Abstract
Purpose: To assess the relationship between the radiofrequency (RF) output power and the intra-oesophageal temperature for hyperthermia of the whole thoracic region, and also to evaluate the patients’ characteristics associated with adequate heating.
Materials and methods: Fifty-nine patients with thoracic cancer treated with radiotherapy plus hyperthermia were retrospectively analysed. The 8-MHz RF capacitive heating device was applied, both the upper and lower electrodes were 300 mm in diameter, placed on opposite sides of the whole thoracic region. All the patients also underwent intra-oesophageal temperature measurements.
Results: All thermal parameters, Tmin, Tmax, Tave, and %T ≥ 41°C, of the intra-oesophageal temperature highly correlated with the median RF output power (p < 0.0001), and the relations were independent in the multivariable analyses including clinical characteristics (p < 0.01). The performance status showed a statistically significant association on Tmax, Tave and %T ≥ 41°C (p < 0.05). The patient age and subcutaneous fat at some levels were inversely correlated with the thermal parameters (p < 0.05).
Conclusion: The RF output power was significantly correlated with the intra-oesophageal temperature; it could be used as a promising parameter to assess the efficacy of hyperthermia for the whole thoracic region. Higher intra-oesophageal temperature may be achieved in patients with good performance status, younger age and thinner subcutaneous fat.
Introduction
Hyperthermia (HT) is known to cause direct cytotoxicity for cancer cells, while also acting as a radiation sensitiser and chemo sensitiser Citation[1]. The efficacy of radiotherapy plus HT in advanced head and neck cancer, locally recurrent breast carcinoma, recurrent or metastatic malignant melanoma and cervical cancer of the uterus was demonstrated and confirmed by randomised phase III clinical trials Citation[1–4]. Promising results have also been reported regarding radiotherapy plus regional HT for Pancoast tumours or lung cancers in contact with the chest wall Citation[5–7]. In contrast, regional HT has rarely been attempted for lung cancers not contacting the chest wall Citation[8], Citation[9], probably because the treatment of those lesions would involve physical difficulties associated with the delivery of heat and measurement of temperature. In most lung cancer treatments, direct intra-tumour measurements tend to be clinically difficult to manipulate, invasive, or uncomfortable for the patients.
The Thermotron RF-8 system (Yamamoto Vinita, Osaka, Japan) is a capacitive heating device operating at 8 MHz, whereby the patient is placed between two electrodes connected to a high-power radiofrequency (RF) generator. With regional HT using this device without direct intra-tumour thermometry, there was a strong positive correlation between the RF output power and the intra-oesophageal temperature in the 22 patients with stage III non-small cell lung cancer (NSCLC), and a higher RF output power could contribute to better clinical outcomes Citation[10]. The correlation between the RF output power and the intra-oesophageal temperature, however, has not been reported for the other types of thoracic tumours. In addition, predictive factors for the higher intra-oesophageal temperature have not been investigated in this device. The first purpose of this study is, therefore, to evaluate the correlation between the RF output power and the intra-oesophageal temperature in 59 patients with various stages of lung cancer and oesophageal cancer on deep regional HT for the whole thoracic region using 8 MHz RF capacitive heating device. The second purpose is to assess the relationship between the clinical characteristics and the intra-oesophageal temperature in this setting of deep regional HT.
Materials and methods
Patients
From March 1991 to April 2008, 59 patients (58 males and one female, age range 36–84 years; median, 62 years) were treated with radiotherapy using deep regional HT of the whole thoracic region guided by the intra-oesophageal temperature; 22 patients with stage III NSCLC who were reported previously were included Citation[10]. The characteristics of the patients are given in . The ECOG performance status and tumour stage Citation[11] were evaluated at the start of this treatment. Patients with subcutaneous fat of 25 mm or greater, or other serious complications such as severe pulmonary, cerebrovascular or renal diseases did not undergo this therapy. The radiotherapy was administered conventionally once daily five times per week and performed using a 4, 6, or 10 MV linear accelerator in all the patients. The total radiation dose ranged from 30–80 Gy (median, 60 Gy), and a daily dose was 1.5–3.4 Gy (median, 2 Gy).
Table I. Patient characteristics.
