Abstract
Purpose: This phase II randomised trial was designed to evaluate the therapeutic efficacy and feasibility of radio frequency regional hyperthermia in combination with chemotherapy for patients with advanced non-small lung cancer (NSCLC).
Methods: Eighty patients with pathologically proven advanced NSCLC, were enrolled and divided into two groups. Group A patients were treated by radio frequency regional hyperthermia in combination with the regimen of gemcitabine and cisplatin (GP). Group B patients were treated with the GP regimen alone.
Results: In group A, one patient achieved a complete response (CR), 18 achieved a partial response (PR), 18 achieved a stable disease and three experienced a progression of the disease. Thirty-three patients had a positive Clinical Benefit Response (CBR). In group B, no patient achieved CR, 17 achieved PR, 19 achieved a stable disease and four experienced a progression of the disease. Nineteen patients had a positive CBR. Significant differences between the two groups were observed for the CBR (P < 0.05), but not for RR. Major toxicities included bone marrow depression, nausea, vomiting, without significant differences between the two groups (P > 0.05).
Conclusions: Radio-frequency regional hyperthermia in combination with chemotherapy (GP) is a safe, well tolerated, and effective therapeutic modality for patients with advanced NSCLC. The addition of hyperthermia improved quality of life.
Introduction
Non-small cell lung cancer (NSCLC) comprises at least 80% of all lung neoplasms and the long-term prognosis for advanced stages (IIIB/IV) which are present in 75% of new cases is poor Citation[1].
Gemcitabine, an anticancer agent with proven efficacy in the treatment of NSCLC, is a prodrug that exerts its cytotoxic effects through its active intracellular metabolites, gemcitabine diphosphate and triphosphateis (a deoxycytidine). The regimen of gemcitabine and cisplatin (GP) is widely used for first-line treatment of advanced NSCLC.
Hyperthermia is known to cause direct cytotoxicity of cancer cells, while it also acts as a radiation and chemo sensitiser. Hyperthermia treatment has been shown to improve the therapeutic effects of treatments for superficial tumours, soft tissue sarcoma, osteosarcoma, prostate cancer, breast cancer and some recurrent and advanced solid tumours Citation[2–4]. Empirical studies indicate that heat is lethal to human tumour cell lines, and enhances the cytotoxicity of radiotherapy, and potentiates the action of some chemotherapeutic drugs such as the alkylating agents (e.g. nitrosoureas), and some antitumour antibiotics. As such, hyperthermia in the temperature range of 40°–46°C may have several potential biological advantages for the treatment of cancer.
Because both GP regimen and hyperthermia cause apoptosis of lung cancer cells, their combination may result in enhanced cell killing. However, the working mechanism of this combination of therapy is not completely understood.
There are rare reports that demonstrate the advantage of combining hyperthermia with chemotherapy for the treatment of advanced NSCLC. Moreover, to our knowledge, there is no previous report that has investigated the improvement in quality of life (QoL) following this combined treatment. Many studies have only reported objective variables (rigid variables), such as mortality rate, survival time, etc., but not subjective variables (soft variables), such as QoL. The reasons for this oversight are that clinical physicians: (1) doubt the feasibility and practical value of QoL studies; (2) are not familiar with the methods or instruments of QoL studies; (3) lack basic dialectical views, which means that they usually pay attention to radical treatments but ignore palliative treatments. In fact, QoL studies could play a pivotal role in clinical oncology research. First, they can help assess the overall therapeutic effect, allowing the most suitable treatment for an individual patient. Second, they help in the selection and evaluation of anti-cancer agents, analgesics, and antiemetic agents. Third, they help highlight the long-term survival status following treatment.
Based on the above ideas, we designed the present phase II study to investigate the overall effect produced by radio-frequency (RF) hyperthermia. We combined GP regimen and RF hyperthermia for patients with advanced NSCLC. The response rate (RR), toxicity and clinical benefit response (CBR) were investigated.
