Abstract
Purpose: The heat-sink effect produced by rapid blood flow through large vessels (diameter (D) ≥ 5 mm) is an important factor that influences ablation zone size after radiofrequency ablation (RFA). Currently, however, the interactions between hepatic RFA lesions and large vessels are not well understood. The purpose of this study was to examine the effects of RFA lesions occurring near large vessels (D ≥ 5 mm) in the canine liver.
Materials and methods: Thirty healthy adult mongrel dogs were used, with 15 dogs randomly assigned to groups I and II. In group I, the closest distance from the tip of the RFA electrode to the large vessel (D ≥ 5 mm) was more than 20 mm; in group II, this distance to the wall of the inferior vena cava (IVC) was no more than 5 mm. RFA was performed on the liver of each dog according to standard procedures. The blood flow velocity of the IVC, the computerised tomography (CT), the pathological characteristics of the RFA lesions and procedure-related complications were examined.
Results: No death or complications occurred in any dogs. Vascular walls were not affected, except for when the tips of the electrode stuck to the IVC. The coagulative necrosis region was decreased, and its shape was fusiform close to the IVC. Some normal hepatic cells were found in the necrotic region near the IVC.
Conclusions: It is both safe and feasible to perform RFA near the IVC. The shape and size of the coagulation zone should be considered when electrodes are placed in this area. Near the IVC, the size of the coagulation zone was decreased, and it was incompletely formed.
Introduction
Radiofrequency ablation (RFA) is a safe, minimally invasive method for treating liver tumors Citation[1–4]. The efficacy of RFA, however, is influenced by the blood flow in large vessels (diameter (D) ≥ 5 mm). Further, the heat-sink effect produced by rapid blood flow is an important factor influencing ablation zone size after RFA Citation[5], Citation[6]. The effects of RFA on an ablation zone located near a large vessel and on a large vessel itself are not currently well understood. Additionally, there is currently no distance between a large vessel and RFA electrodes that is defined as safe for RFA. Although some research has examined the safety and reliability of RFA in these positions, most findings have arisen from clinical observations Citation[7–9].The exploration of these issues has important clinical value in improving RFA efficacy for tumours located near large vessels. In this study, we treated livers of dogs with RFA to determine a safe, near-IVC distance at which RFA can be performed without seriously damaging the IVC or causing serious complications.
Methods
Experimental animal grouping
Thirty healthy adult male and female mongrel dogs (15–21 kg in weight) were raised and provided by the Experimental Animal Center of the Third Military Medical University. Fifteen dogs were randomly assigned to groups I or II. Group I, the control group, was treated with RFA at a distance far from a large vessel in the liver; the closest distance from the tip of the RFA electrode to a large vessel (D ≥ 5 mm) in this group was more than 20 mm. Group II, the experimental group, was treated with RFA near the IVC in the liver; the distance from the tip of the RFA electrode to the wall of the IVC was no more than 5 mm. In group II, ten dogs had electrode-IVC wall distances of 2–5 mm, whereas the daughter needle clung to the wall of the IVC while being monitored by colour Doppler ultrasonography in five dogs.
These experiments were approved by the Animal Care Committee of the Third Military Medical University, Chongqing, China.
Animal treatment
Animals were fasted but permitted to drink water for 12–24 h. Animals received general anaesthesia by intramuscular injection of 0.1 mg/kg SU-MIAN-XIN (xylazole 60 mg/mL, dihydroetorphine 4 µg/nL and haloperidol 2.5 mg/mL; the Military Veterinary Institute, University of Military Supplies of PLA, Changchun, China). After anaesthesia application the hair on the belly and posterior limbs was shaved; residual short hair was removed completely with 8% Na2S. A clinical thermometer was set in the armpit. An electrode plate was attached to the prepared skin on the posterior limbs. A wire was used to connect the electrode plate to a RF2000 current generator (Radio Therapeutics, Mountain View, CA). After abdominal disinfection, the dog's liver was exposed through a midline upper-middle abdominal incision. Haemodynamic parameters of the IVC (i.e. radius and blood flow velocity) were determined with a colour Doppler ultra-sound (US) system (EUB-2000; Hitachi Medical Systems, Tokyo, Japan) equipped with a wideband C5-2 MHz convex probe. Then, in group II, the RFA applicator was placed into the area adjacent to the IVC from the diaphragmatic surface of the liver under US guidance. Finally, RFA was performed (). In group I, US was also used to explore the area around the radiofrequency electrode to ensure that there was no large vessel (with a diameter greater than 5 mm) within 20 mm of the tip of the electrode. After RFA, haemodynamic parameters of the IVC were determined, and the therapeutic area was examined using colour Doppler examination.