Hyperthermia
HT was applied within 15 min after RT once or twice a week. The heat was applied using an 8 MHz radiofrequency capacitive regional HT (Thermotron RF-8, Yamamoto Vinita). The physical features of the RF-8 clinical HT machine and the thermal distribution characteristics in a phantom, as well as in the human body, when heating with this device have been reported previously Citation[12–14]. The number of HT treatments during radiotherapy ranged from 3 to 36 (median 14). In all cases, both the upper and the lower electrodes were 300 mm in diameter, and placed on opposite sides of the whole thoracic region, and the treatment posture was the prone position in order to reduce the degree of pain caused by heating Citation[15]. The overlay boluses were applied in addition to regular boluses attached in front of the metal electrodes to reduce the preferential heating of the subcutaneous fat tissue. The circulating liquid of the overlay boluses was saline water controlled at 5°C. The patients were carefully instructed to alert the operator of any unpleasant sensation such as pain suggestive of a hot spot, respiratory problem and palpitation. The RF output was increased to the maximum level tolerated by the patients, following appropriate adjustments of the treatment setting, and the patient's tolerance threshold was set based on the patient's alert. The goal of the heating was to continue the treatment for at least 30 min after the RF output was increased up to the patient's tolerance threshold, and the maximum total treatment duration was 70 min. Blood pressure and pulse rate were monitored before, during and after hyperthermia. The heating duration was adjusted from 40–70 min based on the patient's tolerance (median 50 min).
The intra-oesophageal temperature was measured in all 59 patients using a 4-point micro-thermocouple sensor (Yamamoto Vinita, LAS-4) which was inserted into the oesophagus, at the level of the bifurcation of the trachea in the patients with lung cancer, or at the level of the tumour in the patients with oesophageal cancer, through a 12 F catheter under X-ray fluoroscopy. The high RF wave filter is inserted in the thermometry system of the Thermotron RF-8, which makes it possible to measure the temperature during hyperthermia. The thermometry system with the 4-point micro-thermocouple sensor connected to an automatic temperature–power feedback controller provides an accuracy of ±0.2°C Citation[14]. The principle of eliminating the disturbance in the measurement of temperature by thermocouples in the presence of RF current was discussed in a previous study Citation[16]. The thermometry wires were aligned perpendicular to the field direction. The temperature was recorded automatically per min during hyperthermia. There was no need for the RF power to be shut off to measure the temperature. The thermometric parameters measured included minimum (Tmin), maximum (Tmax), and mean (Tave) of the four intra-oesophageal temperature measurements at the end of each session, and the proportion of the time during which at least one of the four intra-oesophageal measurements was 41°C or higher during the total heating period of the session(s) with thermometry (%T ≥ 41°C). The measurements were performed once (n = 57) and twice (n = 2) during the whole series in each patient. The median RF output power was obtained between reaching the steady-state and the end of treatment, and the steady-state was defined 20 min after the start of heating.
Statistical analysis
The associations between certain factors, including thermal parameters of intra-oesophageal temperature, median RF output power, patient age, performance status, heating duration, thicknesses of thorax and subcutaneous fat were evaluated using a linear regression analysis. The thicknesses of the thorax and subcutaneous fat were measured on computed tomography scans obtained pre-treatment. Multivariate analyses by a logistic regression were also used to compare the thermal parameters of intra-oesophageal temperature with the patient age, performance status, thickness of subcutaneous fat and median RF output power.
Results
The intra-oesophageal Tmax of their intra-oesophageal temperature ranged from 38.9°C to 48.9°C, with a median of 43.2°C. The Tave ranged from 38.8°C to 47°C, median 42.6°C. The Tmin ranged from 38.6°C to 45.6°C, median 41.7°C. The %T ≥ 41°C ranged from 0% to 98%, median 84%. The median RF output power ranged from 548 W to 1679 W, median 1240 W. and show the relationship between the median RF output power and the intra-oesophageal temperature in all the 59 patients. All thermal parameters, Tmax, Tave, Tmin and %T ≥ 41°C, of intra-oesophageal temperature were highly correlated with the median RF output power. A subset analysis of 27 patients with Stage IV or recurrent thoracic malignant tumours showed that all thermal parameters were significantly correlated with the median RF output power, and all the thermal parameters were also significantly associated in the remaining 32 patients with curative disease. The thermal parameters were not significantly correlated with the median RF output power in 9 patients with oesophageal cancer, although these parameters were significantly associated with the median RF output power in 50 patients with lung cancer.
Figure 1. All the thermal parameters, Tmax (A), Tave (B), Tmin (C) and %T ≥ 41°C (D), of intra-oesophageal temperature were highly correlated with the median radiofrequency output power.
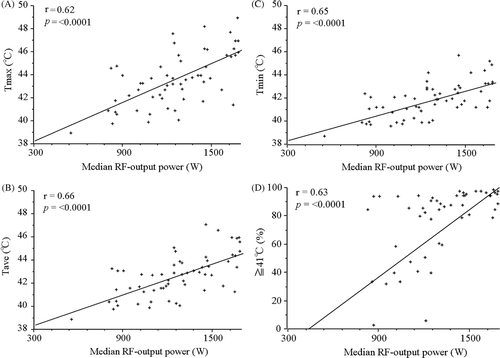
Table II. Correlations between intra-esophageal thermal parameters and clinical characteristics.