Patients and methods
Patient selection
Eligible patients had to have pathological proof of advanced NSCLC based on surgical evaluation (67/80) or lung puncture (13/60), and should have at least one measurable lesion. Patients were enrolled and divided randomly into two groups (A and B), 40 patients to each group. Among them, 16 were referred to our centre for their initial treatment and 64 for retreatment. The 64 patients who had received treatment before enrolment experienced only one platinum-containing regimen as a first-line treatment, accompanied with or without irradiation. These patients failed to respond to the first-line treatment, and proved to be inoperable by helical computerised tomography (CT). In addition, patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 or 1 and a life expectancy of greater than three months. Every patient enrolled in this study signed a written informed consent form approved by the Medical Ethics Committee of Nanjing Medical University, which also approved the study protocol.
Therapeutic regimens
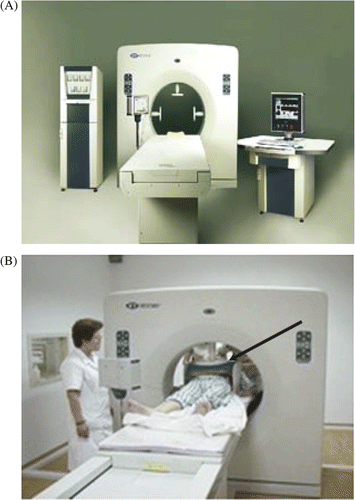
Group A: We used a HY-7000 RF external heat system (working frequency of 40.68 MHz) to perform regional hyperthermia. Primary lesions of the lungs were chosen as the target area, which were covered by an applicator 150–300 mm in diameter. The lesions were selected and located using B-type ultrasonography. No matter the size or symptomatic status of the lesion, they received RF hyperthermia simultaneously. The output power was 300–1100 W and the reflectivity was less than 3%. Hyperthermia was administered 1 h after the anticancer drugs were given. The total heating time was up to 60 min, twice a week, with the interval between the procedures greater than 48 h. The target temperature ranged from 39°C to 42.5°C and the degree of accuracy was controlled to ±0.1°C. The temperature began from 39°C, and increased slowly according to the patient's tolerance but not exceeding 42.5°C. If the tumour (and metastatic masses, if existent were located in the chest, we actually monitored the temperature with electrodes pasted to the surface of skin. If there were metastatic masses in the abdominal cavity, the temperature was monitored using an anal thermometer in the rectum. We instructed each patient to mention any abnormal feelings, so that we could adjust settings, such as power output. As shown in , usually a large enough water bag was used for conducting the heat, so that more than one site would not be a problem. As shown in , the three brackets fixed in the round hole of the machine could hold the water bag and the body, making them approach to each other firmly. If the patient has a good tolerance to heat, the temperature will be controlled relatively high. If not, the temperature will be controlled relatively low.
A regimen of 1000 mg/m2 intravenous gemcitabine on day 1 and day 8 and 80 mg/m2 intravenous cisplatin on day 1 (GP) was performed, with adequate hydration and diuresis repeated every 3 weeks. Gemcitabine was administered intravenously over a period of 30 min. The target sum of chemotherapy was four cycles.
Patients received RF hyperthermia 1 h after gemcitabine was administrated each time. The target sum of hyperthermia was eight times during the whole course of treatment.
Dose adjustment was performed when grade 4 haematological or grade 3, 4 non-haematological toxicities appeared, resulting in a dose reduction of 25% in subsequent cycles.
After each cycle blood tests were performed, including red blood cell, white blood cell, neutrophil, and platelet count as well as determination of haemoglobin concentrations. Routine urine tests, including haematuria and proteinuria, were performed every week, as well as rechecks of all biochemical tests, including tests for alanine and aspartate aminotransferase alkaline phosphatase, bilirubin, albumin and globulin, creatinine and urea and electrolyte levels, as well as electrocardiograms, thoracic CT and abdominal ultrasonographs. Group B patients were treated with a single regimen of GP. The administration and dose were the same as group A.
Evaluation of response and toxicity
The response was estimated within a week after every two cycles of chemotherapy, and confirmed within 4 weeks for responding patients. We used contrast-enhanced helical CT to measure the size of the target tumour (i.e. the longest diameter of the mass), and the response criteria was based on the response evaluation criteria in solid tumours Citation[5]. Toxicity evaluation was performed for all patients according to National Cancer Institute Common Toxicity Criteria version 2.0 Citation[6].