RFA treatment process
A RF 2000TM therapy system was used in all experiments. The electrode was a LeVeen bundled needle, which has ten daughter needles after the divergence. The diameter at divergence was controlled at 20 mm in these experiments. Before treatment, the IVC diameter, blood flow velocity and distance from the tip of the electrode were determined by US. According to standard procedures for clinical RFA Citation[10], RFA was performed with 30 W of power initially; power was then increased by 10 W every min until it reached 90 W, where it was maintained. Thereafter, in response to increases in local tissue impedance, the generator adjusted the output current and power automatically. When the impedance reached a maximum value, the output power became small. When impedance first reached a maximum, the treatment was stopped for 1 min. Then, a second treatment was started using 40 W and the same operating procedure as before (i.e. a 10-W increase every min until the power reached 90 W). When the impedance reached a maximum for the second time, RFA therapy was complete.
Observation indices
Perioperative animal observations were as follows: respiratory parameters; general reactions and temperature; animals’ eating and drinking behaviours; animals’ mental health; complications and mortality rates.
Liver imaging examination: Plain scan and enhanced CT (SOMATOM Plus 4 CT Scanner, Siemens, German) were performed on day 1 and then during the second and fourth weeks after RFA. The liver images after RFA were recorded.
Liver function observation: Peripheral venous blood was collected from animals in groups I and II. Liver function was determined using a Beckman CX-7 automatic biochemistry analyser before RFA and after days 1, 3, 7, 14, 21 and 28.
IVC injured complications: three animals in each group were killed everytime on days 7, 14 and 21, and all remaining animals were killed on day 28 after RFA. All animals underwent open abdominal surgery to identify complications resulting from RFA, changes in the treatment region and IVC using colour Doppler imaging.
Histopathological examination: animals from each group were killed in batches on days 7, 14, 21 and 28 after RFA, and every animal underwent open abdominal surgery. Every liver was removed, fixed with 10% formalin, embedded in paraffin, cut into consecutive 5-micron slices and stained with HE for microscopic examination.
Statistical treatment
SPSS software was used for statistic analysis. Data are expressed as means ± standard deviation (x ± s); a t-test was carried out to detect differences between groups. A value of P < 0.05 was considered statistically significant.
Results
Perioperative animal observation
The operative procedure, from the beginning of the incision to the completion of RFA, lasted from 60–90 min. During the RFA of groups I and II, all animals were stable and displayed no irritability, fever or quickened respiration. Tissues of the treatment region hardened, and no ischaemia or necrosis resulting from thermal damage was observed in the diaphragm or pancreas. Animals were given free access to drinking water 1 day after surgery. Symptoms of appetite loss were observed during the first 3 days after surgery but were not accompanied by irritability or vomiting. There were no post-surgery complications (including intra-abdominal haemorrhage, gastrointestinal perforation, abdominal abscess, thrombosis or stenosis of the IVC related to RFA), and no mortality was observed in any group.
Changes in haemodynamics after RFA
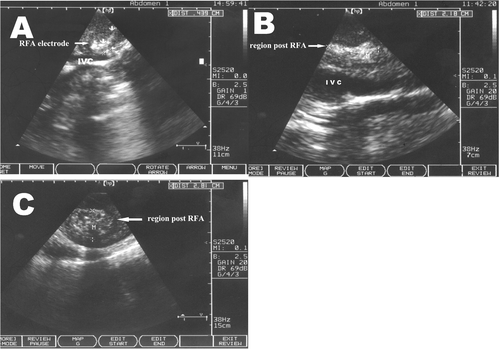
Colour Doppler imaging following RFA revealed filling blood flow in the IVC, a smooth and complete venous wall and no thrombosis. Strong echo light spots were present in the RFA treatment regions of groups I and II, whereas strong echoes extending in the direction of the IVC blood flow and spots appeared as long fusiform shapes along the IVC of group II only (). Spots in group I, however, showed the same round shape as the electrode (). Blood flow parameters of the IVC of group II before and after RFA were compared. The diameter of the IVC near the RFA treatment area did not detectably change. IVC blood flow was slightly faster after RFA than before RFA, but this difference was not statistically significant (220.7 ± 74.5 mm/s before versus 226.5 ± 76.3 mm/s after, P > 0.05) ().
