Citation[18], Citation[19], the results suggest that the combination treatment may be of value in the treatment of malignancy in human patients.
The mechanisms by which either TNF-α or hyperthermia kills cells are not completely clear. The two major types of cell death are apoptosis and necrosis. Apoptosis has been described in some cells treated with some anticancer agents, including heating Citation[20–22]. Apoptosis is an active and programmed physiological process for eliminating superfluous, altered, or malignant cells. In contrast to apoptosis, necrosis has been considered as an uncontrolled form of cell death, which results in release of intracellular components into the microenvironment, an inflammatory response in the corresponding tissue and damage of surrounding cells Citation[23]. In apoptosis, cellular components are systematically broken down without disruption of the cell membrane. The release of intracellular components is avoided as the cell is divided into membrane-bound apoptotic bodies which are then phagocytosed by neighbouring cells or macrophages. Thus, the intense peri-cellular inflammation is avoided Citation[24], Citation[25]. Though both apoptosis and necrosis were detected after the TNF-α treatment in this study, the effect of the combination treatment on apoptosis was focused. It is because apoptosis is a highly controlled process with consequent potential for manipulation, which is vital for the development of effective therapies.
Annexin V-FITC and PI were used to differentiate the stages of apoptosis at different time points after the TNF-α, hyperthermia or combination treatments. At earlier time points the percentage of early apoptosis was similar to that of necrosis plus late apoptosis. At later time points the percentage of early apoptosis became prominent. It revealed that the treatments induced early stage apoptosis in the cells rather than late stage apoptosis and necrosis. At early time points the effect of treatment was not completely exerted and the late apoptotic and necrotic cells detected reflected the baseline.
The combination treatment of TNF-α and hyperthermia overcame the resistance of rL929 cells to TNF-α. A similar phenomenon was observed in other in vitro studies, which showed that hyperthermia rendered TNF-α-resistant mouse breast cancer cells EMT6 tumour cells Citation[8] and human gastric cancer cells MKN45 Citation[26] susceptible to the TNF-α-induced apoptosis. MKN45 is resistant to TNF-α-induced apoptosis due to the activation of NF-kB by TNF-α Citation[26]. Hyperthermia inhibits the NF-kB pathway and results in the sensitisation of MKN45 to TNF-α Citation[26]. A wide range of chemotherapeutic agents activate NF-kB in cancer cells Citation[27], Citation[28]. The NF-kB activation is one of the mechanisms by which tumours are induced to become resistant to chemotherapy Citation[29], Citation[30]. Therefore, the inhibition of the NF-kB pathway by hyperthermia is a promising approach to enhance the efficacy of anticancer therapies.
Typical apoptosis is characterised by shrinkage of cells, segmentation of the nucleus, condensation, and cleavage of DNA into 180-200 base pair fragments by internuclease. Finally, the apoptotic cells break into apoptotic bodies, which are rapidly phagocytosed by neighbouring cells Citation[24], Citation[25]. In our study, correlating the kinetics of cell viability and percentage of apoptotic cells after different treatments, the percentage of dead cells estimated by trypan blue assay rose at earlier time points than percentage of apoptotic cells did. The percentage of apoptotic cells and DNA laddering were the most significant at 12 h and 24 h, while most cells had been stained by trypan blue before 9 h in the combination treatments. We also observed that the cytotoxicity and DNA fragmentation were correlated with the amount of non-adherent cells rendered by different treatments. If we discarded the detached cells, most of which were permeable to trypan blue, the percentage of the apoptosis would greatly decreased and DNA electrophoresis could not show an apoptotic ladder. All these indicate that the cell membrane was impaired prior to the DNA cleavage by endonuclease in L929 and rL929. Other researchers also observed this phenomenon Citation[8], Citation[31]. Fady et al. Citation[31] called it ‘atypical apoptotic cell death’. They studied TNF-α-treated L929 cell using an electron microscope and found that the earliest change after TNF-α incubation was chromatin condensation, while DNA fragmentation occurred later than membrane permeabilisation detected by trypan blue staining.
Even though the increased apoptosis may be one mechanism by which TNF-α and hyperthermia interact to increase cytotoxicity against tumour cells in vitro, that mechanism is not likely to be the sole factor. After TNF-α treatment, whether a cell line displays apoptosis, resistance to apoptosis, or necrosis apparently depends on cell type or lineage Citation[20], Citation[32]. Due to the atypical apoptosis of the L929 and rL929 cells, it is difficult to think that apoptosis is the direct cause of the cell death. It is possible that TNF-α and hyperthermia activate other processes that directly lead to cytolysis, such as generation of membrane damaging free radicals Citation[33–35].