CBR is a clinical index for incurable tumours and can be used to assess the improvement of QoL. The criteria include (1) dosage reduction of analgesics ≥50%, recorded every day; (2) intensity reduction of pain ≥50%, recorded with the memorial pain assessment card (score range 0–100); (3) performance status improvement ≥20, assessed with PS scoring every week and (4) body weight increase ≥7%, measured every day. Patients who met at least one of these indices over a period of greater than 4 weeks, demonstrated no aggravated index and had no fluid retention were assessed as having a positive CBR. These criteria were assessed with objective tables and scored in a blinded fashion, not only patients’ subjective sensation. The treatment was evaluated as effective while a patient's conditions were consistent with at least one of the criteria and all others were stable. The treatment was evaluated as ineffective when a patient's conditions were not consistent with any of the criteria, and as stable when all conditions of a patient were not consistent with the criteria but demonstrated no aggravated index.
Statistical analysis
The primary end points were response rate and grade 3, 4 haematological or non-haematological toxicities, and the secondary end points was CBR. The chi-square test was used to assess statistical differences between groups using SPSS 13.0 software.
Results
Patients’ characteristics
Eighty patients with advanced NSCLC were included in this study. An overview of this study was fully explained to every eligible patient, and every enrolled patient signed an informed consent form and was asked to keep one copy. Patients’ characteristics are presented in . No patient refused hyperthermia treatment because of economic or other reasons.
Table I. Baseline patient characteristics.
Response
All patients in group A and group B received all four planned cycles of chemotherapy. RF hyperthermia was administered twice for each of the four cycles to patients in group A. In group A, one patient achieved complete remission (CR), 18 patients achieved partial remission (PR), 18 patients were evaluated as having a stable disease (SD), and three patients experienced a progression of the disease (PD). In group B, no patient achieved CR, 17 patients achieved PR, 19 patients were evaluated as SD, and seven patients experienced PD. There was no significant difference in the RR between groups. The overall RR was 45%, and the tumour control rate (CR + PR + SD) was 91.3% ().
Table II. Responses of groups A and B.
Toxicities
No patients discontinued the study treatment due to toxicities or side effects related to RF hyperthermia. The main toxicities are summarised in .
Table III. Main toxicities of groups A and B.
Evaluation of quality of life
The improvement rate in QoL for group A was 82.5% and was significantly higher than that for group B (47.5%). However, the differences among various components were not significant ( and ).
Table IV. CRB of groups A and B.
Table V. Impact on each component of the CRB index.
Discussion
The application of RF hyperthermia has rapidly developed in recent years. Several studies have shown that hyperthermia can either sensitise cancer cells to subsequent chemo/radiation therapy Citation[7], Citation[8] or enhance the cytotoxic effect of these interventions Citation[7], Citation[9], leading to loss of cell structure and function. In general, the therapeutic mechanism of hyperthermia on tumours include (1) the direct killing of tumour cells; (2) enhanced expression of apoptosis-modulating genes leading to apoptosis of tumour cells; (3) inhibition of tumour-derived vascular epithelial growth factor expression and its products, resulting in a block to proliferation of the endothelium and remodelling of the extracellular matrix, thereby inhibiting the growth and metastasis of tumours; (4) the formation of hyperoxides and free radicals; (5) reinforcement of immune function by enhancing the function of immune cells and synthesis of cytokines Citation[1].
Tumour blood flow Citation[10], Citation[11] and the permeability of tumour vessels Citation[12–15] are increased at temperatures of 41°–43°C. Some studies have also shown that tumour accumulation of macromolecular and liposome preparations are increased by hyperthermia-induced micro-environmental changes in tumour tissues Citation[16–20]. In general, mild hyperthermia (39°–42°C) induces an increase in tumour-tissue blood flow volume Citation[10], Citation[11], tumour vessel hyperpermeability, and extravasation Citation[21–25]. However, hyperthermia at 43°C or higher induces vascular injury, haemorrhage and collapse, as well as a decrease in extravasation Citation[24], Citation[26–29]. Therefore, in the present study, hyperthermia was conducted at 39°–42.5°C for 60 min.