RFA treatment duration
The average treatment time of group I was 15.4 ± 4.47 (Citation[9–22]) min, whereas the average treatment time of group II was 26.6 ± 4.26 (10–51) min; this difference was statistically significant (P < 0.01).
Changes in liver function after RFA
The levels of alanine transaminase (ALT), aspartate aminotransferase (AST) and glutamyl transpeptidase (GGT) increased significantly after RFA (P < 0.05). ALT, AST and GGT levels reached a maximum during the first 2 days following treatment and then decreased gradually, returning to levels similar to those before treatment around 1 week after RFA. These changes in liver function were similar in groups I and II, with the exception of a significantly greater increase in AST in group I (P < 0.05) ().
Table I. Liver function after RF ablation in the two groups.
CT imaging after RFA
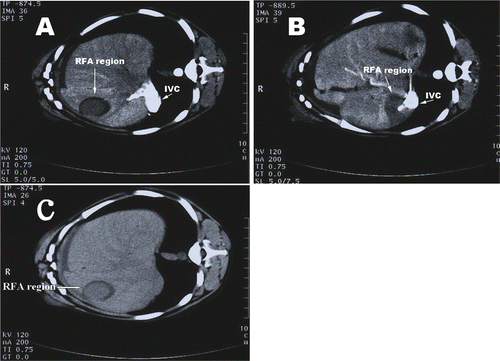
The RFA region of group I was round and had a low density. A hypodense area was present in the areas surrounding the treatment site after RFA, with a clear border between the treatment region and the surrounding liver tissue. An enhanced scan showed no obvious enhancement in the treatment region. The hypodense region generally disappeared after 2 weeks. The shape of the coagulation zone was the same as the outline of the electrode.
Upon CT scanning, the RFA-treated region near the IVC in group II also showed a low density, but its CT value was higher than that of the control group. Additionally, there was no obvious hypodense region in the tissue surrounding the RFA-treated area. Enhanced CT showed that the treatment region was more clearly defined as an irregular long strip, the shape of the treatment region was largely influenced by the surrounding large vessel. The shape of the coagulation zone was not the same as the shape of the electrode. No thrombus or ruptures were observed along the IVC ().
Figure 2. CT scans after RFA. (A) Enhanced CT scans show that the RFA region away from the IVC is round; (B) The RFA region near the IVC shows that the treatment region is more clearly defined as an irregular long strip. No thrombus or ruptures were observed along the IVC; (C) A hypodense area was present in the surrounding area after RFA.
Pathological results
Mild swelling after RFA was confirmed by naked-eye observation of the treatment region in group II. Pathological sections showed drying and reddish-brown coagulative necrosis in the central region of the ablation zone, with a surrounding dark red hemorrhagic infarction. Sections of normal liver tissue were dark red, and the shape of the necrotic region near the IVC was irregular. When the distance between the electrode and vessel wall was 2–5 mm (n = 10), the wall of the nearby IVC was smooth and normal. When a set of electrodes clung to the venous wall (n = 5), the wall was dry, matte and murky grey rather than the normal silvery white. No thrombosis was observed, however, on the surface of the tunica intima in any group after treatment. There was no blood vessel rupture or bleeding in either the experimental or the control group. A fibrous capsule was present around RFA-treated regions, forming a border separating these regions from surrounding liver tissue for 2–4 weeks after RFA. This capsule, however, was sometimes thin and incomplete by about week 2 after RFA.
The size of the ablation zone after RFA was directly related to therapeutic efficacy. The size of the coagulative necrosis was determined as the mean value between the maximum and minimum diameters of each necrotic zone. There were significant differences in the size of coagulative necrotic areas determined at different times after RFA between the experimental group and the control group (P < 0.05) (). The shape of the RFA-treated region in group I was spherical and the average ellipticity was 0.793 ± 0.06 (0.72–0.86), whereas that of group II was fusiform and the average ellipticity was 0.516 ± 0.156 (0.27–0.71), shape thus differed significantly between groups (P < 0.05).
Table II. Difference in the lesion size post-RF ablation between the two groups.