Another possible mechanism by which hyperthermia enhances the antitumour effect of TNF-α is the overexpression of heat shock proteins (HSPs) Citation[36]. HSPs are highly conserved proteins which are synthesised to protect cells against the harmful consequences of stress stimuli, including those imposed by heat shock Citation[37]. Hsp70 is expressed by almost all the cells and may exert both pro-apoptotic Citation[38], Citation[39] and anti-apoptotic Citation[37], Citation[40] roles. Through the interaction with TNF receptor (TNFR)-associated factor 2 (TRAF2), Hsp70 differentially regulates TNF-α induced activation of NF-kB (antiapoptotic signal) and cJun N-terminal kinase (JNK, apoptotic signal) Citation[36]. Within the cell membrane, specialised microdomains, known as lipid rafts, coordinate various signalling pathways involved in cancer development Citation[41]. Overexpression of Hsp70i, the inducible form of Hsp70, greatly reduces the level of TRAF2 in lipid rafts but increases the level of TRAF2 in soluble fractions Citation[36]. The localisation of TRAF2 in soluble fractions may facilitate oligomerisation and ubiquitination of TRAF2, promoting the TNF-α-induced activation of JNK pathway.
When TNF-α is used in ILP, both regional and systemic toxicity in the patients have been reported Citation[42–44]. A grading system described by Wieberdink is commonly used to classify the symptoms related to regional toxicity (). Melphalan-based ILP with TNF-α leads to Grade II reactions most frequently Citation[46]. On the other hand, the leakage of TNF-α into the systemic circulation leads to systemic toxicity including haemodynamic alterations, inflammatory response and respiratory complications Citation[47]. The patients’ haemodynamic and metabolic variables should be monitored to access the toxic side-effects of the combination treatment of TNF-α and hyperthermia. The leakage of TNF-α into systemic circulation should be monitored by injecting inert compound 99mTc-radiolabelled albumin or erythrocytes into the circuit Citation[48].
Table I. Grading of regional toxicity according to Weiberdink et al. Citation[45].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore H, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 1975; 72: 3666–3670
- Old LJ. Tumor necrosis factor (TNF). Science 1985; 230: 630–632
- Spriggs DR, Yates SW. Tumor necrosis factor: The molecules and their emerging role in medicine. Tumor necrosis factor: The molecules and their emerging role in medicine, B Beutler. Raven Press, New York 1992; 383–406
- Van Ostade X, Tavernier J, Fiers W. Structure-activity studies of human tumour necrosis factors. Protein Eng 1994; 7: 5–22
- Tomasovic SP, Barta M, Klostergaard J. Neutral red uptake and clonogenic survival assays of the hyperthermic sensitization of tumor cells to tumor necrosis factor. Radiat Res 1989; 119: 325–337
- Tomasovic SP, Barta M, Klostergaard J. Temporal dependence of hyperthermic augmentation of macrophage-TNF production and tumor cell-TNF sensitization. Int J Hyperthermia 1989; 5: 625–639
- Watanabe N, Niitsu Y, Umeno H, Sone H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Synergistic cytotoxic and antitumor effects of recombinant human tumor necrosis factor and hyperthermia. Cancer Res 1988; 48: 650–653
- Tomasovic SP, Vasey TA, Story MD, Stephens LC, Klostergaard J. Cytotoxic manifestations of the interaction between hyperthermia and TNF: DNA fragmentation. Int J Hyperthermia 1994; 10: 247–262
- Vaglini M, Belli F, Ammatuna M, Inglese MG, Manzi R, Prada A, Persiani L, Santinami M, Santoro N, Cascinelli N. Treatment of primary or relapsing limb cancer by isolation perfusion with high-dose alpha-tumor necrosis factor, gamma-interferon, and melphalan. Cancer 1994; 73: 483–492
- Hill S, Fawcett WJ, Sheldon J, Soni N, Williams T, Thomas JM. Low-dose tumour necrosis factor alpha and melphalan in hyperthermic isolated limb perfusion. Br J Surg 1993; 80: 995–997
- Alexander HR, Jr. Isolation perfusion. Cancer: Principles and practice of oncology, VT De Vita, Jr, S Hellman, SA Rosenberg. Lippincott, Williams & Wilkins, Philadelphia 2001; 769–776
- Grünhagen DJ, van Etten B, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM. Efficacy of repeat isolated limb perfusions with tumor necrosis factor alpha and melphalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol 2005; 12: 609–615
- Di Filippo F, Rossi CR, Santinami M, Cavaliere F, Garinei R, Anzà M, Perri P, Botti C, Di Angelo P, Pasqualoni R, Di Filippo S. Hyperthermic isolation limb perfusion with TNFalpha in the treatment of in-transit melanoma metastasis. In Vivo 2006; 20: 739–742
- Hayes AJ, Neuhaus SJ, Clark MA, Thomas JM. Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol 2007; 14: 230–238
- Taeger G, Grabellus F, Podleska LE, Müller S, Ruchholtz S. Effectiveness of regional chemotherapy with TNF-alpha/melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia 2008; 24: 193–203
- Kong SK, Suen YK, Chan YM, Chan CW, Choy YM, Fung KP, Lee CY. Concanavalin A-induced apoptosis in murine macrophages through a Ca(2+)-independent pathway. Cell Death Differ 1996; 3: 307–314