Furthermore, the vascular structure of malignant tissue is greatly different from that of normal tissue, as the blood flow rate in normal tissue is 30 times greater than that of tumour tissues. This difference makes the temperature of tumour tissues significantly higher than normal tissue under the same conditions, which also increases the effect of hyperthermia.
The HY7000 external heat system utilises non-interventional physiotherapy, producing heat that is powered by electromagnetic waves of a certain wavelength. When a part of the body is put between two radiators, the heat system can produce and deliver heat to the part rapidly (). Because of the differences in blood circulation between tumour and normal tissues, the temperature of the tumour tissue is much higher than that of normal tissue. When temperature rises to the effective curative temperature and is maintained for a certain period of time, the tumour tissue will be destroyed and the normal tissue will remain alive.
Furthermore, regional RF hyperthermia can also relieve side effects caused by cytotoxic chemotherapy. In another way, it helps patients receive necessary chemotherapy. According to the theory of Chinese traditional medicine, hyperthermia can activate the blood flow and dredge stasis of blood fluids.
We determine skin or rectal temperature, but not the actual tumour temperature because the RF hyperthermia we perform is a non-invasive treatment modality, so we would not allow any invasion or wound to be made during the process of treatment. In the eight-year practice of RF hyperthermia, we have found that the tumour temperature is 2°C higher than skin temperature. We have performed measurement of tumour temperature on mice, but to the best of our knowledge, there has not been any report about the measurement of tumour temperature in vivo. Comparing the effect of RF hyperthermia with the harm of the wound made by invasive measurement of tumour temperature, we do not adopt invasive measurement, and usually the skin temperature is more precise than rectum temperature if the mass is located in the chest.
The cytotoxic effects of several antitumour agents, including gemcitabine, can be enhanced by raising the tissue temperature, and the synergism between hyperthermia and chemotherapy has been clearly demonstrated for gemcitabine. In vivo experiments reveal that (1) combination therapy significantly delays tumour growth, (2) extracted tissue has a greater frequency of apoptosis, lower levels of mitosis, lower levels of Hsp70 and increased activation of caspase-3; and (3) multiple combination therapies prevent tumour growth during the experimental period Citation[1].
Results in the present study suggested that the regimen of GP combined with hyperthermia had the advantage of remarkably improving the patients’ QoL. The 80 patients were assigned to the two arms randomly, so the major difference between the two arms was receiving hyperthermia or not. We think this improvement in QoL was a result of the RF hyperthermia, but this conclusion requires a progressive clinical trial with a larger sample size. In fact, in May 2009 we initiated another randomised trial investigating the effects of regional RF hyperthermia for the treatment of advanced NSCLC, which involves a substantially greater number of patients.
We chose CBR to be the measure of QoL because although the number of criteria was limited to four, these criteria contained the main aspects of QoL. Pain is the most common syndrome directly impacting QoL. Among cancer patients, 30–60% endure pain and most do not have satisfactory relief. Also, PS scoring is the common used instrument for evaluating QoL. As for weight, it not only is the appearance of QoL, but also can indicate the tolerance of enduring various therapeutic modalities.
To the best of our knowledge, the present study is the first that indicates a remarkable improvement in QoL, which is very important to patients. Because the duration of follow-up was short, we did not compare the survival times and survival rates between the two groups, but results of this study were encouraging.
In summary, the regimen of GP combined with regional RF hyperthermia for advanced NSCLC was well tolerated and safe. The results of the present study show remarkable advantages in QoL for this combined treatment regimen. These data suggest that hyperthermia could become an additional modality in the multidisciplinary approach for the treatment of advanced NSCLC. Prospective studies are warranted to further these conclusions.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Vertrees RA, Das GC, Popov VL, Coscio AM, Goodwin TJ, Logrono R, Zwischenberger JB, Boor PJ. Synergistic Interaction of Hyperthermia and Gemcitabine. Cancer Biol Ther 2005; 4: 1144–1153
- Pereira S, Fernandes PA, Ramos MJ. Mechanism for ribonucleotide reductase inactivation by the anticancer drug gemcitabine. J Comput Chem 2004; 25: 1286–1294
- Rosell R, Danenberg KD, Alberola V, Bepler G, Sanchez JJ, Camps C, Provencio M, Isla D, Taron M, Diz P, Artal A. Spanish Lung Cancer Group. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced nonsmall cell lung cancer patients. Clin Cancer Res 2004; 10: 1318–1325
- Pace E, Melis M, Siena L, Bucchieri F, Vignola AM, Profita M, Gjomarkaj M, Bonsignore G. Effects of gemcitabine on cell proliferation and apoptosis in nonsmall-cell lung cancer (NSCLC) cell lines. Cancer Chemother Pharmacol 2000; 46: 467–476
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026.