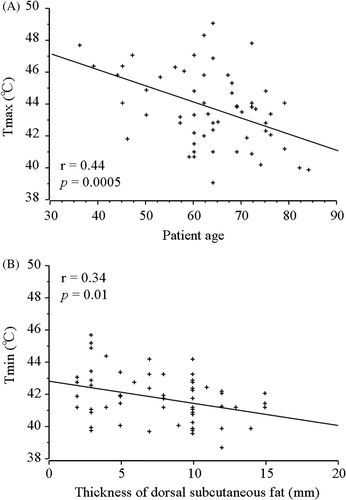
summarises the correlations between intra-oesophageal temperatures and clinical characteristics. The patient age was inversely correlated with all thermal parameters of intra-oesophageal temperature (). The performance status also was inversely correlated with the %T ≥ 41°C of intra-oesophageal temperature. The thickness of the thorax was not significantly correlated with any thermal parameters of intra-oesophageal temperature. The thicknesses of the subcutaneous fat at the levels of the tracheal bifurcation, the nipple and the cardia of the stomach were inversely correlated with the Tmin of intra-oesophageal temperature (). The thickness of the dorsal subcutaneous fat at the levels of the tracheal bifurcation and the ventral subcutaneous fat at the cardia of the stomach were also inversely correlated with the Tave.
Figure 2. The correlations between the intra-oesophageal temperatures and clinical characteristics. (A) The patient age was inversely correlated with the Tmax as thermal parameters of intra-oesophageal temperature. (B) The thicknesses of dorsal subcutaneous fat at the levels of the tracheal bifurcation was inversely correlated with Tmin.
presents the results of the multivariate analysis by logistic regression to evaluate the effects of the patient characteristics on the thermal parameters of intra-oesophageal temperature. The median RF output power was strongly correlated with all thermal parameters of the intra-oesophageal temperature. The performance status also showed a statistically significant association with the thermal parameters of Tmax and %T ≥ 41°C.
Table III. Multivariate analyses by logistic regression to evaluate effects of clinical factors on the thermal parameters in patients with thoracic malignant tumour.
Discussion
The use of regional deep heating has been investigated, especially for the treatment of pelvic tumours and soft tissue sarcoma Citation[1]. Randomised trials of radiotherapy with or without deep regional HT for cervical cancer have been reported to demonstrate positive results concerning survival Citation[2], Citation[3]. However, there is little information reported in the literature on the use of deep regional HT for the treatment of patients with thoracic malignant tumours Citation[6–8], Citation[10], Citation[17], probably because most available devices for regional deep heating are structurally difficult to apply to the thoracic region. In addition, the use of thermometry is more invasive when used to those tumours than it is when used for pelvic tumours or for a soft tissue sarcoma of an extremity.
Van der Zee et al. reported that the direct intra-tumour measurements for deep-seated tumours have the possibility of causing severe complications (e.g. subcutaneous or deep infection, intolerable pain, and bleeding) while providing information of limited disproportional clinical value Citation[18]. On the other hand, Fatehi et al. reported that intra-luminal thermometry provides sufficient information to apply deep regional HT to individual patients with pelvic tumours, since the intra-tumour and intra-luminal temperatures during individual treatments were highly correlated, and the average intra-tumour and intra-luminal temperatures were not different Citation[19].
Some investigators have shown the importance of RF power as a parameter to assess the treatment of deep regional HT in abdominal or pelvic tumours [15–17]. Hamazoe et al. reported that the 39 patients with liver, pancreatic or bile duct tumours were treated with deep regional HT using an 8 MHz RF capacitive heating device; there was a positive correlation between the maximum RF output power and maximum temperature of the tumours Citation[20]. Fatehi et al. also observed a positive correlation between normalised net integrated RF power and intra-vaginal temperature in the patients with advanced cervical cancer treated with the BSD2000-3D at frequencies 70–120 MHz Citation[21]. The current study confirmed that the RF output power was highly correlated with the intra-oesophageal temperature in 59 patients with thoracic malignant tumours, and the relations were independent in the multivariable analyses including clinical characteristics; therefore, the RF output power as well as the intra-oesophageal temperature may be used as a promising parameter to assess the treatment of deep regional HT for the thoracic region, if deep heating using an 8 MHz RF capacitive heating device is enforced with the same size of electrodes and the same body posture. This strategy of regional HT, which is less invasive and causes less distress, may be suitably incorporated into the clinical combined modality therapy.