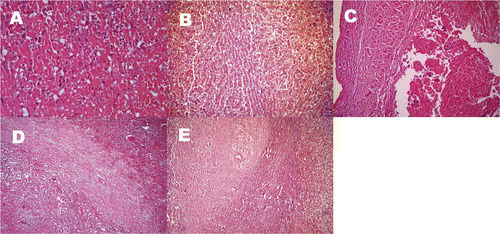
Bleeding and coagulative necrosis of massive tissue in the RFA region, loose cytoplasm and obvious swelling of surrounding liver cells as well as obvious hyperaemia in the hepatic sinusoid were microscopically observed (). Some samples of treated regions from animals in group II showed hepatic cord residue, indicating incomplete necrosis (). RFA with an electrode attached directly to the IVC wall produced incomplete endothelial denudation and damaged endothelial continuity. In cases where the electrode was 2–5 mm from the vessel wall, endothelial cell morphology was normal, and there was no obvious damage to the vessel wall after RFA (). Inflammatory and fibroblast cells proliferated and gathered, fibrosis of local tissue was present and necrotic foci were gradually encapsulated by fibrous tissue in the necrotic region during weeks 1–2 after RFA (). Collagen hyperplasia, fibrosis and glassy degeneration, with some hepatocyte fatty degeneration in the necrotic region, were observed during the 3–4 weeks after RFA ().
Figure 3. Microscope display of RFA regions: (A) Bleeding and coagulative necrosis of tissue in the RFA region, loose cytoplasm and obvious swelling of surrounding liver cells, and obvious hyperaemia in the hepatic sinusoid were microscopically observed (HE × 200). (B) Some samples of Group II treatment regions showed hepatic cord residue, which indicated incomplete necrosis (HE × 100). (C) In cases where the electrode was 2–5 mm from the vessel wall, endothelial cell morphology was normal and there was no obvious damage to the vessel wall after RFA (HE × 100). (D) Two weeks after RFA, necrotic foci were gradually encapsulated by fibrous tissue in the necrotic region (HE × 100). (E) Four weeks after RFA, collagen hyperplasia, fibrosis, and glassy degeneration with some hepatocyte fatty degeneration are present in the necrosis region (HE × 100).
Discussion
The principle of RFA is that radiofrequency currents produce heat that induces ion oscillation and friction in tissue, resulting in coagulative necrosis of local tissue. RFA, therefore, is a thermal treatment Citation[11–12]. One associated limitation of RFA is the influence of the heat-sink effect of blood flow. In theory, this is also an important factor in assuring that nearby large vessels are not damaged. Venous walls are thin; accordingly, their heat conductivity is good. Thus, heating tissue near veins that carry rapid blood flow provides the structural basis for the assumption that RFA will not result in heat-induced damage. In the current experiment, the venous wall was not damaged, except when the electrode was directly attached to the wall of the IVC, supporting the presence of the heat-sink effect. When the electrode was too close to the vessel wall (distance ≤ 2 mm), the local current was so high that it produced endothelial denudation and damaged the continuity of the endothelia; however, no thrombosis or stenosis was observed. For safety reasons, however, we conclude that a distance of more than 2 mm from the IVC should be maintained to ensure a safe RFA procedure.
Respiratory and other overall physiological responses are good measures of safety in an RFA procedure near a large vessel, as heat can be rapidly conducted through blood flow through the heat-sink effect Citation[13]. All RFA procedures were completed with no quickened respiration or agitated activity recorded, and the surface temperature of the large vessels in the limbs did not significantly increase. The body weight and blood volume of the dogs are, of course, less than those of humans, so adult humans should exhibit even more tolerance to RFA near a large vessel.
We also found that the time of the RFA procedure near the IVC was significantly prolonged with respect to the control group. Because RFA acts through heating of local tissue to the point of coagulation necrosis, completion of the therapy is judged by the increase in electric resistance of the tissue. If the heat-sink effect of blood flow results in too great a loss of local heat, the temperature will increase slowly. Electric resistance will thus change slowly, prolonging therapy time. The results of this study indicate that increased therapy time did not negatively influence tolerance to RFA. Good tolerance to RFA near the IVC was demonstrated by the absence of obvious interference with the haemodynamics of the IVC and lack of significant change in haemodynamics after RFA.
Since most patients with liver cancer also have hepatitis and liver cirrhosis, their liver function is in a decompensation stage (or compensation function is severely decreased) Citation[14–15]. It is thus necessary to monitor changes in liver function after RFA near a large vessel. The levels of ALT and AST increased significantly after RFA. Compared with the control group, the level of the experimental group was lower and returned to a normal level faster. In this study, continuous observation of liver function during the 3 weeks after RFA indicated that the influence of RFA on liver function was decreased in the experimental group compared to the control group. This result was closely related to the decrease in the size of the RFA-affected area induced by the heat-sink effect due to the large amount of blood flow near a large vessel.