- Kaba H, Fukuda H, Yamamoto S, Ohashi Y. Reliability at the National Cancer Institute-Common Toxicity Criteria version 2.0. Gan To Kagaku Ryoho. 2004 Aug;31(8):1187–1192.
- Van der Zee J. Heating the patient A promising approach?. Ann Oncol 2002; 13: 1173–1184
- Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003; 10: 463–468
- Nagle VLJ, Berry JM, Bull JM. Whole body hyperthermia with carboplatin (CBDCA) for treatment of advanced or metastatic GI adenocarcinomas. Proc Am Assoc CA Res 1999; 40: 345
- Karino T, Koga S, Maeta M. Experimental studies of the effects of local hyperthermia on blood flow, oxygen pressure and pH in tumors. Jpn J Surg 1988; 18: 276–283
- Song CW. Effect of local hyperthermia on blood flow and micro-environment. Cancer Res 1984; 44: 4721–4730
- Engin K. Biological rationale and clinical experience with hyperthermia. Control Clin Trials 1996; 17: 316–342
- Feyerabend T. Rationale and clinical status of local hyperthermia, radiation and chemotherapy in locally advanced malignancies. Anticancer Res 1997; 17: 2895–2897
- Issels R. Hyperthermia combined with chemotherapy: Biological rationale, clinical application and treatment results. Oncology 1999; 22: 374–381
- Matthew CB. Hyperthermia-induced changes in the vascular permeability of rats: A model system to examine therapeutic interventions. J Thermal Biol 2000; 25: 381–386
- Massing U. Cancer therapy with liposomal formations of anti-cancer drugs. Int J Clin Pharm Ther 1997; 35: 87–90
- Huang SK, Stauffer PR, Hong K, Guo JW, Phillips TL, Huang A, Papahadjopoulos D. Liposomes and hyperthermia in mice: Increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res 1994; 54: 2186–2191
- Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia 1999; 15: 345–370
- Maruyama K, Unezaki S, Takahashi N, Iwatsuru M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim Biophys Acta 1993; 1149: 209–216
- Unezaki S, Maruyama K, Takahashi N, Koyama M, Yuda T, Suginaka A, Iwatsuru M. Enhanced delivery and antitumor activity of doxorubicin using long-circulating thermosensitive liposomes containing amphipathic polyethylene glycol in combination with local hyperthermia. Pharm Res 1994; 11: 1180–1185
- Fujiwara K, Watanabe T. Effects of hyperthermia radiotherapy and thermoradiotherapy on tumor microvascular permeability. Acta Pathol Jpn 1990; 40: 79–84
- Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys 1996; 36: 1177–1187
- Hauck ML, Coffin DO, Dodge RK, Dewhirst MW, Mitchell JB, Zalutsky MR. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumor interstitial fluid pressure. Int J Hyperthermia 1997; 13: 307–316
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Lefor A, Makohon S, Ackerman N. The effects of hyperthermia on vascular permeability in experimental liver metastasis. J Surg Oncol 1985; 28: 297–300
- Dudar T, Jain R. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res 1984; 44: 605–612
- Eddy HA. Alterations in tumor microvasculature during hyperthermia. Radiology 1980; 137: 515–521
- Moriyama E. Cerebral blood flow changes during localized hyperthermia. Neurol Med Chir 1990; 30: 923–929
- Nishimura Y, Hiraoka M, Jo S, Akuta K, Yukawa Y, Shibamoto Y, Takahashi M, Abe M. Microangiographic and histologic analysis of the effects of hyperthermia on murine tumor microvasculature. Int J Radiat Oncol Biol Phys 1988; 15: 411–420