A Dutch phase III trial showed an improvement of the local efficacy in a mixed cohort of patients with locally advanced cervical, bladder or rectal carcinoma, and a major survival benefit was obtained in patients with cancer of the uterine cervix by adding deep regional HT to radiotherapy Citation[2]. However, no significant improvement was seen in patients with rectal and bladder cancer. As discussed elsewhere, the value of deep regional HT in these patients might have been underestimated for the problem of patients who received insufficient HT treatment Citation[22]. Recently, van Haaren et al. reported that intra-oesophageal temperatures during locoregional hyperthermia using a 70 MHz AMC-4 phased array system were inversely related to patients’ body size parameters, where fat percentage proved to be the most significant predictor Citation[17], Citation[23]. The current study showed that good performance status, younger age and thinner subcutaneous fat are significant predictive factors for a good thoracic regional heating of elevating intra-oesophageal temperature. Future clinical trials for regional HT for thoracic region using 8 MHz RF capacitive heating device should be directed to those patients.
One of the disadvantages of an RF capacitive device is the preferential heating of the subcutaneous fat tissue, although Asian patients are considered to be relatively suitable due to their slender constitution. The excessive power deposition in the fatty tissue limits the effectiveness of the capacitive technique. There is a depth limit to the skin-cooling ability of the overlay bolus in the 8 MHz RF capacitive heating device Citation[24], Citation[25]. Therefore, regional HT was not applied to the patients with a subcutaneous fat thickness more than 25 mm; our combined therapy was rarely performed for women because the thickness of subcutaneous fat was over 25 mm in most women. In the current study, the thickness of the subcutaneous fat was inversely correlated with the RF output power, even in patients with a subcutaneous fat thickness of <25 mm. It may therefore be necessary to assess the effect of regional HT on patients with such limited indications.
Some reports have described the feasibility and efficacy of regional HT with RT for lung cancer Citation[5–8] and noted the tumour temperature by direct measurements tended to correlate with the tumour response of lung cancer Citation[7], Citation[8]. Numerous reports in superficial or pelvic tumours treated with radiotherapy plus HT indicated a positive interrelationship between thermal parameters and clinical parameters concerning the effectiveness Citation[1], Citation[26], Citation[27]. A previous report also showed that the median RF output power (≥1200 W) was a significantly good prognostic factor for the local control rate and survival rates in the patients with stage III non-small cell lung cancer treated with radiotherapy plus regional HT Citation[10]. Radiotherapy combined with deep regional HT for the whole thoracic region using a higher RF output power may contribute to better clinical outcomes in thoracic malignant tumours.
This study could not assess the relationship between the thermal data and clinical outcomes, because treatment regimen and clinical tumour stage were varied in our treatment data. A formal phase II trial is consequently needed to determine the efficacy for this combined therapy in the patients with thoracic malignant tumours. The underlying factors for the inverse relation between age and performance status and the thermal parameters also could not be analysed because of the lack of detail data for the patient's tolerance threshold of the heating. Subset analysis showed that the nine patients with oesophageal cancer did not show significant relationship between the median RF output power and thermal parameters. However, no conclusions can be made with regard to the patients with oesophageal cancer because of the small number of cases.