Because blood flow from a large vessel influences an RFA region, some clinical groups block or reduce the liver's blood flow, using procedures such as Pringle manipulation, to increase the size of the coagulative necrosis region and further improve the efficacy of RFA Citation[16–18]. However, it is not easy to block the blood flow of a large vessel in percutaneous RFA.
The controllability of the treated region in RFA makes this procedure preferable to chemical ablation, in which heterogeneous diffusion of the ablative reagent may occur. However, some groups have reported temperature increases in tissue that occur in the direction of the blood flow in RFA Citation[19–20]. In our experiment, we also found that the shape of the coagulative necrosis region was irregular along the direction of IVC blood flow and was different from a spherical region that was located away from a large vessel. If we placed the electrode near the IVC, as is the usual practice, the coagulative necrosis region may not be located at the site we are aiming for, which influences our ability to control the ablation zone after RFA. When we performed RFA near a large vessel, the size of the coagulation region was significantly smaller than away from a large vessel and the shape of the region irregular and shifted downstream. Because a local high-temperature region could not be maintained when the radiofrequency heat was dissipated by blood flow, the distance range within which we successfully inactivated tumours was small, and some activated tumour cells were not treated Citation[21]. This heat-sink effect weakens the effectiveness of RFA for inactivating a tumour. In the current study, histopathological examination confirmed the presence of hepatic cord residue in the treatment region. The efficacy of RFA in treating liver tumours depends not only on the size of the coagulative necrosis region but also on the shape of the ablated foci. Thus, it is important to properly adjust the site of the RF electrode in RFA therapy to ensure that the region upstream of the tumour is completely included in the treatment. This practice may reduce the risk of lesion residue in the treatment process.
CT re-examination is generally used to evaluate the efficacy of RFA treatment and to identify possible complications following RFA Citation[22–24]. This study reports differences in CT scan results and RFA region characteristics between control and experimental groups after RFA near the IVC. CT scans showed a lower density region in the early stages after RFA in the control group than in the experimental group, with a surrounding hypodense region of bleeding and swelling; therefore, the treatment region was easier to identify in the control group than in the experimental group. In contrast, this hypodense region was absent in the experimental group, although a lower density region was present. CT scans of the experimental group showed no difference in the treated area and the surrounding normal liver tissue, demonstrating the importance of enhanced CT scans in evaluating the efficacy of RFA. Generally, because of the strong echo light spots of the local region after RFA, colour Doppler imaging is not considered an ideal tool for the re-examination of RFA therapy. We also noticed that the area with strong echo light spots measured by colour Doppler imaging was larger than that of the real necrotic area; therefore, colour Doppler imaging is not a viable tool for estimating RFA efficacy. Some groups Citation[25–28] recently suggested that a contrast agent can help to detect residual tumour tissue and tumour recurrence with ultrasound and can be used differentiate tumour tissue from the coagulation necrosis region of ischaemia; however, the clinical value of this technique must be verified through further study.
As this experiment only employed the normal liver tissue of canines, our conclusions should be further confirmed in human patients suffering from liver cancer near the IVC.
Conclusion
Because of the heat-sink effect produced by the large amount of blood flow in the IVC, RFA is relatively safe when the distance between the probe tip and the IVC is more than 2 mm. However, RFA therapy in this position produces different properties than does RFA in other regions of the liver. To effectively destroy the focus of disease, the RF probe should be placed in a position that is based on the shape and size of coagulative necrosis region. With RFA near the IVC, the resulting ablation zone was decreased and necrosis was incomplete.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Forner A, Bruix J. Locoregional treatment for hepatocellular carcinoma: From clinical exploration to robust clinical data, changing standards of care. Hepatology 2008; 47: 5–9