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998; 31: 1–9
- Niitsu Y, Watanabe N, Umeno H, Sone H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Synergistic effects of recombinant human tumor necrosis factor and hyperthermia on in vitro cytotoxicity and artificial metastasis. Cancer Res 1988; 48: 654–657
- Lin JC, Park HJ, Song CW. Combined treatment of IL-1 alpha and TNF-alpha potentiates the antitumour effect of hyperthermia. Int J Hyperthermia 1996; 12: 335–344
- Rubin BY, Smith LJ, Hellermann GR, Lunn RM, Richardson NK, Anderson SL. Correlation between the anticellular and DNA fragmenting activities of tumor necrosis factor. Cancer Res 1988; 48: 6006–6010
- Zimmerman RJ, Chan A, Leadon SA. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res 1989; 49: 1644–1648
- Bellomo G, Perotti M, Taddei F, Mirabelli F, Finardi G, Nicotera P, Orrenius S. Tumor necrosis factor alpha induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation. Cancer Res 1992; 52: 1342–1346
- Leist M, Jäättelä M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2001; 2: 589–598
- Gerschenson LE, Rotello RJ. Apoptosis: A different type of cell death. FASEB J 1992; 6: 2450–2455
- Wyllie AH, Kerr JF, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol 1980; 68: 251–306
- Kokura S, Yoshida N, Ueda M, Imamoto E, Ishikawa T, Takagi T, Naito Y, Okanoue T, Yoshikawa T. Hyperthermia enhances tumor necrosis factor alpha-induced apoptosis of a human gastric cancer cell line. Cancer Lett 2003; 201: 89–96
- Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, Naito Y, Yoshikawa T. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperthermia 2009; 25: 210–219
- Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS, Jr. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: Implications for systemic nuclear factor-kappa B inhibition. Cancer Res 2001; 61: 3535–3540
- Chendil D, Das A, Dey S, Mohiuddin M, Ahmed MM. Par-4, a pro-apoptotic gene, inhibits radiation-induced NF kappa B activity and Bcl-2 expression leading to induction of radiosensitivity in human prostate cancer cells PC-3. Cancer Biol Ther 2002; 1: 152–160
- Hochwald SN, Lind DS, Malaty J, Copeland EM, 3rd, Moldawer LL, MacKay SL. Antineoplastic therapy in colorectal cancer through proteasome inhibition. Am Surg 2003; 69: 15–23
- Fady C, Gardner A, Jacoby F, Briskin K, Tu Y, Schmid I, Lichtenstein A. Atypical apoptotic cell death induced in L929 targets by exposure to tumor necrosis factor. J Interferon Cytokine Res 1995; 15: 71–80
- Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 1988; 141: 2629–2634
- Matthews N, Neale ML, Jackson SK, Stark JM. Tumour cell killing by tumour necrosis factor: Inhibition by anaerobic conditions, free-radical scavengers and inhibitors of arachidonate metabolism. Immunology 1987; 62: 153–155
- Yamauchi N, Kuriyama H, Watanabe N, Neda H, Maeda M, Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res 1989; 49: 1671–1675
- Zimmerman RJ, Chan A, Leadon SA. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res 1989; 49: 1644–1648
- Dai S, Jiang L, Wang G, Zhou X, Wei X, Cheng H, Wu Z, Wei D. Hsp70 interacts with TRAF2 and differentially regulates TNF alpha signalling in human colon cancer cells. J Cell Mol Med 2010; 14: 710–25
- Beere HM. ‘The stress of dying’: The role of heat shock proteins in the regulation of apoptosis. J Cell Sci 2004; 117: 2641–2651
- Liossis SN, Ding XZ, Kiang JG, Tsokos GC. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J Immunol 1997; 158: 5668–5675
- Chant ID, Rose PE, Morris AG. Susceptibility of AML cells to in vitro apoptosis correlates with heat shock protein 70 (hsp 70) expression. Br J Haematol 1996; 93: 898–902
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: Essential proteins for apoptosis regulation. J Cell Mol Med 2008; 12: 743–761
- Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta 2008; 1785: 182–206
- Vrouenraets BC, Klaase JM, Nieweg OE, Kroon BB. Toxicityand morbidity of isolated limb perfusion. Semin Surg Oncol 1998; 14: 224–231
- Eggimann P, Chiolero R, Chassot PG, Lienard D, Gerain J, Lejeune F. Systemic and hemodynamic effects of recombinant tumor necrosis factor alpha in isolation perfusion of the limbs. Chest 1995; 107: 1074–1082
- Lewis JM, Sander G, Reintgen D, Letson DG. Hyperthermic limb perfusion: Evolving concepts in the treatment of extremity soft tissue sarcoma. Curr Opin Orthop 2006; 17: 1–5
- Weiberdink J, Benckhuysen C, Braat R, Van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 1982; 18: 905–910
- Taeger G, Grabellus F, Podleska LE, Müller S, Ruchholtz S. Effectiveness of regional chemotherapy with TNF-alpha/melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia 2008; 24: 193–203
- Möller MG, Lewis JM, Dessureault S, Zager JS. Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperthermia 2008; 24: 275–289
- Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Liénard D, van Geel AN, Hoekstra HJ, Meller I, Nieweg OE, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996; 224: 756–764