In summary, the current study confirmed that the RF output power significantly correlated with the intra-oesophageal temperature in the 59 patients with thoracic malignant tumours, and the relations were independent in the multivariable analyses. Therefore, the RF output power may be used as a promising parameter to assess the treatment of deep regional HT if deep heating using an 8 MHz RF capacitive heating device is applied with the same size of electrodes and the same body posture. This strategy of regional HT, which is less invasive, may therefore, be suitably incorporated into the combined clinical therapeutic modality. Higher intra-oesophageal temperature may be achieved in the patients with good performance status, younger age and thinner subcutaneous fat, and such patients may therefore be good candidates for thermo-radiotherapy using deep regional HT for the whole thoracic region.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jones EL, Samulski TV, Leonard RP, Dewhirst MW. Hyperthermia, 4th. Lippincott Williams & Wilkins, Philadelphia 2003
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996; 12: 3–20
- Terashima H, Nakata H, Yamashita S, Imada H, Tsuchiya T, Kunugita N. Pancoast tumour treated with combined radiotherapy and hyperthermia – A preliminary study. Int J Hyperthermia 1991; 7: 417–424
- Sakurai H, Hayakawa K, Mitsuhashi N, Tamaki Y, Nakayama Y, Kurosaki H, Nasu S, Ishikawa H, Saitoh JI, Akimoto T, Niibe H. Effect of hyperthermia combined with external radiation therapy in primary non-small cell lung cancer with direct bony invasion. Int J Hyperthermia 2002; 18: 472–483
- Hiraoka M, Masunaga S, Nishimura Y, Nagata Y, Jo S, Akuta K, Li YP, Takahashi M, Abe M. Regional hyperthermia combined with radiotherapy in the treatment of lung cancers. Int J Radiat Oncol Biol Phys 1992; 22: 1009–1014
- Karasawa K, Muta N, Nakagawa K, Hasezawa K, Terahara A, Onogi Y, Sakata K, Aoki Y, Sasaki Y, Akanuma A. Thermoradiotherapy in the treatment of locally advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 1994; 30: 1171–1177
- Ohguri T, Imada H, Narisada H, Yahara K, Morioka T, Nakano K, Miyaguni Y, Korogi Y. Systemic chemotherapy using paclitaxel and carboplatin plus regional hyperthermia and hyperbaric oxygen treatment for non-small cell lung cancer with multiple pulmonary metastases: Preliminary results. Int J Hyperthermia 2009; 25: 160–167
- Ohguri T, Imada H, Yahara K, Morioka T, Nakano K, Terashima H, Korogi Y. Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer: The radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int J Radiat Oncol Biol Phys 2009; 73: 128–135
- Sobin LH, Witterkind CH. International Union Against Cancer. TNM Classification of Malignant Tumours, 5th edn. (Japanese edn.). Tokyo, Japan: Kinbara, 1997.
- Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer 1987; 60: 121–127
- Song CW, Rhee JG, Lee CK, Levitt SH. Capacitive heating of phantom and human tumors with an 8 MHz radiofrequency applicator (Thermotron RF-8). Int J Radiat Oncol Biol Phys 1986; 12: 365–372
- Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, Morita K, Kakehi M, Sugahara T. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 1986; 58: 1589–1595
- Imada H, Nomoto S, Tomimatsu A, Ostapenko VV, Terashima H. Importance of patient positioning in hyperthermia for deep-seated intrathoracic tumors using an 8 MHz RF capacitive heating device. Jpn J Hyperthermic Oncol 1999; 15: 15–19
- Chakraborty DP, Brezovich IA. Error sources affecting thermocouple thermometry in RF electromagnetic fields. J Microw Power 1982; 17: 17–28
- Hulshof MC, Van Haaren PM, Van Lanschot JJ, Richel DJ, Fockens P, Oldenborg S, Geijsen ED, Van Berge Henegouwen MI, Crezee J. Preoperative chemoradiation combined with regional hyperthermia for patients with resectable esophageal cancer. Int J Hyperthermia 2009; 25: 79–85
- van der Zee J, Peer-Valstar JN, Rietveld PJ, de Graaf-Strukowska L, van Rhoon GC. Practical limitations of interstitial thermometry during deep hyperthermia. Int J Radiat Oncol Biol Phys 1998; 40: 1205–1212
- Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol 2007; 183: 479–486
- Hamazoe R, Maeta M, Murakami A, Yamashiro H, Kaibara N. Heating efficiency of radiofrequency capacitive hyperthermia for treatment of deep-seated tumors in the peritoneal cavity. J Surg Oncol 1991; 48: 176–179
- Fatehi D, van der Zee J, van der Wal E, Van Wieringen WN, Van Rhoon GC. Temperature data analysis for 22 patients with advanced cervical carcinoma treated in Rotterdam using radiotherapy, hyperthermia and chemotherapy: A reference point is needed. Int J Hyperthermia 2006; 22: 353–363
- Hildebrandt B, Wust P, Rau B, Schlag P, Riess H. Regional hyperthermia for rectal cancer. Lancet 2000; 356: 771–772
- van Haaren PM, Hulshof MC, Kok HP, Oldenborg S, Geijsen ED, Van Lanschot JJ, Crezee J. Relation between body size and temperatures during locoregional hyperthermia of oesophageal cancer patients. Int J Hyperthermia 2008; 24: 663–674
- Ohguri T, Imada H, Yahara K, Kakeda S, Tomimatsu A, Kato F, Nomoto S, Terashima H, Korogi Y. Effect of 8-MHz radiofrequency-capacitive regional hyperthermia with strong superficial cooling for unresectable or recurrent colorectal cancer. Int J Hyperthermia 2004; 20: 465–475
- Kroeze H, van de Kamer JB, de Leeuw AA, Kikuchi M, Lagendijk JJ. Treatment planning for capacitive regional hyperthermia. Int J Hyperthermia 2003; 19: 58–73
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, Budach V, Felix R, Schlag PM. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 2000; 48: 381–391