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005; 21: 755–760
- Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: A critical review of the literature. Am J Surg 2008; 195: 508–511
- Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: A systematic review. Arch Surg 2006; 141: 181–184
- Nakai M, Sato M, Sahara S, Kawai N, Tanihata H, Kimura M, Terada M. Radiofrequency ablation in a porcine liver model: Effects of transcatheter arterial embolization with iodized oil on ablation time, maximum output, and coagulation diameter as well as angiographic characteristics. World J Gastroenterol 2007; 13: 2841–2845
- Yoshimoto T, Kotoh K, Horikawa Y, Kohjima M, Morizono S, Yamashita S, Enjoji M, Nakamuta M. Decreased portal flow volume increases the area of necrosis caused by radio frequency ablation in pigs. Liver Int 2007; 27: 368–372
- Thanos L, Mylona S, Galani P, Pomoni M, Pomoni A, Koskinas I. Overcoming the heat‐sink phenomenon: Successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn Interv Radiol 2008; 14: 51–54
- Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006; 43: 1101–1105
- Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. Am J Roentgenol 2008; 190: W187–W192
- Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000; 232: 381–385
- Schirmang TC, Dupuy DE. Image-guided thermal ablation of nonresectable hepatic tumors using the Cool-Tip radiofrequency ablation system. Expert Rev Med Devices 2007; 4: 803–806
- Crocetti L, Lencioni R. Thermal ablation of hepatocellular carcinoma. Cancer Imaging 2008; 8: 19–24
- Quan-Da Liu, Kuan-Sheng Ma, Zhen-Ping He, Jun Ding, Xue-Quan Huang, Jia-Hong Dong. Experimental study on the feasibility and safety of radiofrequency ablation for secondary splenomegaly and hypersplenism. World Gastroenterol, 2003; 9: 813–817
- McMahon BJ. Natural history of chronic hepatitis B - clinical implications. Medscape J Med 2008; 10: 91–95
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: Summary of a clinical research workshop. Hepatology 2007; 45: 1056–1060
- Shen P, Fleming S, Westcott C, Challa V. Laparoscopic radiofrequency ablation of the liver in proximity to major vasculature: Effect of the Pringle maneuver. J Surg Oncol 2003; 83: 36–40
- Burdío F, Navarro A, Sousa R, Burdío JM, Güemes A, Gonzalez A, Cruz I, Castiella T, Lozano R, Berjano E, et al. Evolving technology in bipolar perfused radiofrequency ablation: Assessment of efficacy, predictability and safety in a pig liver model. Eur Radiol 2006; 16: 1826–1831
- Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg 2006; 93: 440–446
- Lee JM, Han JK, Eoh H, Kim SH, Lee JY, Lee MW, Choi BI. Intraoperative radiofrequency ablation using a loop internally cooled-perfusion electrode: In vitro and in vivo experiments. J Surg Res 2006; 131: 215–220
- Raggi MC, Schneider A, Härtl F, Wilhelm D, Wirnhier H, Feussner H. A family of new instruments for laparoscopic radiofrequency ablation of malignant liver lesions. Minim Invasive Ther Allied Technol 2006; 15: 42–46
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559
- Casaril A, Abu Hilal M, Harb A, Campagnaro T, Mansueto G, Nicoli N. The safety of radiofrequency thermal ablation in the treatment of liver malignancies. Eur J Surg Onco 2008; l34: 668–672
- Kobayashi M, Ikeda K, Kawamura Y, Hosaka T, Sezaki H, Yatsuji H, Akuta N, Suzuki F, Suzuki Y, Arase Y, et al. Randomized controlled trial for the efficacy of hepatic arterial occlusion during radiofrequency ablation for small hepatocellular carcinoma - Direct ablative effects and a long-term outcome. Liver Int 2007; 27: 353–257
- Poggi G, Riccardi A, Quaretti P, Teragni C, Delmonte A, Amatu A, Saini G, Mazzucco M, Bernardo A, Palumbo R, et al. Complications of percutaneous radiofrequency thermal ablation of primary and secondary lesions of the liver. Anticancer Res 2007; 27: 2911–2915
- Nouso K, Shiraga K, Uematsu S, Okamoto R, Harada R, Takayama S, Kawai W, Kimura S, Ueki T, Okano N, et al. Prediction of the ablated area by the spread of microbubbles during radiofrequency ablation of hepatocellular carcinoma. Liver Int 2005; 25: 967–971
- Kisaka Y, Hirooka M, Kumagi T, Uehara T, Hiasa Y, Kumano S, Tanaka H, Michitaka K, Horiike N, Mochizuki T, et al. Usefulness of contrast-enhanced ultrasonography with abdominal virtual ultrasonography in assessing therapeutic response in hepatocellular carcinoma treated with radiofrequency ablation. Liver Int 2006; 26: 1241–1245
- Nicolau C, Vilana R, Bianchi L. Early-stage hepatocellular carcinoma: The high accuracy of real-time contrast-enhanced ultrasonography in the assessment of response to percutaneous treatment. Eur Radiol 2007; 17: F80–F85
- Lencioni R, Della Pina C, Crocetti L, Bozzi E, Cioni D. Clinical management of focal liver lesions: The key role of real-time contrast-enhanced US. Eur Radiol 2007; 17: F73–F77